Articles
- Page Path
- HOME > Restor Dent Endod > Volume 47(1); 2022 > Article
- Research Article Morphological characteristics of the mesiobuccal root in the presence of a second mesiobuccal canal: a micro-CT study
-
Lucas P. Lopes Rosado1
 , Matheus Lima Oliveira1
, Matheus Lima Oliveira1 , Karla Rovaris2
, Karla Rovaris2 , Deborah Queiroz Freitas1
, Deborah Queiroz Freitas1 , Frederico Sampaio Neves1,3
, Frederico Sampaio Neves1,3
-
Restor Dent Endod 2022;47(1):e6.
DOI: https://doi.org/10.5395/rde.2022.47.e6
Published online: January 18, 2022
1Department of Oral Diagnosis, Division of Oral Radiology, Piracicaba Dental School, University of Campinas (UNICAMP), Piracicaba, São Paulo, Brazil.
2Department of Pathology and Dentistry Clinic, School of Dentistry, Federal University of Piauí (UFPI), Teresina, Piauí, Brazil.
3Department of Propedeutics and Integrated Clinic, Division of Oral Radiology, School of Dentistry, Federal University of Bahia (UFBA), Salvador, Bahia, Brazil.
- Correspondence to Lucas P. Lopes Rosado, DDS, MSc. Ph.D Student, Department of Oral Diagnosis, Division of Oral Radiology, Piracicaba Dental School, University of Campinas (UNICAMP), Av. Limeira, 901, Piracicaba, São Paulo 13414-903, Brazil. lucaslopesrosado@gmail.com
Copyright © 2022. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives This study investigated the internal morphology of mesiobuccal (MB) roots of maxillary molars with a second mesiobuccal (MB2) canal.
-
Materials and Methods Forty-seven maxillary first or second molars from Brazilians were scanned using micro-computed tomography. The following measurements were obtained from the MB roots: root thickness, root width, and dentin thickness of the buccal aspect of the first mesiobuccal (MB1) canal, between the MB1 and MB2 canals, and the palatal aspect of the MB2 and MB1 canals at 3 mm from the root apex and in the furcation region. For statistical analysis, the Student’s t-test and analysis of variance with the post-hoc Tukey test were used (α = 0.05).
-
Results In maxillary molars with an MB2 canal, MB roots were significantly thicker (p = 0.0014) and narrower (p = 0.0016) than in maxillary molars without an MB2 canal. The dentin thickness of the palatal aspect of the MB1 canal was also significantly greater than that of MB roots without an MB2 canal at 3 mm from the root apex (p = 0.0007) and in the furcation region (p < 0.0001). In the furcation region of maxillary molars with an MB2 canal, the dentin thickness between the MB1 and MB2 canals was significantly smaller than that in the buccal and palatal aspects (p < 0.0001).
-
Conclusions The internal morphology of MB roots of maxillary molars with an MB2 canal revealed differences in dentin thickness, root diameter, and distance between the canals when compared with maxillary molars without an MB2 canal.
INTRODUCTION
MATERIALS AND METHODS
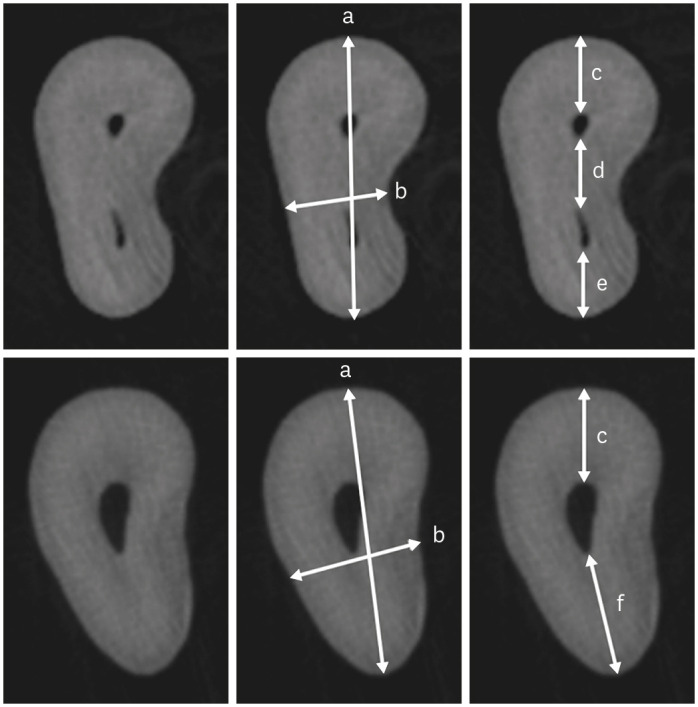
Cropped micro-CT axial sections at 3 mm from the apex of the mesiobuccal root with (upper row) and without an MB2 canal (lower row). For better visualization, the same root is shown in triplicate. (a) Root thickness, (b) Root width, (c) MB1 buccal segment, (d) MB1-MB2 segment, (e) MB2 palatal segment, (f) MB1 palatal segment.
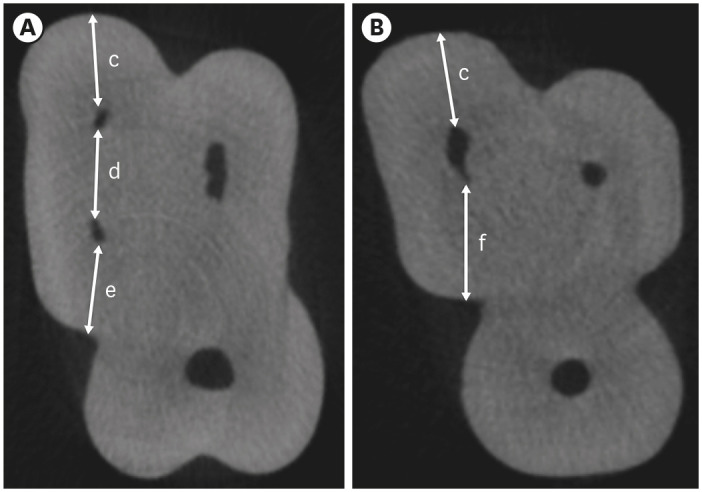
Cropped axial sections of measurements taken from the furcation region of maxillary molars with an MB2 canal (A) and without an MB2 canal (B). (c) MB1 buccal segment, (d) MB1-MB2 segment, (e) MB2 palatal segment, (f) MB1 palatal segment.
RESULTS
Mean (variance) values of the MB root thickness and width in maxillary molars with and without an MB2 canal
| Measurement | MB2 canal | p value | |
|---|---|---|---|
| With | Without | ||
| Root thickness | 5.18 (0.81) | 4.30 (0.42) | 0.0014 |
| Root width | 2.05 (0.20) | 2.55 (0.34) | 0.0016 |
Mean (variance) values of the MB1 buccal and palatal segments in maxillary molars with and without an MB2 canal
Mean, variance, minimum, and maximum values of dentin thickness at 2 axial levels of teeth with and without an MB2 canal
Fraction (as a percentage) of dentin thickness of MB root segments in relation to the total root thickness of teeth with an MB2 canal at 2 axial levels
| Axial level | Root segment | p value | ||
|---|---|---|---|---|
| MB1 buccal | MB1-MB2 | MB2 palatal | ||
| 3 mm from the apex | 0.35 (0.09) | 0.33 (0.15) | 0.32 (0.11) | 0.2710 |
| Furcation region | 0.36 (0.05)A | 0.26 (0.10)B | 0.38 (0.07)A | < 0.0001 |
DISCUSSION
CONCLUSIONS
-
Funding: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Rosado LPL, Oliveira ML, Rovaris K, Freitas DQ, Neves FS.
Data curation: Freitas DQ, Neves FS.
Formal analysis: Rosado LPL, Rovaris K.
Funding acquisition: Rosado LPL, Oliveira ML, Rovaris K, Freitas DQ, Neves FS.
Investigation: Rosado LPL, Oliveira ML, Rovaris K.
Methodology: Rosado LPL, Oliveira ML, Rovaris K.
Project administration: Freitas DQ, Neves FS.
Resources: Rosado LPL, Oliveira ML, Rovaris K.
Software: Rosado LPL.
Supervision: Freitas DQ, Neves FS.
Validation: Rosado LPL, Oliveira ML.
Visualization: Rosado LPL, Oliveira ML, Rovaris K.
Writing - original draft: Rosado LPL, Oliveira ML.
Writing - review & editing: Freitas DQ, Neves FS.
- 1. Karabucak B, Bunes A, Chehoud C, Kohli MR, Setzer F. Prevalence of apical periodontitis in endodontically treated premolars and molars with untreated canal: a cone-beam computed tomography study. J Endod 2016;42:538-541.ArticlePubMed
- 2. Nascimento EHL, Gaêta-Araujo H, Andrade MFS, Freitas DQ. Prevalence of technical errors and periapical lesions in a sample of endodontically treated teeth: a CBCT analysis. Clin Oral Investig 2018;22:2495-2503.ArticlePubMedPDF
- 3. do Carmo WD, Verner FS, Aguiar LM, Visconti MA, Ferreira MD, Lacerda MF, Junqueira RB. Missed canals in endodontically treated maxillary molars of a Brazilian subpopulation: prevalence and association with periapical lesion using cone-beam computed tomography. Clin Oral Investig 2021;25:2317-2323.ArticlePubMedPDF
- 4. Tomaszewska IM, Jarzębska A, Skinningsrud B, Pękala PA, Wroński S, Iwanaga J. An original micro-CT study and meta-analysis of the internal and external anatomy of maxillary molars-implications for endodontic treatment. Clin Anat 2018;31:838-853.ArticlePubMedPDF
- 5. Martins JNR, Alkhawas MAM, Altaki Z, Bellardini G, Berti L, Boveda C, Chaniotis A, Flynn D, Gonzalez JA, Kottoor J, Marques MS, Monroe A, Ounsi HF, Parashos P, Plotino G, Ragnarsson MF, Aguilar RR, Santiago F, Seedat HC, Vargas W, von Zuben M, Zhang Y, Gu Y, Ginjeira A. Worldwide analyses of maxillary first molar second mesiobuccal prevalence: a multicenter cone-beam computed tomographic study. J Endod 2018;44:1641-1649.e1.ArticlePubMed
- 6. Das S, Warhadpande MM, Redij SA, Jibhkate NG, Sabir H. Frequency of second mesiobuccal canal in permanent maxillary first molars using the operating microscope and selective dentin removal: a clinical study. Contemp Clin Dent 2015;6:74-78.ArticlePubMedPMC
- 7. Estrela C, Holland R, Estrela CR, Alencar AH, Sousa-Neto MD, Pécora JD. Characterization of successful root canal treatment. Braz Dent J 2014;25:3-11.ArticlePubMed
- 8. Sipavičiūtė E, Manelienė R. Pain and flare-up after endodontic treatment procedures. Stomatologija 2014;16:25-30.PubMed
- 9. Somma F, Leoni D, Plotino G, Grande NM, Plasschaert A. Root canal morphology of the mesiobuccal root of maxillary first molars: a micro-computed tomographic analysis. Int Endod J 2009;42:165-174.ArticlePubMed
- 10. Verma P, Love RM. A Micro CT study of the mesiobuccal root canal morphology of the maxillary first molar tooth. Int Endod J 2011;44:210-217.ArticlePubMed
- 11. Degerness RA, Bowles WR. Dimension, anatomy and morphology of the mesiobuccal root canal system in maxillary molars. J Endod 2010;36:985-989.ArticlePubMed
- 12. Kim Y, Chang SW, Lee JK, Chen IP, Kaufman B, Jiang J, Cha BY, Zhu Q, Safavi KE, Kum KY. A micro-computed tomography study of canal configuration of multiple-canalled mesiobuccal root of maxillary first molar. Clin Oral Investig 2013;17:1541-1546.ArticlePubMedPDF
- 13. Wolf TG, Paqué F, Woop AC, Willershausen B, Briseño-Marroquín B. Root canal morphology and configuration of 123 maxillary second molars by means of micro-CT. Int J Oral Sci 2017;9:33-37.ArticlePubMedPMCPDF
- 14. Ordinola-Zapata R, Martins JNR, Versiani MA, Bramante CM. Micro-CT analysis of danger zone thickness in the mesiobuccal roots of maxillary first molars. Int Endod J 2019;52:524-529.ArticlePubMedPDF
- 15. Harris SP, Bowles WR, Fok A, McClanahan SB. An anatomic investigation of the mandibular first molar using micro-computed tomography. J Endod 2013;39:1374-1378.ArticlePubMed
- 16. Hiebert BM, Abramovitch K, Rice D, Torabinejad M. Prevalence of second mesiobuccal canals in maxillary first molars detected using cone-beam computed tomography, direct occlusal access, and coronal plane grinding. J Endod 2017;43:1711-1715.ArticlePubMed
- 17. van der Vyver PJ, Paleker F, Vorster M, de Wet FA. Root canal shaping using nickel titanium, M-wire, and Gold wire: a micro-computed tomographic comparative study of one shape, ProTaper Next, and WaveOne gold instruments in maxillary first molars. J Endod 2019;45:62-67.ArticlePubMed
- 18. Zhang Y, Liu J, Gu Y, Wang J, Xu H, Zhang G. Analysis of second mesiobuccal root canal instrumentation in maxillary first molars with three nickel-titanium rotary instruments: a micro-computed tomographic study. Odontology 2021;109:496-505.ArticlePubMedPDF
- 19. Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod 1989;15:512-516.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Effectiveness and safety of three NiTi systems in endodontic retreatment of MB1 and MB2 root canals: a micro-CT and CBCT combined analysis
Airton Oliveira Santos-Junior, Rocharles Cavalcante Fontenele, Karina Ines Medina Carita Tavares, Fernanda Ferrari Esteves Torres, Jáder Camilo Pinto, Pedro Luis Busto Rosim, Andréa Gonçalves, Marco Antonio Hungaro Duarte, Juliane Maria Guerreiro-Tanomaru
Clinical Oral Investigations.2025;[Epub] CrossRef - Cone-beam computed tomography evaluation of root and canal morphology of maxillary molars in a Chinese kazakh population
Shuchun Yang, Chenye Li, Hui Shi, Ming Liu, Xu Wang
BMC Oral Health.2025;[Epub] CrossRef - Can maxillary molar dimensions predict the presence of the second mesiobuccal canal?
Lucas P. Lopes Rosado, Deborah Queiroz Freitas, Karla Rovaris, Matheus L. Oliveira, Frederico Sampaio Neves
Oral Radiology.2023; 39(3): 482. CrossRef - Can the detection of second mesiobuccal canals be enhanced based on the volume of adjacent canals?
Lucas P. Lopes Rosado, Deborah Q. Freitas, Karla Rovaris, Matheus L. Oliveira, Frederico S. Neves
Archives of Oral Biology.2023; 146: 105604. CrossRef - Assessment of the coronal root canal morphology of permanent maxillary first molars using digital 3D-reconstruction technology based on micro-computed tomography data
Mudan Wang, Yuxuan Gao, Qi Deng, Yuan Gao, Dongzhe Song, Dingming Huang
Journal of Dental Sciences.2023; 18(2): 586. CrossRef
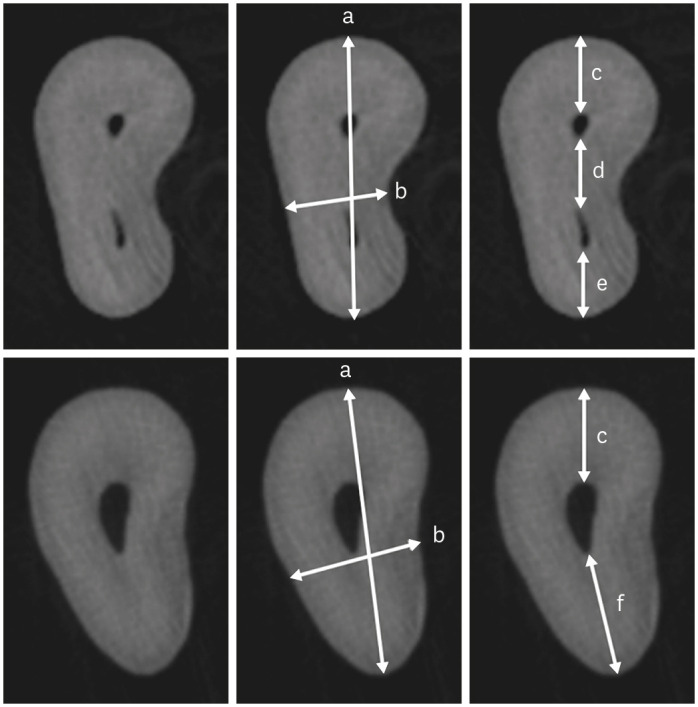
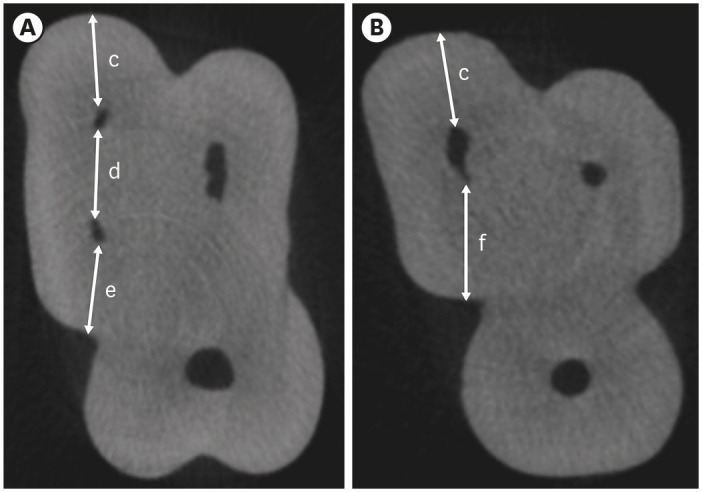
Figure 1
Figure 2
Mean (variance) values of the MB root thickness and width in maxillary molars with and without an MB2 canal
| Measurement | MB2 canal | ||
|---|---|---|---|
| With | Without | ||
| Root thickness | 5.18 (0.81) | 4.30 (0.42) | 0.0014 |
| Root width | 2.05 (0.20) | 2.55 (0.34) | 0.0016 |
Student’s
MB, mesiobuccal.
Mean (variance) values of the MB1 buccal and palatal segments in maxillary molars with and without an MB2 canal
| Axial level | Root segment | MB2 canal | ||
|---|---|---|---|---|
| With | Without | |||
| 3 mm from the apex | MB1 buccal | 1.44 (0.10) | 1.58 (0.12) | 0.1314 |
| MB1 palatal | 2.55 (0.58)* | 1.94 (0.12) | 0.0007 | |
| Furcation region | MB1 buccal | 2.14 (0.11) | 2.32 (0.13) | 0.0850 |
| MB1 palatal | 3.73 (0.33)* | 2.84 (0.20) | < 0.0001 | |
Student’s
MB, mesiobuccal.
*Sum of the MB1-MB2 and MB2 palatal segments.
Mean, variance, minimum, and maximum values of dentin thickness at 2 axial levels of teeth with and without an MB2 canal
| MB2 canal | Axial level | Root segment | Mean | Variance | Minimum | Maximum |
|---|---|---|---|---|---|---|
| With | 3 mm from the apex | MB1 buccal | 1.44 | 0.10 | 0.90 | 2.08 |
| MB1-MB2 | 1.33 | 0.65 | 0.29 | 3.48 | ||
| MB2 palatal | 1.26 | 0.22 | 0.25 | 2.06 | ||
| Furcation region | MB1 buccal | 2.14 | 0.11 | 1.28 | 2.88 | |
| MB1-MB2 | 1.56 | 0.53 | 0.32 | 2.79 | ||
| MB2 palatal | 2.29 | 0.23 | 1.64 | 3.59 | ||
| Without | 3 mm from the apex | MB1 buccal | 1.58 | 0.12 | 1.00 | 2.11 |
| MB1 palatal | 1.94 | 0.12 | 1.23 | 2.60 | ||
| Furcation region | MB1 buccal | 2.32 | 0.13 | 1.66 | 3.09 | |
| MB1 palatal | 2.84 | 0.20 | 2.17 | 3.64 |
MB, mesiobuccal.
Fraction (as a percentage) of dentin thickness of MB root segments in relation to the total root thickness of teeth with an MB2 canal at 2 axial levels
| Axial level | Root segment | |||
|---|---|---|---|---|
| MB1 buccal | MB1-MB2 | MB2 palatal | ||
| 3 mm from the apex | 0.35 (0.09) | 0.33 (0.15) | 0.32 (0.11) | 0.2710 |
| Furcation region | 0.36 (0.05)A | 0.26 (0.10)B | 0.38 (0.07)A | < 0.0001 |
One-way analysis of variance; values followed by different letters are significantly different between the root segments (horizontal comparisons).
Power analysis (0.05) = 0.90.
MB, mesiobuccal.
Student’s
MB, mesiobuccal.
Student’s
MB, mesiobuccal.
*Sum of the MB1-MB2 and MB2 palatal segments.
MB, mesiobuccal.
One-way analysis of variance; values followed by different letters are significantly different between the root segments (horizontal comparisons).
Power analysis (0.05) = 0.90.
MB, mesiobuccal.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

