Abstract
-
Objectives
This study aimed to investigate the endodontic characteristics of mandibular premolars with dens evaginatus (DE) that require endodontic treatment.
-
Materials and Methods
Patients who underwent endodontic treatment were enrolled. The inclusion criteria were patients who underwent root canal treatment in the lower permanent teeth with DE and were followed up for at least 1 year. Preoperative clinical and radiographic variables were obtained. The frequency distribution of the preoperative variables was compared using the χ2 or Fisher’s exact tests. The significance of the change in periapical health index (PAI) and root development stages before and after treatment was examined using the Wilcoxon signed-rank test.
-
Results
A total of 150 teeth of 134 patients with an average age of 15.3 years were included. The percentage distribution comparison of the preoperative variables and obturation techniques revealed significant differences in pulpal and periapical diagnosis, and percussion, and especially regarding age, root development stage, and PAI. Age was the only statistically significant preoperative variable associated with root growth (p < 0.05).
-
Conclusions
Approximately, 60% of DEs requiring endodontic treatment had immature roots. Age being the most significant predisposing factor, early treatment provides the greatest opportunity for full root development.
-
Keywords: Bicuspid; Dental pulp; Endodontics; Humans; Periapical peridontitis; Retrospective studies
INTRODUCTION
Dens evaginatus (DE), a developmental dental abnormality, is a supernumerary tubercle on the palatal surface of the anterior teeth or occlusal surface of the premolars. DE occurs when the pulp extends into the tubercle, and the internal enamel epithelium and dental papilla exfoliate into the stellate reticulum of the enamel organ during the bell stage of tooth formation [
1]. Mandibular premolars account for the majority of bilateral DE cases (59.4%–70.8%), with the second mandibular premolar having the highest prevalence [
2,
3,
4]. DE prevalence varies from 0.1%–15% depending on ethnicity and differences in diagnostic criteria, with a high prevalence (4.3%) in the Mongoloid descendants (Chinese, Japanese, Malay, and Filipino); whereas it is a rare anomaly in the Caucasians [
5,
6,
7].
According to Oehler, the pulp horn extends approximately 70% into the tubercle of the DE; therefore, if the pulp horn is exposed by fracture or wear, or if caries or infection occurs within the microscopic tooth structure, the tooth may gradually become necrotic [
8]. Furthermore, pulpal necrosis can occur in teeth with DE at an early age, when the root is underdeveloped and immature. Wide canals, thin root dentin walls, and open apices impede endodontic therapy when pulpal and periapical diseases affect immature DE teeth. A thin dentin root wall makes the tooth more prone to fracture. In addition, the presence of an open apex makes chemical-mechanical preparation, determination of the working length, and obturation difficult [
9]. These characteristics contribute to the complexity and difficulty of endodontic treatment of DE in young permanent teeth with open apices.
Regarding the endodontic treatment of immature permanent teeth, the usual technique is apexification with periodic changes of calcium hydroxide-based endodontic medication or placement of an apical plug using mineral trioxide aggregate (MTA) [
10]. Both treatments aim to form an apical calcific barrier; they leave the tooth with a thin, fragile dentin wall and do not permit continued root development [
11]. However, regenerative endodontic procedure (REP) is a viable alternative to conventional treatments. REP successfully promotes sustained root development and periapical lesion repair in nonviable immature teeth [
12,
13].
The root growth between different treatment techniques has been compared; however, most studies investigated necrotic immature permanent teeth with DE [
14,
15,
16]. Diseases of pulp tissues are dynamic and progressive, and if left untreated, each disease condition can lead to another. As a result, the signs and symptoms will differ depending on the stage of the disease when the patient presents for treatment [
17]. Various stages of pulpal and periapical diseases exist; for example, symptoms such as pulpitis can occur before pulp necrosis, and periapical disease can occur before any symptoms of pulpal infection. Moreover, research on root growth and relieving periapical inflammation as a function of preoperative tooth conditions are lacking. Notably, preoperative factors, such as the stage of root development, level of periapical inflammation, and clinical symptoms can influence the treatment outcomes.
Therefore, we aimed to investigate the preoperative status of the teeth with DE requiring endodontic treatment. Additionally, concerning the continuation of root development, we aimed to examine the obturation techniques used in permanent DE with open apices based on the preoperative condition of the teeth. Ultimately, this will allow clinicians to know when a tooth requires treatment, so that they can identify the condition of the tooth before an infection occurs, prevent it, and determine the appropriate treatment methods. The null hypothesis was that preoperative factors do not have a significant effect on root growth.
MATERIALS AND METHODS
The present study protocol was reviewed and approved by the Institutional Review Board of Institutional Review Board of the Daejeon Dental Hospital, Wonkwang University College of Dentistry (approval No. W2204/001-1). This retrospective study used patient records from the Wonkwang University Daejeon Dental Hospital database, to protect their privacy and confidentiality. The requirement for informed consent was waived owing to the retrospective nature of the study.
Study population
Patients with pulpal problems in the teeth with DE between November 2012 and 2022 were enrolled. To diagnose DE, we recorded cases in which a fracture of the DE tubercle was observed radiographically or clinically. The following criteria were used to diagnose DE: clinically identified cusp fracture in the electronic record or radiographs showed attrition or fracture of the DE cusp when compared to previous images. Individuals who had received root canal treatment in the lower permanent teeth with DE and with > 1-year follow-up were included. The clinical records eligible for this study included responses to percussion, mobility, and ice tests that confirmed the pulpal and periapical diagnoses. Radiographic images were evaluated using panoramic and periapical radiographs at the initial and final visits after treatment. Teeth extracted during root canal treatment, teeth with incomplete treatment, and primary teeth were excluded from the study. Based on these criteria, from 2012–2022, 134 patients (150 teeth) consulted for treatment of DE in the lower teeth, and they were included in the demographic data. After collecting pre-treatment demographic data, 6 cases were discarded during post-treatment data collection because the dental records lacked postoperative radiographs, or the patient did not complete the treatment. Consequently, 144 teeth were used to compare the radiographic changes before and after treatment.
Data collection
1. Clinical evaluation
Each dental record was obtained by an investigator with special training in endodontics. The clinical and radiographic variables included age at treatment initiation, sex, tooth location, percussion, mobility, swelling or sinus tract, pulpal and periapical diagnosis, stage of root development, radiographic periapical health index (PAI), and obturation technique. Subsequently, 150 teeth were grouped according to the endodontic obturation modality performed. Pulpal and periapical diagnoses were identified by clinical symptoms recorded using the pulpal and periapical diagnostic terminology introduced by the American Association of Endodontists (AAE) [
18]. Each pulpal and periapical diagnoses were associated with a single tooth.
Endodontic therapeutic modalities used in 5 groups
Technique 1: MTA apexification (apexification)
Technique 2: REP
Technique 3: continuous wave technique (CWT)
Technique 4: apicoectomy
Technique 5: pulpotomy with MTA
Regarding clinical records, each variable was graded based on the following criteria commonly used in the clinic.
• Percussion in response to the standard percussion test: no discomfort or pain - 0, slight discomfort or feelings different from normal - 1, moderate discomfort and pain - 2, and severe pain - 3.
• Mobility (grading proposed by Miller [19] classification, 1950): no detectable movement apart from physiologic tooth movement - 0, mobility greater than normal/physiologic - 1, mobility up to 1 mm in the buccolingual direction - 2, and mobility > 1 mm in the buccolingual direction in combination with vertical deformability is present - 3.
• Swelling: no symptoms of swelling or redness - 0, presence of swelling and redness - 1, and fluctuant swelling or abscess - 2.
2. Radiographic evaluation
The radiographs were measured from panoramic and periapical images using INFINIT PACs software (INFINITT Healthcare, Seoul, Korea). To minimize the effect of different observation time points, the preoperative radiographs were used from the first visit, and the postoperative radiographs were chosen from the final radiographs taken after 1 year of treatment. Radiographic comparisons between before and after treatment were analyzed using ImageJ software (version 1.53e, Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). To correct the difference in the acquisition angle of the 2 periapical radiographs, TurboReg plugin (Philippe Thévenaz, Biomedical Imaging Group, Swiss Federal Institute of Technology Lausanne, Lausanne, Switzerland) was used to align 2 images according to a previous study (
Figure 1) [
20].
Figure 1Periapical radiographs of tooth #35. (A) Preoperative radiograph reveals tooth #35 with an open apex and is diagnosed as symptomatic apical periodontitis with pulpal necrosis. (B) Preoperative radiograph obtained after image alignment correction using TurboReg plug-in of Image J (version 1.53e, Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). (C) Final radiograph obtained at follow-up, 32 months postoperatively.
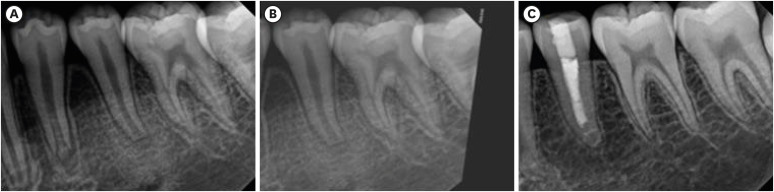
The Tsilingaridis classification was used to determine the stages of root development on preoperative and last-recall radiographs [
21]. The stages of root development were classified as follows: 1 = 1-quarter root formation; 2 = 1-half root formation; 3 = 3-quarters root formation; 4 = full root formation with open apex; 5 = full root formation with half-closed apex; and 6 = full root formation with closed apex. Stages 1–4 indicated very immature, 5 was immature, and 6 was mature roots. To assess the radiographic periapical condition, PAI proposed by Orstavik
et al. [
22] was used. Apical periodontitis was classified as healed (PAI ≤ 2) or diseased (PAI ≥ 3).
Lin
et al. [
12] divided the root development into 4 categories. An increase in root length and a decrease in the apical foramen were characteristics of type I. Types II and III were identified as either an increase in root length or a decrease in the apical foramen alone, while type IV was defined as neither of these changes. Types I, II, and III resulted in further root development.
All radiographs were evaluated preoperatively and at the last follow-up and interpreted by an investigator with special training as a second-year resident in the Department of Endodontics; the interpretation was repeated a month later. Any discrepancies between the results were evaluated by a second investigator (another investigator who was board-certified in endodontics) and finalized by consensus. The intra-examiner reliability was assessed using the intraclass correlation coefficient (ICC).
Statistical analysis
The statistical analysis was performed using the IBM SPSS 25.0 software. The percentage distribution of the patient demographic data was investigated. Following the obturation technique used, the frequencies and percentages of the variables of interest (absolute and relative) were obtained and categorized. The distribution of these frequencies in each of the 5 treatment techniques was compared using the χ2 and Fisher’s exact tests.
The significance of the variations in the PAI and stage of root development before and after treatment was examined using the Wilcoxon signed-rank test. A total of 144 teeth were analyzed for pre- and post-operative PAI changes based on obturation techniques, and 89 immature teeth were analyzed for changes in root development.
Furthermore, the correlation between the stage of root development and preoperative variables using correlation coefficients specific to each type of variable was determined. We used Cramer's V for the 4 nominal qualitative variables (sex, tooth location, pulpal diagnosis, and periapical diagnosis) and Kendall's Tau-B for the 2 common quantitative variables (age and preoperative PAI). Statistical significance was set at p value ≤ 0.05 for all analyses.
RESULTS
Regarding the root development stage and PAI, ICC values were very reliable, with k = 0.967 and 0.937 before treatment and 0.985 and 0.986 after treatment, respectively.
The clinical and demographic characteristics of 134 patients with 150 teeth are presented in
Table 1. Seventy-eight teeth (52%) were from females and 72 (48%) were from males. The average age was 15.3 years (range, 9–51 years), with the largest number of cases in the age group of 10–14 years (54.0%). Cases involving mature teeth or patients aged > 20 years were also observed (21.3%). A total of 150 teeth were distributed across the 4 Tsilingaridis stages. Regarding root development, most cases were categorized as stage 6 (
n = 61, 40.7%), followed by stages 4 (
n = 38, 25.3%) and 5 (
n = 36, 24.0%). Only a few patients were classified as having stage 3 (
n = 15, 10.0%). The most observed clinical symptoms and statuses of periapical health were degree 1 for percussion (90 teeth, 60%), degree 1 for swelling (91 teeth, 60.7%), and degree 3 for PAI (77 teeth, 51.3%), which were statistically significant (
p < 0.05). Based on radiographic PAI, 39.6% of the cases were classified as having healthy periapical tissue (scores 1 and 2) and 60.4% were classified as having unhealthy periapical tissue (scores 3, 4, and 5).
Table 1 Demographic data of the study population
|
Variables |
Categories |
Values |
|
Age |
Mean ± SD |
15.3 ± 6.6 |
|
Sex |
Female |
78 (52.0) |
|
Male |
72 (48.0) |
|
Tooth type |
Mandibular first premolars |
16 (10.7) |
|
Mandibular second premolars |
134 (89.3) |
|
Pulp diagnosis |
Pulpal necrosis |
97 (64.7) |
|
Irreversible pulpitis |
50 (33.3) |
|
Reversible pulpitis |
1 (0.7) |
|
Normal pulp |
2 (1.3) |
|
Periapical diagnosis |
Symptomatic apical periodontitis |
60 (40.0) |
|
Asymptomatic apical periodontitis |
16 (10.7) |
|
Acute apical abscess |
26 (17.3) |
|
Chronic apical abscess |
31 (20.7) |
|
Normal apical tissue |
17 (11.3) |
|
Percussion |
0 |
26 (17.3) |
|
1 |
90 (60.0) |
|
2 |
32 (21.3) |
|
3 |
2 (1.3) |
|
Mobility |
0 |
81 (54.0) |
|
1 |
44 (29.3) |
|
2 |
19 (12.7) |
|
3 |
6 (4.0) |
|
Swelling |
0 |
5 (3.3) |
|
1 |
91 (60.7) |
|
2 |
54 (36.0) |
|
Stages of root development |
3 |
15 (10.0) |
|
4 |
38 (25.3) |
|
5 |
36 (24.0) |
|
6 |
61 (40.7) |
|
Radiographic periapical health |
1 |
17 (11.3) |
|
2 |
42 (28.0) |
|
3 |
77 (51.3) |
|
4 |
14 (9.4) |
|
5 |
0 (0.0) |
All studied cases were distributed according to the 5 endodontic therapeutic modalities technique 1 (apexification) = 31, technique 2 (REP) = 54, technique 3 (CWT) = 63, technique 4 (apical surgery) = 1, and technique 5 (pulpotomy) = 1. As techniques 4 and 5 had only 1 observation each, they were excluded from the results in
Tables 2,
3, and
4. Similarly, regarding stages of root development, stages 1 and 2 were not observed, and as a result, they are not represented in
Table 2. Regarding the comparison between the treatment modalities and 9 preoperative variables' percentage distribution, particularly, age (
p < 0.001), stage of root development (
p < 0.001), and PAI (
p = 0.005) showed significant differences. Other significant differences were seen in pulpal (
p = 0.040) and periapical diagnosis (
p = 0.030), and percussion (
p = 0.019).
Table 2 Distribution of the preoperative variables according to the endodontic obturation techniques
|
Variables |
Obturation technique modality total |
Total |
χ2 (p) |
|
Apexification |
REP |
CWT |
|
Age groups (yr) |
|
|
|
|
85.535† (0.000) |
|
< 10 |
2 |
6 |
0 |
8 (5.3) |
|
10–14 |
24 |
41 |
15 |
81 (54.0) |
|
15–19 |
2 |
7 |
19 |
29 (19.3) |
|
20–29 |
2 |
0 |
24 |
26 (17.3) |
|
> 30 |
1 |
0 |
5 |
6 (4.0) |
|
Stages of root development |
|
|
|
|
119.138† (0.000) |
|
3 |
3 |
11 |
0 |
15 (10.0) |
|
4 |
15 |
20 |
2 |
38 (25.3) |
|
5 |
10 |
20 |
6 |
36 (24.0) |
|
6 |
3 |
3 |
55 |
61 (40.7) |
|
Percussion |
|
|
|
|
23.508* (0.019) |
|
0 |
5 |
6 |
13 |
26 (17.3) |
|
1 |
23 |
28 |
39 |
90 (60.0) |
|
2 |
3 |
18 |
11 |
32 (21.3) |
|
3 |
0 |
2 |
0 |
2 (1.3) |
|
Mobility |
|
|
|
|
17.436 (0.135) |
|
0 |
19 |
21 |
39 |
81 (54.0) |
|
1 |
10 |
17 |
17 |
44 (29.3) |
|
2 |
2 |
11 |
6 |
19 (12.7) |
|
3 |
0 |
5 |
1 |
6 (4.0) |
|
Swelling |
|
|
|
|
14.020 (0.075) |
|
0 |
2 |
3 |
0 |
5 (3.3) |
|
1 |
18 |
27 |
45 |
91 (60.7) |
|
2 |
11 |
24 |
18 |
54 (36.0) |
|
Periapical health index |
|
|
|
|
25.148† (0.005) |
|
1 |
2 |
1 |
13 |
17 (11.4) |
|
2 |
8 |
20 |
14 |
42 (28.2) |
|
3 |
18 |
30 |
28 |
76 (51.0) |
|
4 |
3 |
3 |
7 |
14 (9.4) |
|
5 |
0 |
0 |
0 |
0 (0.0) |
Table 3 Periapical health index according to the endodontic obturation techniques of 144 teeth
|
Obturation technique |
Preoperative |
Postoperative |
Z |
p value |
|
Apexification |
2.71 ± 0.74 |
1.52 ± 0.77 |
−4.210 |
0.000*
|
|
REP |
2.65 ± 0.62 |
1.54 ± 0.72 |
−5.591 |
0.000*
|
|
CWT |
2.44 ± 0.98 |
1.63 ± 0.96 |
−4.738 |
0.000*
|
Table 4 Root development stage according to the endodontic obturation techniques of 89 immature teeth
|
Obturation technique |
Preoperative |
Postoperative |
Z |
p value |
|
Apexification |
4.25 ± 0.65 |
4.79 ± 1.13 |
−2.696 |
0.007†
|
|
REP |
4.18 ± 0.77 |
4.94 ± 0.97 |
−4.686 |
0.000†
|
|
CWT |
4.75 ± 0.46 |
5.25 ± 0.46 |
−2.000 |
0.046*
|
Six cases were excluded from the degree of PAI comparison before and after the procedure. The changes in the pre- and post-operative PAI for all 144 teeth are presented in
Table 3, and the degree of root growth in the 89 immature teeth is presented in
Table 4. All obturation techniques showed a significant reduction in PAI and root growth after treatment (
p < 0.05) (
Tables 3 and
4). When we examined the correlation between the preoperative variables and root growth in this study, age was the only statistically significant factor (
p < 0.001) (
Table 5).
Table 5 Correlation between root growth and preoperative variables
|
Variables |
Value |
p value |
Correlation coefficient |
|
Sex |
0.150 |
0.625 |
Cramer's V |
|
Tooth location |
0.150 |
0.737 |
Cramer's V |
|
Pulpal diagnosis |
0.232 |
0.118 |
Cramer's V |
|
Periapical diagnosis |
0.249 |
0.163 |
Cramer's V |
|
Pre-treatment PAI |
0.112 |
0.241 |
Kendall's tau B |
|
Age |
0.290 |
0.000*
|
Kendall's tau B |
DISCUSSION
DE occurs most frequently in the mandibular premolars of young patients, especially the second premolars, which is consistent with this study population containing 135 mandibular second premolars (89.3%) in a study participants group. The most observed clinical symptoms were degree 1 for percussion (90 teeth, 60%), degree 1 for swelling (91 teeth, 60.7%), and degree 3 for PAI (77 teeth, 51.3%), which were statistically significant (p < 0.05), and 60.4% of total were classified as having unhealthy periapical tissue (scores 3, 4, and 5). This can be explained by the fact that swelling, pain, and tenderness are typical onset signs of periapical inflammation and disease.
In this study, we assessed pulpal and periapical diagnoses as possible prognostic factors. Using the most recent AAE diagnostic terminology, all cases were classified into 4 clinical groups based on the periapical conditions: asymptomatic apical periodontitis, symptomatic apical periodontitis, chronic apical abscess, and acute apical abscess. A retrospective study by Chrepa
et al. [
23] demonstrated that periapical diagnosis according to the AAE criteria was a significant predictor of radiographic periapical area (RRA) change after REP, indicating that the infection/inflammatory status of the periapical region may affect the control over root development. The demographics in our study indicated that the most observed conditions were pulpal necrosis (97 teeth, 64.7%) and symptomatic apical periodontitis (60 teeth, 40%). However, in the present study, diagnosis had no significant effect on root development, suggesting that clinical diagnosis may not be used to select treatment techniques such as REP. There are several reasons that may have contributed to the conflicting results, including the heterogeneous variables and different statistical techniques applied across studies. Moreover, the diagnosis was evaluated based on clinical records and radiographic examinations, which can be biased by the evaluator and lead to inaccurate judgments if there are insufficient records. In addition, individuals’ reactions and perceptions of pain vary depending on their emotional state and coping strategies. The signs and symptoms of different pulp conditions often overlap due to the disease’s progressive nature and dynamic interactions [
17]. As a result, in this study, we identified the most likely diagnoses based on study participants’ clinical findings and radiographic evaluations commonly presented in AAE criteria within limitations.
The prognostic significance of root development status was also assessed. In the present study, all teeth were distributed across the 4 Tsilingaridis stages, from stage 3 to 6, and 59.3% (89 teeth) were immature. The stage of root development had no significant effect on root growth, which is inconsistent with the results of previous studies [
23,
24]. Chan
et al. [
13] explained that REPs in immature teeth can help achieve the expected root growth by controlling infection; however, root growth does not increase beyond the prescribed amount. Therefore, the more advanced the root development, the less tooth growth remains; accordingly, apexification with MTA plugs is a more reliable prognosis than REPs [
25]. In our study, the preoperative status of pulpal necrosis in symptomatic apical periodontitis with PAI degree 3 was dominant, which means that pulpal necrosis and periapical inflammation were already quite advanced by the time treatment was required. When pulpal necrosis progresses in immature teeth, the teeth remain open at the apex and growth is inhibited, which can affect the stage of root development. Therefore, regardless of age, the teeth become underdeveloped, which explains why immature permanent teeth with open apices can develop in adults.
Nevertheless, immature permanent teeth have large root canals and apical foramina that allow for regenerative pulp tissue and vascular growth. Younger people also have looser cancellous bone and thinner cortical laminae, which lead to a more rapid infection, which produces pain and swelling quickly in the early stages of infection [
26]. Similarly, periapical disease can also occur when the apical foramen is open, allowing communication between periapical tissues and resulting in partial pulpal necrosis. Therefore, even in a necrotic root canal, the removal of necrotic tissue and resolution of periapical inflammation promptly are beneficial for root growth because viable pulp tissue may still be present in a wide canal.
Younger teeth have more potential for stem cell pulp regeneration, which improves their ability to heal. Regardless of the preoperative apical diameter, Estefan
et al. [
25] investigated the impact of age (9–18 years) on the success of REP and revealed a significant increase in length in the younger group (9–13 years) compared to the older group (14–18 years). In a San Antonio study involving study participants aged 7–26 years, Chrepa
et al. [
23] discovered that age was a significant predictor of failure and RRA change, and that the gain in RRA decreased with increasing age. Consistent with previous studies, age was the only preoperative variable that significantly affected root growth in the immature teeth in this study, therefore, the null hypothesis was rejected.
DE is not only a developmental abnormality but has several clinical implications. Oehlers
et al. [
27] performed a histological examination of 22 extracted teeth due to pulpal/periapical disease or orthodontic reasons out of 153 DE premolars, and only 15 teeth had the possibility of pulpal extension into the DE tubercles. The prevalence of pulp exposure after tubercle fracture was < 10% (15/153 teeth). Tubercle wear and fracture can result in pulpal complications with incomplete root formation seriously affecting tooth survival. However, not all cases of tubercle fractures cause dental problems, and approximately 1.1% of teeth eventually develop pulpal complications [
12]. Moreover, DE occurs primarily in Asian populations (including Chinese, Malay, Thai, Japanese, Filipino, and Indians), with prevalence rates ranging from 0.5%–4.3%, depending on the population studied [
28]. Considering that the prevalence of DE is 4.3% or less, and pulpal complications occur in only 1.1%–10% of DE cases, it was unusual for our study to collect a large number of cases (150 teeth in 134 patients). We aimed to investigate demographic data and dental conditions at the onset time of pulpal complications caused by DE so that they could be prevented and treated in a timely manner.
Several studies have examined the prognostic factors influencing the outcome of REP to provide guidance for clinical practice; however, the limitations of the various study designs and sample sizes (50, 62, and 46 cases, respectively) prevented these studies from reaching firm conclusions [
3,
29,
30]. Therefore, to determine the preoperative factors that might have an impact on the outcome depending on the treatment approach, this retrospective study was designed based on a relatively large sample size containing 134 patients. This study focused on the effects of preoperative factors on root development and periapical inflammation resolution following each treatment method based on the standard procedures followed by the patients included in the follow-up.
This study included patients who were followed up for > 12 months after root canal treatment. Sang
et al. [
31] reported that root growth gradually slowed after 6 months of regenerative root canal therapy. Chueh
et al. [
24] reported that healing of periapical lesions was observed at an average of 8 months after regenerative endodontic treatment. The quality of evidence for treatment factors influencing the outcomes of primary endodontic treatment was analyzed by Ng
et al. [
32]. Studies mentioned above have recommended standardization of the study design, data collection, and results, emphasizing the need for a minimum 1 year follow-up period and, ideally, a minimum of 3 years of observation. Therefore, 12 months after endodontic treatment can be considered the minimum time to identify changes in periapical healing and root growth in our study.
The present study has several limitations. First, identifying a specific histopathologic condition of the pulp based on clinical diagnosis is impossible. Inflammation of the pulp may have passed a reversible stage; therefore, preventive measures for teeth with DE may be insufficient, leading to failure. Further, root growth measurements using periapical imaging are limited. Periapical images are limited in their analysis towing to overlapping of surrounding structures, and errors can occur depending on the position of the sensor and canal. To compensate for this, we used the Turboreg plugin; however, it was difficult to make comparisons when there were severe differences in shooting conditions.
For clinicians, how to manage DE may be challenging. The mean age of symptomatic pulpal complications with DE was 15.3 ± 6.6 in the present study. As a result, individuals with DE, even if asymptomatic, should be closely monitored beginning around the age of 15 years. The early detection and prophylaxis of DE teeth while the pulp status is still normal are important to preserve pulp vitality and continued root formation, as well as to prevent the development of pulpal pathology [
1]. To prevent DE fracture, it is recommended to check the occlusion on a regular basis to remove tubercle interference. When the tubercle is fractured or worn, the vitality of DE should be preserved by performing prep-and-fill techniques to block the pathway of bacterial ingress and allow the pulp to heal [
33]. Considering slight discomfort to percussion and swelling were the most observed clinical symptoms in this study, the presence of pulp inflammation should be suspected. Immature teeth were present in 59.3% of the DEs that required treatment, and age had a significant impact on root development. If these clinical signs are detected, treatment should begin as soon as possible, with shorter intervals between visits and frequent monitoring of the symptoms' progression. Treatment options for immature teeth can vary including apical plugs with MTA and REPs. Overall success rates of these techniques ranged from 50% to 98% and the survival rates were between 94% and 100%, if treated properly [
34,
35].
CONCLUSIONS
DE is a developmental abnormality with several clinical implications. Early diagnosis and effective treatment of affected DE teeth are very important in root development. This study found that 59.3% of DEs (average age, 15.3 years) requiring treatment had immature teeth. As age is the most significant predisposing factor, periodic observation and prompt intervention upon symptomatic onset provide the greatest opportunity for full root development while avoiding pathological conditions. Regular examinations and an increased awareness of DE-affected teeth can help dentists make informed decisions regarding prevention and treatment strategies.
-
Funding: This paper was sponsored by Soongsan Fellowship in Wonkwang University in 2023.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Kim M, Seo MS.
Data curation: Kim M.
Formal analysis: Kim M.
Funding acquisition: Seo MS.
Investigation: Kim M.
Methodology: Kim M, Seo MS.
Project administration: Kim M.
Resources: Kim M.
Software: Kim M.
Supervision: Jeon S, Seo MS.
Validation: Kim M.
Visualization: Kim M.
Writing - original draft: Kim M.
Writing - review & editing: Jeon S, Seo MS.
REFERENCES
- 1. Levitan ME, Himel VT. Dens evaginatus: literature review, pathophysiology, and comprehensive treatment regimen. J Endod 2006;32:1-9.ArticlePubMed
- 2. Yip WK. The prevalence of dens evaginatus. Oral Surg Oral Med Oral Pathol 1974;38:80-87.ArticlePubMed
- 3. Reichart P, Tantiniran D. Dens evaginatus in the Thai. An evaluation of fifty-one cases. Oral Surg Oral Med Oral Pathol 1975;39:615-621.PubMed
- 4. Arunyanart O. Dens evaginatus in Bangkok metropolitan schoolchildren in Bangkhaen District. J Dent Assoc Thai 2002;52:120-125.
- 5. Temilola DO, Folayan MO, Fatusi O, Chukwumah NM, Onyejaka N, Oziegbe E, et al. The prevalence, pattern and clinical presentation of developmental dental hard-tissue anomalies in children with primary and mix dentition from Ile-Ife, Nigeria. BMC Oral Health 2014;14:125.ArticlePubMedPMCPDF
- 6. Uslu O, Akcam MO, Evirgen S, Cebeci I. Prevalence of dental anomalies in various malocclusions. Am J Orthod Dentofacial Orthop 2009;135:328-335.ArticlePubMed
- 7. Lin CS, Llacer-Martinez M, Sheth CC, Jovani-Sancho M, Biedma BM. Prevalence of premolars with dens evaginatus in a Taiwanese and Spanish population and related complications of the fracture of its tubercle. Eur Endod J 2018;3:118-122.ArticlePubMedPMC
- 8. Law AS. Considerations for regeneration procedures. J Endod 2013;39:S44-S56.ArticlePubMed
- 9. Alobaid AS, Cortes LM, Lo J, Nguyen TT, Albert J, Abu-Melha AS, et al. Radiographic and clinical outcomes of the treatment of immature permanent teeth by revascularization or apexification: a pilot retrospective cohort study. J Endod 2014;40:1063-1070.ArticlePubMedPMC
- 10. Shabahang S. Treatment options: apexogenesis and apexification. J Endod 2013;39:S26-S29.ArticlePubMed
- 11. Silujjai J, Linsuwanont P. Treatment outcomes of apexification or revascularization in nonvital immature permanent teeth: a retrospective study. J Endod 2017;43:238-245.ArticlePubMed
- 12. Lin J, Zeng Q, Wei X, Zhao W, Cui M, Gu J, et al. Regenerative endodontics versus apexifcation in immature permanent teeth with apical periodontitis: a prospective randomized controlled study. J Endod 2017;43:1821-1827.PubMed
- 13. Chan EK, Desmeules M, Cielecki M, Dabbagh B, Ferraz Dos Santos B. Longitudinal cohort study of regenerative endodontic treatment for immature necrotic permanent teeth. J Endod 2017;43:395-400.ArticlePubMed
- 14. Nolla CM. The development of the permanent teeth. J Dent Child 1960;27:254-266.
- 15. Moorrees CF, Fanning EA, Hunt EE Jr. Age variation of formation for ten permanent teeth. J Dent Res 1963;42:1490-1502.ArticlePubMedPDF
- 16. Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol 1992;8:45-55.ArticlePubMed
- 17. Abbott PV, Yu C. A clinical classification of the status of the pulp and the root canal system. Aust Dent J 2007;52:S17-S31.ArticlePubMed
- 18. American Association of Endodontists. Guide to clinical endodontics. 6th ed. Chicago, IL: American Association of Endodontists; 2013. p. 16.
- 19. Miller SC. Textbook of periodontia. 3rd ed. Philadelphia, PA: The Blakeston Co.; 1950. p. 125.
- 20. Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 2009;35:1343-1349.ArticlePubMed
- 21. Tsilingaridis G, Malmgren B, Andreasen JO, Wigen TI, Maseng Aas AL, Malmgren O. Scandinavian multicenter study on the treatment of 168 patients with 230 intruded permanent teeth - a retrospective cohort study. Dent Traumatol 2016;32:353-360.ArticlePubMed
- 22. Orstavik D, Kerekes K, Eriksen HM. The periapical index: a scoring system for radiographic assessment of apical periodontitis. Endod Dent Traumatol 1986;2:20-34.ArticlePubMed
- 23. Chrepa V, Joon R, Austah O, Diogenes A, Hargreaves KM, Ezeldeen M, et al. Clinical outcomes of immature teeth treated with regenerative endodontic procedures-a San Antonio study. J Endod 2020;46:1074-1084.ArticlePubMed
- 24. Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod 2009;35:160-164.ArticlePubMed
- 25. Estefan BS, El Batouty KM, Nagy MM, Diogenes A. Influence of age and apical diameter on the success of endodontic regeneration procedures. J Endod 2016;42:1620-1625.ArticlePubMed
- 26. Cheng J, Yang F, Li J, Hua F, He M, Song G. Treatment outcomes of regenerative endodontic procedures in traumatized immature permanent necrotic teeth: a retrospective study. J Endod 2022;48:1129-1136.ArticlePubMed
- 27. Oehlers FA, Lee KW, Lee EC. Dens evaginatus (evaginated odontome). Its structure and responses to external stimuli. Dent Pract Dent Rec 1967;17:239-244.PubMed
- 28. Kocsis G, Marcsik A, Kokai E, Kocsis K. Supernumerary occlusal cusps on permanent human teeth. Acta Biol Szeged 2002;46:71-82.
- 29. Reynolds K, Johnson JD, Cohenca N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J 2009;42:84-92.ArticlePubMed
- 30. Curzon ME, Curzon JA, Poyton HG. Evaginated odontomes in the Keewatin Eskimo. Br Dent J 1970;129:324-328.ArticlePubMedPDF
- 31. Sang EJ, Song JS, Shin TJ, Kim YJ, Kim JW, Jang KT, et al. Ratio and rate of induced root growth in necrotic immature teeth. J Korean Acad Pediatr Dent 2018;45:225-234.Article
- 32. Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature - part 1. Effects of study characteristics on probability of success. Int Endod J 2007;40:921-939.ArticlePubMed
- 33. Swift EJ Jr, Trope M, Ritter AV. Vital pulp therapy for the mature tooth – can it work? Endod Topics 2003;5:49-56.Article
- 34. Nicoloso GF, Pötter IG, Rocha RO, Montagner F, Casagrande L. A comparative evaluation of endodontic treatments for immature necrotic permanent teeth based on clinical and radiographic outcomes: a systematic review and meta-analysis. Int J Paediatr Dent 2017;27:217-227.ArticlePubMedPDF
- 35. Rojas-Gutiérrez WJ, Pineda-Vélez E, Agudelo-Suárez AA. Regenerative endodontics success factors and their overall effectiveness: an umbrella review. Iran Endod J 2022;17:90-105.PubMedPMC
 , Sujin Jeon
, Sujin Jeon , Min-Seock Seo
, Min-Seock Seo



 KACD
KACD
 ePub Link
ePub Link Cite
Cite

