Search
- Page Path
- HOME > Search
- High-plasticity mineral trioxide aggregate and its effects on M1 and M2 macrophage viability and adherence, phagocyte activity, production of reactive oxygen species, and cytokines
- Betânia Canal Vasconcellos, Layara Cristine Tomaz Tavares, Danilo Couto da Silva, Francielen Oliveira Fonseca, Francine Benetti, Antônio Paulino Ribeiro Sobrinho, Warley Luciano Fonseca Tavares
- Restor Dent Endod 2023;48(1):e6. Published online December 29, 2022
- DOI: https://doi.org/10.5395/rde.2023.48.e6
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
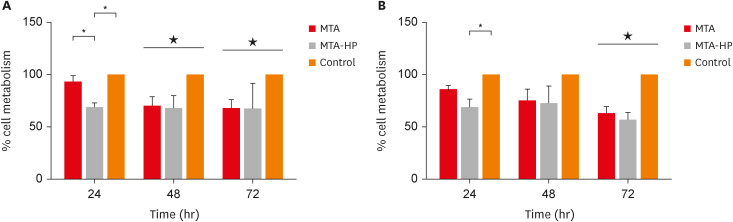
ePub Objectives This study evaluated the effects of high-plasticity mineral trioxide aggregate (MTA-HP) on the activity of M1 and M2 macrophages, compared to white MTA (Angelus).
Materials and Methods Peritoneal inflammatory M1 (from C57BL/6 mice) and M2 (from BALB/c mice) macrophages were cultured in the presence of the tested materials. Cell viability (MTT and trypan blue assays), adhesion, phagocytosis, reactive oxygen species (ROS) production, and tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β production were evaluated. Parametric analysis of variance and the non-parametric Kruskal-Wallis test were used. Results were considered significant when
p < 0.05.Results The MTT assay revealed a significant decrease in M1 metabolism with MTA-HP at 24 hours, and with MTA and MTA-HP later. The trypan blue assay showed significantly fewer live M1 at 48 hours and live M2 at 48 and 72 hours with MTA-HP, compared to MTA. M1 and M2 adherence and phagocytosis showed no significant differences compared to control for both materials. Zymosan A stimulated ROS production by macrophages. In the absence of interferon-γ, TNF-α production by M1 did not significantly differ between groups. For M2, both materials showed higher TNF-α production in the presence of the stimulus, but without significant between-group differences. Likewise, TGF-β production by M1 and M2 macrophages was not significantly different between the groups.
Conclusions M1 and M2 macrophages presented different viability in response to MTA and MTA-HP at different time points. Introducing a plasticizer into the MTA vehicle did not interfere with the activity of M1 and M2 macrophages.
-
Citations
Citations to this article as recorded by- Local Immune Response to Mineral Trioxide Aggregate: A Narrative Review
Shankargouda Patil, Shilpa Bhandi, Oladapo T Okareh
World Journal of Dentistry.2023; 14(4): 382. CrossRef
- Local Immune Response to Mineral Trioxide Aggregate: A Narrative Review
- 1,952 View
- 28 Download
- 1 Crossref

- The effect of mineral trioxide aggregate on the production of growth factors and cytokine by human periodontal ligament fibroblasts
- Ji-Yoon Kwon, Sung-Sam Lim, Seung-Ho Baek, Kwang-Shik Bae, Myung-Hoe Kang, Woocheol Lee
- J Korean Acad Conserv Dent 2007;32(3):191-197. Published online May 31, 2007
- DOI: https://doi.org/10.5395/JKACD.2007.32.3.191
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Mineral trioxide aggregate (MTA) would influence healing of periapical tissues by modulating the production of growth factors and cytokines from PDL fibroblasts, however, the studies are insufficient. Therefore, the purpose of this study was to monitor the expression of transforming growth factor-beta1 (TGF-β1), fibroblast growth factor-2 (FGF-2), and interleukin-6 (IL-6) from PDL fibroblasts in the presence of MTA. The human PDL fibroblasts were seeded onto the set MTA or IRM at a level of 1 × 105 cells per unit well, and further incubated for 6, 12, 24, and 48 hours. The levels of TGF-β1, FGF-2, and IL-6 from the supernatant were measured by enzyme-linked immunosorbent assay (ELISA). The data were analyzed using one-way ANOVA. The level of TGF-β1 was down-regulated when the cells were grown in the presence of MTA except at 6 hours. The levels of FGF-2 release were significantly suppressed when PDL fibroblasts were grown in the presence of MTA or IRM at all time intervals (p < 0.05). The expressions of IL-6 from MTA treated cells were comparable to those of untreated control cells throughout the observation periods. We presume that this material inhibits the stimulatory function of growth factors on granulation tissue formation and in turn, it promotes the healing process modulated by other bone-remodeling cells.
-
Citations
Citations to this article as recorded by- Osteo/odontogenic Differentiation of Human Mesenchymal Stem Cells with Platelet-rich Plasma and Mineral Trioxide Aggregate
Shanthi Vanka, Amit Vanka, Sandeep Kumar Vishwakarma, Manohar K Bhat, Othman Wali, Aleem A Khan
The Journal of Contemporary Dental Practice.2019; 20(10): 1171. CrossRef - The effect of several root-end filling materials on MG63 osteoblast-like cells
Jeong-Ho Lee, Won-Jun Shon, WooCheol Lee, Seung-Ho Baek
Journal of Korean Academy of Conservative Dentistry.2010; 35(3): 222. CrossRef - Biocompatibility of experimental mixture of mineral trioxide aggregate and glass ionomer cement
Min-Jae Oh, Yu-Na Jeong, In-Ho Bae, So-Young Yang, Bum-Jun Park, Jeong-Tae Koh, Yun-Chan Hwang, In-Nam Hwang, Won-Mann Oh
Journal of Korean Academy of Conservative Dentistry.2010; 35(5): 359. CrossRef - Biocompatibility of bioaggregate cement on human pulp and periodontal ligament (PDL) derived cells
Choo-Ryung Chung, Euiseong Kim, Su-Jung Shin
Journal of Korean Academy of Conservative Dentistry.2010; 35(6): 473. CrossRef - Effects of condensation techniques and canal sizes on the microleakage of orthograde MTA apical plug in simulated canals
Deuk-Lim Nam, Jeong-Kil Park, Bock Hur, Hyeon-Cheol Kim
Journal of Korean Academy of Conservative Dentistry.2009; 34(3): 208. CrossRef
- Osteo/odontogenic Differentiation of Human Mesenchymal Stem Cells with Platelet-rich Plasma and Mineral Trioxide Aggregate
- 1,404 View
- 2 Download
- 5 Crossref

-
IL-1 and TNF-α release in human polymorphonuclear leukocytes after exposure to calcium hydroxide treated
Porphyromonas endodontalis lipopolysaccharide - Chan-Je Park, Dong-Sung Park, Hyeon-Mee Yoo, Tae-Seok Oh, Sung-Sam Lim
- J Korean Acad Conserv Dent 2002;27(5):463-472. Published online September 30, 2002
- DOI: https://doi.org/10.5395/JKACD.2002.27.5.463
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Bacterial lipopolysaccharide (LPS) plays a major role in stimulating the synthesis and release of the principal osteoclast-activating cytokines, namely, interleukin 1 and tumor necrosis factor-α from immune cells. Although monocytes/macrophages are the main producers of these cytokines, recent evidence has indicated that polymorphonuclear leukocytes (PMN) have the ability to release IL-1 and TNF-α. Calcium hydroxide has been shown to be an effective medicament in root canal infections, reducing the microbial titre within the canal. It has been proposed that the therapeutic effect of Ca(OH)2 may also be the result of direct inactivation of LPS. The purpose of this study was to investigate whether treatment of Porphyromonas endodontalis LPS with calcium hydroxide alters its biological action as measured by human PMN secretion of IL-1 and TNF-α, and it was compared with Escherichia coli LPS.
P. endodontalis ATCC 35406 was cultured in anaerobic condition, and LPS was extracted using the hot-phenol water extraction method and purified. Purchased E. coli LPS was also purified. 100 µg/ml of each LPS in pyrogen free water were incubated with 25mg/ml Ca(OH)2 at 37℃ for 7 days. The supernatants were subjected to ultrafiltration, and the isolates were lyophilized and weighed. PMNs were obtained from peripheral blood by centrifugation layered over Lymphoprep. The cells were resuspended (4×106 cells/ml) in RPMI 1640 followed by treatment with various concentrations of LPS (0, 0.1, 1, 10µg/ml) for 24 hours at 37℃ in 5% CO2 incubator. The supernatants of cells were collected and the levels of IL-1α, IL-1β and TNF-α were measured by enzyme-linked immunosorbent assay.
The results were as follows;
1. The levels of IL-1α, IL-1β, TNF-α from PMN treated with each LPS were significantly higher than those released from unstimulated PMN of the control group (p<0.05).
2. The levels of all three cytokines released from PMN stimulated with each calcium hydroxide treated LPS were significantly lower than those released from PMN stimulated with each untreated LPS (p<0.05), while they were not significantly different from those released from unstimulated PMN of the control group (p>0.05).
3. The levels of secretion for all three cytokines were affected in a dose-dependent manner in PMN stimulated with each LPS (p<0.05), but not in PMN stimulated with each calcium hydroxide treated LPS (p>0.05).
4. The levels of all three cytokines released from PMN stimulated with P. endodontalis LPS were significantly lower than those released from PMN stimulated with E. coli LPS (p<0.05).
-
Citations
Citations to this article as recorded by- Lipopolysaccharide from Porphyromonas gingivalis, but Not from Porphyromonas endodontalis, Induces Macrophage M1 Profile
Pablo Veloso, Alejandra Fernández, Jessica Astorga, David González-Quintanilla, Alfredo Castro, Alejandro Escobar, Anilei Hoare, Marcela Hernández
International Journal of Molecular Sciences.2022; 23(17): 10011. CrossRef
- Lipopolysaccharide from Porphyromonas gingivalis, but Not from Porphyromonas endodontalis, Induces Macrophage M1 Profile
- 1,035 View
- 0 Download
- 1 Crossref


 KACD
KACD

 First
First Prev
Prev


