Abstract
-
Objectives
This study evaluated the effects of high-plasticity mineral trioxide aggregate (MTA-HP) on the activity of M1 and M2 macrophages, compared to white MTA (Angelus).
-
Materials and Methods
Peritoneal inflammatory M1 (from C57BL/6 mice) and M2 (from BALB/c mice) macrophages were cultured in the presence of the tested materials. Cell viability (MTT and trypan blue assays), adhesion, phagocytosis, reactive oxygen species (ROS) production, and tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β production were evaluated. Parametric analysis of variance and the non-parametric Kruskal-Wallis test were used. Results were considered significant when p < 0.05.
-
Results
The MTT assay revealed a significant decrease in M1 metabolism with MTA-HP at 24 hours, and with MTA and MTA-HP later. The trypan blue assay showed significantly fewer live M1 at 48 hours and live M2 at 48 and 72 hours with MTA-HP, compared to MTA. M1 and M2 adherence and phagocytosis showed no significant differences compared to control for both materials. Zymosan A stimulated ROS production by macrophages. In the absence of interferon-γ, TNF-α production by M1 did not significantly differ between groups. For M2, both materials showed higher TNF-α production in the presence of the stimulus, but without significant between-group differences. Likewise, TGF-β production by M1 and M2 macrophages was not significantly different between the groups.
-
Conclusions
M1 and M2 macrophages presented different viability in response to MTA and MTA-HP at different time points. Introducing a plasticizer into the MTA vehicle did not interfere with the activity of M1 and M2 macrophages.
-
Keywords: Cytokines; Macrophages; Mineral trioxide aggregate
INTRODUCTION
Mineral trioxide aggregate (MTA) is a calcium silicate-based material used for pulp capping, pulpotomy, apexogenesis, apical barrier formation in teeth with open apexes, repair of root perforations, and as a root canal filling material [
1]. MTA has a bioactive nature, which makes it able to induce interactions between tissues interfaces, culminating in the deposition of a layer of carbonate apatite, which constitutes the mineral phase of hard tissues, such as bone, dentin, and cementum [
2,
3,
4]. This biomaterial also induces the release of ionic components that interfere with cellular enzymatic activity, therefore allowing cell adhesion, proliferation, and growth, as well as the production of a mineralized matrix, which is related to its marginal sealing ability [
5,
6,
7]. However, MTA has shown some clinical limitations, such as difficulty in handling and lack of flow [
8,
9,
10].
In order to improve the physical-chemical properties and material handling, high-plasticity MTA repair cement (MTA-HP, Angelus, Londrina, PR, Brazil) was developed using a vehicle containing water and a plasticizing polymer to be mixed with the MTA powder. Clinically, this new material presents HP and, therefore, greater ease of insertion and handling [
8,
9,
10].
Due to the indications of MTA, it is usually applied in direct contact with cells in the periodontal ligament. Macrophages are present in inflamed pulp and periradicular tissues, and they may modulate the immune-inflammatory response [
11,
12,
13,
14]. Among the functions of these cells are the elimination of invasive bacteria and the recruitment of other cells to the site of inflammation [
15].
Macrophages can be split into 2 subtypes: M1 and M2 cells. Type 1 macrophages (M1) are activated in response to lipopolysaccharide (LPS) in the presence of tumor necrosis factor (TNF)-α. These cells present a greater phagocytic capacity due to the production of high levels of reactive oxygen species (ROS), which promote the elimination of aggressive agents, in addition to inducing the activation of the T-helper 1 lymphocyte response [
14,
16].
In contrast, type 2 macrophages (M2) are activated through the alternative route. This activation occurs when macrophages are exposed to a microenvironment consisting of interleukin (IL)-4, IL-13, IL-10, or corticosteroids, promoting the conversion of arginase into ornithine and urea during arginine metabolism, with collagen and cell proliferation as the final products. These cells favor the production of anti-inflammatory cytokines, such as IL-10 and transforming growth factor (TGF)-β [
16,
17,
18]. Thus, M1 and M2 are essential in the healing process and in modulating the inflammatory response [
19].
Previous studies have evaluated the responses of macrophages and other cell types, such as human dental pulp stem cells and fibroblasts, in contact with MTA [
17,
18,
20,
21]. However, only 1 study evaluated the response of macrophages to MTA-HP, demonstrating that the use of MTA-HP was not related to an increase in the inflammatory response associated with the activity of metalloproteinases [
22]. However, the influence of this material on the immune-inflammatory response of M1 and M2 macrophages has not yet been elucidated.
This study aimed to evaluate the influence of MTA-HP on cell viability, adherence, phagocytosis, and the production of ROS, TNF-α, and TGF-β in M1 and M2 macrophages, compared to white MTA. The null hypothesis was that macrophages would not show different results in the presence of MTA-HP and conventional white MTA.
MATERIALS AND METHODS
Mice
The experimental protocol was submitted to and approved by the ethics committee on the use of animals of the Universidade Federal de Minas Gerais (CEUA – UFMG) under protocol #15/2018. A total of 16 female mice were used in this study. The sample size was based on previous studies [
12,
13,
14] and on statistical power calculations. Sixteen female mice 4 to 8 weeks of age (8 C57BL/6 mice for M1 macrophages and 8 BALB/c mice for M2 macrophages) (CEBIO – UFMG, Belo Horizonte, MG, Brazil) were housed with barriers in a temperature-controlled environment (22°C ± 1°C, 70% humidity, and a 12-hour light-dark cycle). Water and food were offered
ad libitum. All protocols of this study were approved by the local Ethics Committee (CEUA/UFMG – 15\2018).
To obtain inflammatory macrophages, 2 mL of 3% thioglycolate broth containing 1% sterile agar (Biobras S.A., Montes Claros, MG, Brazil) was injected into the peritoneal cavity of C57BL/6 and BALB/c mice. After 5 days, which is the time needed to induce the local inflammatory process with macrophage recruitment, the animals were euthanized by an anesthesia overdose.
In order to obtain the highest possible cell suspension content, 10 mL of sterile phosphate-buffered saline (PBS) medium was injected into the peritoneal cavity of the mice using a syringe attached to a 40 × 16 mm needle. Afterward, the cells were centrifuged at 350 rotations per minute for 10 minutes at 4°C. The supernatant was discarded; the cells were resuspended in RPMI 1640 complete medium (Sigma Chemicals Co., St Louis, MO, USA), supplemented with 10% fetal calf serum (Nutricell, Campinas, SP, Brazil), 0.1% of 0.05 mg/mL. Mercaptoethanol (Sigma Chemicals Co.), 0.2% penicillin (100 U/mL)/streptomycin (0.1 mg/mL), and 200 mg/mL glutamine and counted in a Newbauer chamber, using an optical microscope. It was observed that more than 90% of the cells had the morphological characteristics of macrophages [
13]. The cell concentration was adjusted to 5 × 10
5 cells for assays of cell viability, phagocytosis, and ROS production, 1 × 10
6 cells for cell adherence testing, and 2 × 10
6 cells for the measurement of cytokines [
12,
13,
14]. All incubations were carried out in a humidified atmospheric oven, containing 5% CO
2, at 37°C [
12].
White MTA and MTA-HP were manipulated according to the manufacturer’s instructions under sterile conditions in a laminar flow chamber. Soon after preparation, MTA and MTA-HP were inserted into the tips of previously sectioned sterilized capillary tubes to ensure that their contact with the cell suspension could be standardized [
13]. Empty capillary tubes were used in control cultures.
Two methods were used to analyze cell viability: the trypan blue exclusion assay and the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, at 3 different times (after 24, 48, and 72 hours of incubation). MTT was used to test the viability of cells in the presence of capillary tubes by culturing 1 × 10
6 cells in 96-well culture plates. Specifically, 100 µL of RPMI 1640 supplemented with 10% fetal bovine serum, penicillin, and streptomycin containing 1 × 10
6 cells/well, together with the capillary tubes, was seeded in 96-well plates and incubated for 24 hours at 37°C. Additionally, 200 µL of the cell suspension and capillaries for each tested group were added to 96-well culture plates in triplicate. After 24 hours, the culture medium was removed, and the cells were gently washed with PBS. A volume of 100 mL of MTT-succinate solution (1 mg/mL) was added to each well, and the cells were incubated for 4 additional hours. Dimethyl sulfide (100 mL, Sigma-Aldrich, St Louis, MO, USA) was added to each well, and the readings were performed using a microplate reader (Bio-Rad 2550, Bio-Rad, Hercules, CA, USA) [
12].
Cell viability assayed by trypan blue exclusion was performed in 24-well culture plates (2 × 10
5 cells /mL) for 24, 48, and 72 hours [
12,
15]. Briefly, cells were incubated in the presence of capillary tubes in 1 mL of RPMI (Sigma Chemical Co.) containing 10% fetal calf serum (Nutricell), 2 mM L-glutamine, 100 U/mL of penicillin, and 100 µg mg/mL of streptomycin at 37°C in a 5% CO
2 humidified atmosphere. After incubation, 100 µL of 0.25% trypan blue (Sigma Chemical Co.) in saline was added, and cultures were examined under an inverted microscope. At least 300 cells were counted per culture (performed in triplicate), and the results were expressed as the percentage of viability. The experiment was repeated 3 times [
13,
14,
23].
Sterile polypropylene tubes, to which the cell suspension and capillaries were added, were incubated for 2 hours in an incubator with a humidified atmosphere containing 5% CO
2 at 37°C. The tubes were incubated and vortexed at a low speed for 5 seconds. Then, 20 μL from each tube was removed and placed into Newbauer chambers and incubated for 18 hours at 37°C. The percentage of adherent and non-adherent macrophages was then established by counting under an optical microscope [
24].
In 24-well culture plates (Nunclon, Nalge Nunc International, Miami, FL, USA), cells (1 × 10
6 in 1 mL) were incubated for 2 hours. A sterile round glass coverslip was placed in each well. Non-adherent cells were removed by washing with warm complete medium; afterward, 10
7 colony-forming units of
Saccharomyces boulardii (Floratil, Merck S.A., Rio de Janeiro, RJ, Brazil) and capillaries with or without sealers were added to the medium, and the plates were incubated for 1 hour. Then, the plates were washed with phenol red-free RPMI 1640 and subsequently filled with 1 mL of the same substance, in addition to the cement-containing capillaries and 10 µL of
S. boulardii suspension at 10
7 cells/mL (Floratil, Merck SA). Unbound yeast cells were removed by washing with a complete medium, and the coverslips were covered for 1 minute with 1 mL of tannic acid at 1% (Merck, Rahway, NJ, USA), so that extracellular and intracellular yeast cells could be observed. One drop of fetal calf serum was applied onto each coverslip. The dried coverslips were stained with Panotico Rapido (Laborclin Ltd., Pinhais, PR, Brazil) and glued to microscope glass slides with Entellan (Merck) [
13,
23]. The coverslips were analyzed under optical microscopy in oil immersion at ×100 magnification, through which a minimum of 200 macrophages were counted, and the percentage of macrophages with phagocytosed
S. boulardii was assessed [
25].
Cells were cultured, with or without sealers, in an opaque 96-well plate, at 24 hours. Then, 1 × 10
6 cells were transferred to a C96 White Maxisorp (Nalgene, Rochester, NY, USA) plate in 100 µL, and 10
7 zymosan A particles (Sigma Chemical Co.) and 0.05 mmol/L luminol in 1640 RPMI without phenol red were added to each well. The plates were read every 2 minutes for 118 minutes in a luminometer (LumiCount, Packard Instrument Company Inc., Downers Grove, IL, USA) [
26]. The results were expressed as the area under each curve obtained in the 118-minute period [
23]. The experiments were performed 3 times in triplicate.
In a 24-well plate, a peritoneal cell suspension (2 × 106 cells/well) and capillaries, with or without sealers, were cultured in a CO2 incubator at 37°C. For the TNF-α assay, cells were cultured in the presence or absence of 10 U/mL of recombinant murine interferon (IFN)-γ (Pharmingem, San Diego, CA, USA), in a final volume of 1 mL, for 24 hours. The supernatants were harvested, and cytokine readings were performed using the Duo Set Elisa TNF kit (B&D Industrial, Macon, GA, USA), following the manufacturer’s guidelines. To evaluate TGF-β detection, half of the plate was stimulated with 50 μL of LPS at a concentration of 100 ng/mL to activate the macrophages. The supernatant was collected after 72 hours of incubation, while the other half remained without any stimulation. Cytokines were detected in the supernatant using the R&D Systems enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s recommendations.
Statistical analysis
The results were analyzed using GraphPad Prism v. 7.04 (GraphPad Software, San Diego, CA, USA). The Shapiro-Wilk test was used to check for normality, and parametric analysis of variance test was used for multiple comparisons; the proposed analyses were considered significant when
p < 0.05 [
27,
28].
RESULTS
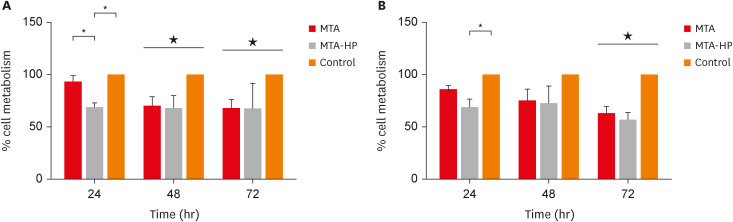
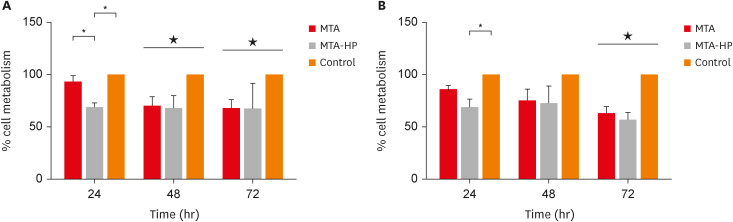
MTT assay
For M1 macrophages, MTA-HP decreased cell viability in comparison to MTA and the control group at 24 hours (
p < 0.05). Subsequently, at 48 and 72 hours, both sealers (MTA and MTA-HP) reduced M1 cell viability compared to control cells (
p < 0.05). For M2 macrophages, MTA-HP decreased cell viability at 24 hours compared to the control (
p < 0.05), but at 48 hours, both sealers behaved similarly to the control group (
p > 0.05). As observed in M1 macrophages, at 72 hours, both sealers decreased M2 cell viability (
p < 0.05) (
Figure 1).
Figure 1
Percentage of living M1 (A) and M2 (B) macrophages after incubation in 96-well culture plates with capillaries containing MTA and MTA-HP by the MTT assay. Controls were cultured with empty capillaries. Cultures were maintained for 24, 48, and 72 hours, as described in the Materials and Methods. Bars represent the mean of 2 experiments; lines denote the standard error of the mean.
MTA, mineral trioxide aggregate; HP, high-plasticity.
*A statistically significant difference in cell viability between the 2 macrophage culture conditions and cell viability between both macrophage cultures stimulated by MTA and MTA-HP compared to control (p < 0.05), by analysis of variance, and ★ indicates difference between the two materials compared to control group (P<0.05).
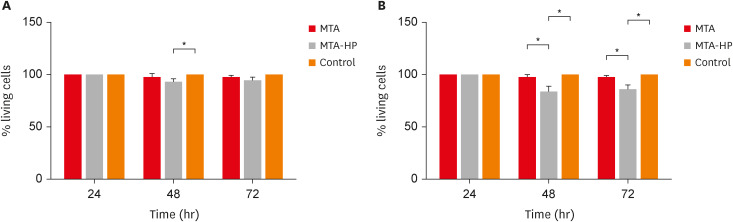
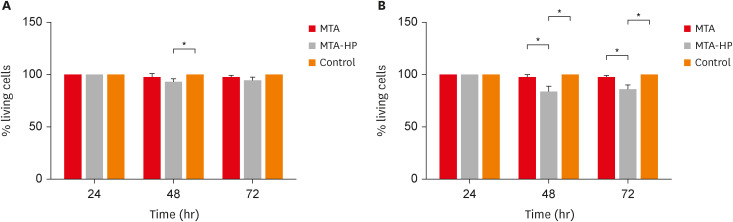
Trypan blue exclusion
The results for cell viability evaluated using the trypan blue exclusion method are shown in
Figure 2. For M1 macrophages, MTA-HP decreased cell viability compared to control cells only at 48 hours (
p < 0.05). However, both sealers (MTA and MTA-HP) reduced M2 cell viability at 48 and 72 hours (
p < 0.05) (
Figure 2).
Figure 2
Percentage of living M1 (A) and M2 (B) macrophages after incubation in 24-well culture plates with capillaries containing MTA and MTA-HP by the trypan blue exclusion assay. The controls were cultured with empty capillaries. Cultures were maintained for 24, 48, and 72 hours as described in the Materials and Methods. Bars represent the mean of 2 experiments; lines denote the standard error of the mean.
MTA, mineral trioxide aggregate; HP, high-plasticity.
*A statistically significant difference in cell viability between the 2 macrophage culture conditions and cell viability between both macrophage cultures stimulated by MTA and MTA-HP compared to control (p < 0.05), by analysis of variance.
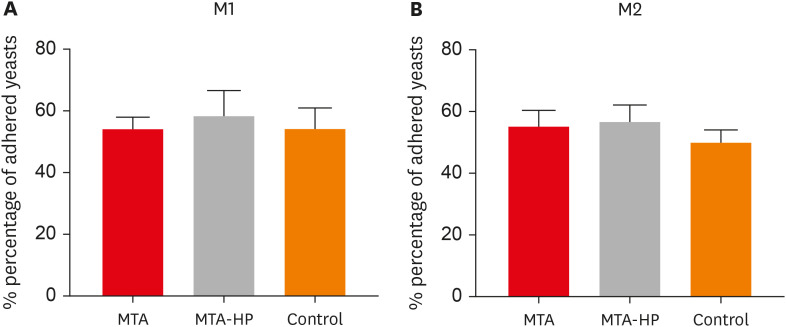
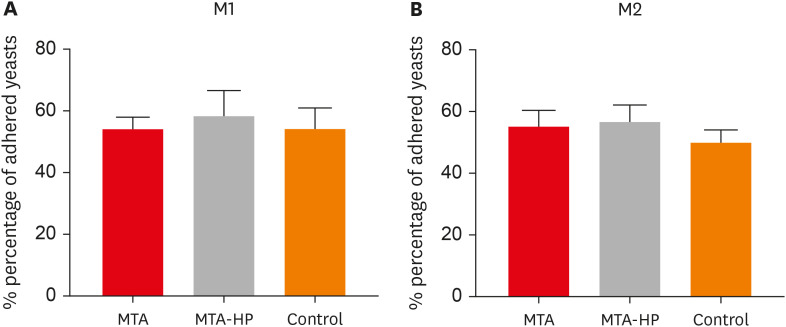
Adherence of macrophage in the presence of sealers
The adherence of mouse peritoneal macrophages to glass was tested for 18 hours of culture in the presence of sealers and control (empty capillaries). Neither sealer interfered with M1 or M2 cell adherence at any time point (
p > 0.05) (
Figure 3).
Figure 3
Percentage of adherent M1 (A) and M2 (B) macrophages after incubation in culture plates with capillaries containing MTA and MTA-HP. The control were cultures with empty capillaries. Cultures were performed as described in the Materials and Methods. Bars denote the mean of results of 3 experiments performed in duplicate. Lines indicate the standard error of the mean (p < 0.05).
MTA, mineral trioxide aggregate; HP, high-plasticity.
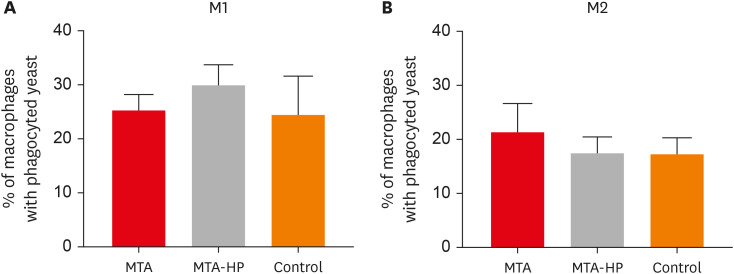
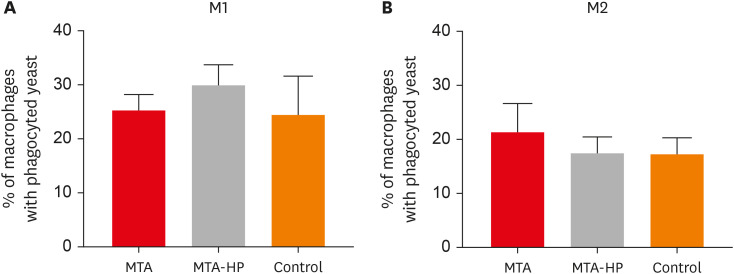
Yeast phagocytosis activity
The ability of M1 and M2 mouse macrophages to take up
S. boulardii in the presence or absence of sealers was assayed. Macrophages bound to yeast similarly in all groups, with or without sealers (
Figure 4).
Figure 4
Percentages of M1 (A) and M2 (B) macrophages displaying phagocytosed yeast cells after incubation in culture plates with capillaries containing MTA and MTA-HP. Controls were cultured with empty capillaries. Cultures were performed as described in the Materials and Methods. Bars denote the mean results of 3 experiments performed in duplicate. Lines indicate the standard error of the mean. No statistically significant difference between M1 and M2 cells under the same conditions was observed (p > 0.05).
MTA, mineral trioxide aggregate; HP, high-plasticity.
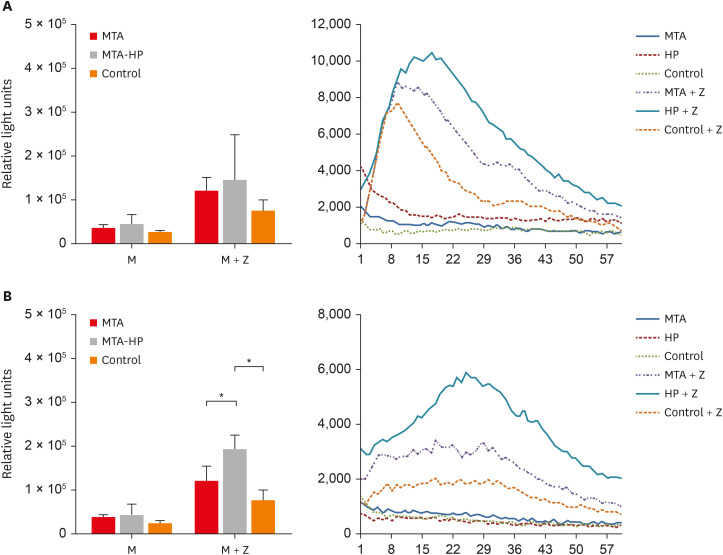
Production of ROS
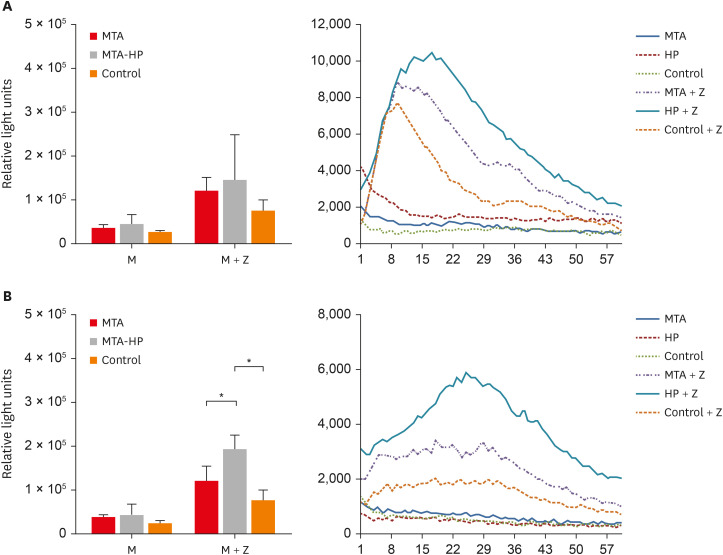
Analysis of the area under the curve for ROS production in each condition showed that, in general, M2 macrophages produced more ROS than M1 The addition of zymosan A (positive control) to cultures induced higher levels of ROS production in both cell types. However, statistically significant differences among the MTA, MTA-HP, and control groups were observed only for M2 macrophages. MTA-HP led to a statistically significant increase in ROS production in MTA and control cells (p < 0.05).
The production of ROS by M1 and M2 macrophages is shown in
Figure 5. For M1, there was no significant difference between the sealers and the control group (
p > 0.05). For M2, higher ROS production was seen with MTA-HP than in the MTA and control groups in the stimulated condition (
p < 0.05). M2 macrophage ROS production was consistently significantly higher than that of M1 cells (
p < 0.05) (
Figure 5).
Figure 5
Kinetic of ROS production by M1 (A) and M2 (B) macrophages. Cells were cultured with capillaries containing MTA and MTA-HP and stimulated with zymosan A, as described in the Materials and Methods. Bars denote the mean results of 3 experiments performed in duplicate. Lines indicate the standard error of the mean. Different symbols indicate a statistically significant difference (p < 0.05) for ROS production compared with M1 or M2 macrophages in medium with or without stimulation, by analysis of variance.
M, macrophage culture containing the materials or not (control); M + Z, macrophage cultures stimulated with zymosan A in the presence or absence of materials (control); ROS, reactive oxygen species; MTA, mineral trioxide aggregate; HP, high-plasticity.
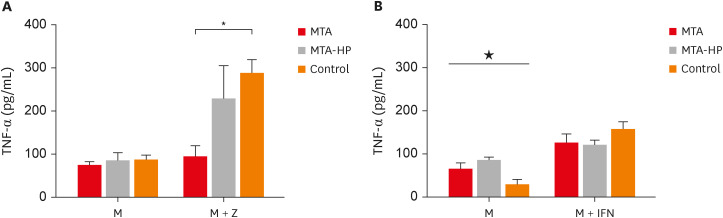
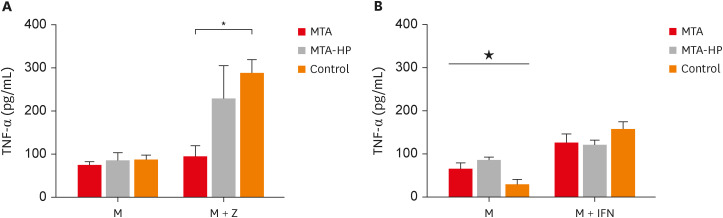
TNF-α production
M1 macrophages released similar levels of TNF-α in the presence of sealers in cultures without IFN-γ (
Figure 6A) (
p > 0.05). However, MTA statistically decreased TFN-α production in a comparison with IFN-γ-stimulated control cells. Both sealers increased TNF-α production in M2 cells in the absence of IFN-γ compared to control (
p < 0.05), while no significant differences were observed in cultures stimulated by IFN-γ (
p > 0.05) (
Figure 6).
Figure 6
Mean production of TNF-α by M1 (A) and M2 (B) macrophages cultured in the absence (control) or presence of MTA and MTA-HP. The cells were cultured in the medium alone or in the presence of IFN-γ. Bars denote the mean results of 3 experiments performed in duplicate. Lines indicate the standard error of the mean.
M, the presence of macrophages; M + IFN, the presence of macrophages and interferon-γ in the mineral trioxide aggregate, high-plasticity mineral trioxide aggregate, and control groups; TNF, tumor necrosis factor; IFN, interferon; MTA, mineral trioxide aggregate; HP, high-plasticity.
* indicates a statistically significant difference (p < 0.05) in TNF-α mean production when specific cultured M2 macrophages were compared with other M1 or M2 macrophages in medium with or without stimulation, by analysis of variance. ★ indicates difference between the two materials compared to control group (P<0.05).
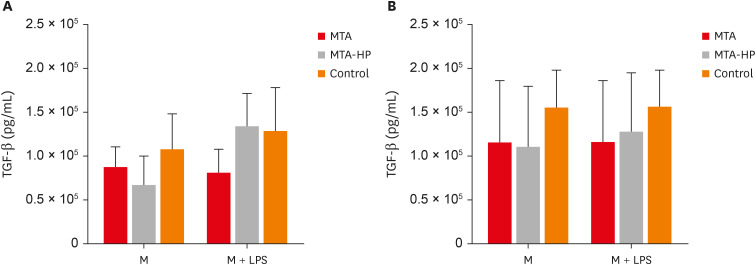
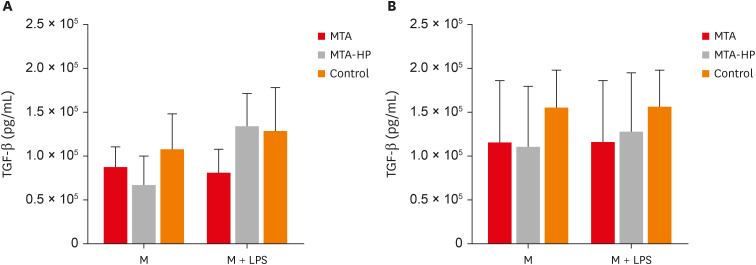
TGF-β production
Data on the production of TGF-β by M1 and M2 macrophages are illustrated in
Figure 7. In the absence and presence of the LPS stimulus, M1 and M2 macrophages showed no statistically significant differences when in contact with the sealers compared to the control group (
p > 0.05) (
Figure 7).
Figure 7
Mean production of TGF-β by M1 (A) and M2 (B) macrophages cultured in the absence (control) or presence of MTA and MTA-HP. The cells were cultured in the medium alone or in the presence of LPS. Bars denote the mean results of 3 experiments performed in duplicate. Lines indicate the standard error of the mean. No statistically significant differences were observed (p > 0.05) by analysis of variance.
M, the presence of macrophages; M + LPS, the presence of macrophages and lipopolysaccharide; TGF, transforming growth factor; MTA, mineral trioxide aggregate; HP, high-plasticity; LPS, lipopolysaccharide.
DISCUSSION
Several studies have shown that MTA is accepted as the gold standard in endodontics compared with other materials due to its outstanding biocompatibility [
1,
10,
14,
18]. The choice of Angelus white MTA as a comparison material in this study was based on previous studies and the fact that it is produced by the same manufacturer of MTA-HP. In 2019, the manufacturer changed the radiopacifier of Angelus MTA from bismuth oxide to calcium tungstate, which is less toxic [
12,
18,
21,
29]. Thus, the cement extracts of MTA and MTA-HP are the same. The difference between the 2 materials is the presence of a plasticizer in the liquid (
Table 1), which improves the handling and insertion properties of the material (
Figure 8). Of note, in a previous study that compared the cytotoxicity of the 2 materials in 2017, the radiopacifier used in the Angelus MTA was still bismuth oxide [
21].
Table 1 Composition of MTA and MTA-HP
|
MTA |
MTA-HP |
|
Tricalcium silicate |
Tricalcium silicate |
|
Dicalcium silicate |
Dicalcium silicate |
|
Tricalcium aluminate |
Tricalcium aluminate |
|
Calcium oxide |
Calcium oxide |
|
Calcium tungstate |
Calcium tungstate |
|
Liquid |
Liquid |
|
Distilled water |
Water and organic plasticizer |
Figure 8
Manipulation and consistency of MTA-HP.
MTA, mineral trioxide aggregate; HP, high-plasticity.
Macrophages play a pivotal role in the phagocytosis and clearance of pathogens, the stimulation and activation of immune cells such as polymorphonuclear neutrophils and T lymphocytes, and tissue repair processes [
30]. In this study, 2 lines of macrophages were evaluated: one activated by the classic pathway (M1) and the other by the alternative route (M2), with stimulation by MTA and MTA-HP or the absence of such stimulation in the control group. M1 and M2 macrophages present different actions in the periapical immune response. While M1 cells are responsible for protection against infection, M2 cells play a significant role in the healing process, presenting an anti-inflammatory profile [
16,
31]. The balance between M1 and M2 macrophages is paramount for the host's defense [
13,
18].
Macrophages need to be viable during experimental procedures. In this study, the trypan blue exclusion assay showed significant difference in viable M1 macrophages. A similar result was found by Rezende
et al. [
14] when comparing 2 MTA formulations (gray and white). However, regarding M2 cells, we noticed significantly greater viability for MTA than for MTA-HP at 48 and 72 hours. However, in the MTT assay, MTA and MTA-HP promoted similar cell metabolism for M1 (48 and 72 hours) and M2 (24, 48 and 72 hours) at most analyzed time points. Lower cell metabolism with MTA-HP was observed only at 24 hours for M1 cells. These findings may be related to the hydration process in the initial period when the calcium silicate reacts to form calcium hydroxide and calcium silicate gel, and the medium reaches an alkaline pH [
1]. In accordance with these findings, it was demonstrated that MTA-HP in contact with stem cells from the human periodontal ligament presented acceptable cell viability in neutral and acidic environments [
32]. Moreover, the cytotoxicity of MTA and MTA-HP materials was also evaluated by the MTT assay in the presence of human osteoblasts, and results revealed that MTA and MTA-HP extracts promoted similar osteoblast viability [
22].
In their kinetics of activity in the immune system, macrophages first adhere and subsequently phagocyte [
30]. None of the tested materials interfered with cell adhesion, which highlights the biocompatible characteristics of these cements. Similar results were previously observed for MTA Angelus [
18].
Furthermore, the repair material must not alter the phagocytic activity of macrophages, which could suppress their clearance activity. In the present study, we observed that none of the tested materials inhibited the phagocytosis capacity of M1 or M2 macrophages. Similar findings were shown by Rezende
et al. [
14] when evaluating the phagocytic activity of M1 and M2 macrophages in the presence of ProRoot MTA and MTA Angelus [
18].
The production of oxygen free radicals plays a vital role in eliminating pathogens, inducing cell apoptosis and gene expression, and activating cell signaling cascades [
33]. We observed that ROS production was upregulated in M2 macrophages stimulated by MTA-HP compared to the white MTA and control cells. Thus, MTA-HP can improve the immune responses during the healing process in the presence of infection.
TNF-α induces neutrophil and macrophage migration to the site of infections to eliminate microorganisms and, indirectly, induces osteoclast activation [
34,
35]. Interestingly, M1 macrophages in the MTA group, when stimulated by IFN-γ, did not increase TNF-α production, maintaining the same basal levels. In contrast, the MTA-HP and control cells induced TNF-α production. This finding suggests that MTA-HP may improve microbial clearance by antigen-presenting cells. However, MTA Repair HP did not increase the pro-inflammatory response of macrophages [
22].
Many cell types secrete TGF-β, including macrophages. TGF-β stimulates resting monocytes and inhibits activated macrophages [
36]. The effects of TGF-β are contradictory, depending on the context: for monocytes, TGF-β functions as a chemoattractant and an upregulator of an inflammatory response, while TGF-β also downregulates inflammatory cytokine production in monocytes and macrophages [
37,
38]. In this study, both macrophage profiles, M1 and M2, whether stimulated or not by LPS, presented similar production levels of TGF-β despite being pro- and anti-inflammatory cells, respectively, due to this contradictory aspect of the profile of TGF-β.
In vitro cytotoxicity assays comprise the first level of a biocompatibility analysis of a material and can be influenced by the choice of cell used. The addition of plasticizer to MTA-HP did not interfere with the tissue repair process [
39]. The similar biocompatibility of both materials in this study can be causally related to their composition. In order to confirm the biocompatibility of these materials and enable their intended clinical use, further research regarding their cytotoxicity
in vitro and subsequent preclinical
in vivo tests with mice are required.
CONCLUSIONS
This study showed that M1 and M2 macrophages presented differences in viability in response to MTA and MTA-HP at different time points. The introduction of the plasticizer into the vehicle of MTA did not interfere with the adherence, phagocytosis, or production of ROS, TNF-α, and TGF-β in M1 and M2 macrophages.
ACKNOWLEDGEMENTS
The authors would like to thank LQ Vieira (Department of Biochemistry and Immunology, Institute of Biological Sciences, Federal University of Minas Gerais [UFMG]) for providing the space and laboratory equipment for carrying out the experiments and the graduate program of dentistry at UFMG.
-
Funding: This work received financial support from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Data curation: Tavares WLF, Sobrinho APR.
Formal analysis: Vasconcellos BC, Tavares LCT, Tavares WLF.
Funding acquisition: Tavares WLF, Sobrinho APR.
Investigation: Vasconcellos BC, Tavares LCT, Tavares WLF.
Methodology: Vasconcellos BC, Tavares LCT, Tavares WLF, da Silva DC, Fonseca FO, Benetti F.
Project administration: Tavares WLF.
Resources: Tavares WLF, Sobrinho APR.
Software: Vasconcellos BL.
Supervision: Tavares WLF, Sobrinho APR.
Validation: Tavares WLF, Sobrinho APR.
Visualization: Tavares WLF, Sobrinho APR.
Writing - original draft: Vasconcellos BL, Tavares WLF, Sobrinho APR.
Writing - review & editing: Tavares WLF, Sobrinho APR.
REFERENCES
- 1. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part I: chemical, physical, and antibacterial properties. J Endod 2010;36:16-27.ArticlePubMed
- 2. Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc 1991;74:1487-1510.Article
- 3. Reyes-Carmona JF, Felippe MS, Felippe WT. Biomineralization ability and interaction of mineral trioxide aggregate and white Portland cement with dentin in a phosphate-containing fluid. J Endod 2009;35:731-736.ArticlePubMed
- 4. Emara R, Elhennawy K, Schwendicke F. Effects of calcium silicate cements on dental pulp cells: a systematic review. J Dent 2018;77:18-36.ArticlePubMed
- 5. Felippe WT, Felippe MC, Rocha MJ. The effect of mineral trioxide aggregate on the apexification and periapical healing of teeth with incomplete root formation. Int Endod J 2006;39:2-9.ArticlePubMed
- 6. Scarparo RK, Haddad D, Acasigua GA, Fossati AC, Fachin EV, Grecca FS. Mineral trioxide aggregate-based sealer: analysis of tissue reactions to a new endodontic material. J Endod 2010;36:1174-1178.ArticlePubMed
- 7. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod 1999;25:197-205.ArticlePubMed
- 8. Guimarães BM, Prati C, Duarte MA, Bramante CM, Gandolfi MG. Physicochemical properties of calcium silicate-based formulations MTA Repair HP and MTA Vitalcem. J Appl Oral Sci 2018;26:e2017115.PubMedPMC
- 9. Jiménez-Sánchez MC, Segura-Egea JJ, Diaz-Cuenca A. Physicochemical parameters-hydration performance relationship of the new endodontic cement MTA Repair HP. J Clin Exp Dent 2019;11:739-744.
- 10. Palczewska-Komsa M, Kaczor-Wiankowska K, Nowicka A. New bioactive calcium silicate cement mineral trioxide aggregate repair high plasticity (MTA HP)-a systematic review. Materials (Basel) 2021;14:4573.ArticlePubMedPMC
- 11. Escobar-García DM, Medina-Rosas MG, González-Amaro AM, Méndez-González V, Flores H, Pozos-Guillén A. MTA-based cements: biocompatibility and effects on the gene expression of collagen type 1 and TGF-β1. BioMed Res Int 2022;2022:2204698.PubMedPMC
- 12. Braga JM, Oliveira RR, Martins RC, Ribeiro Sobrinho AP. The effects of a mineral trioxide aggregate-based sealer on the production of reactive oxygen species, nitrogen species and cytokines by two macrophage subtypes. Int Endod J 2014;47:909-919.ArticlePubMed
- 13. de Oliveira Mendes ST, Ribeiro Sobrinho AP, de Carvalho AT, de Souza Côrtes MI, Vieira LQ. In vitro evaluation of the cytotoxicity of two root canal sealers on macrophage activity. J Endod 2003;29:95-99.ArticlePubMed
- 14. Rezende TM, Vargas DL, Cardoso FP, Sobrinho AP, Vieira LQ. Effect of mineral trioxide aggregate on cytokine production by peritoneal macrophages. Int Endod J 2005;38:896-903.ArticlePubMed
- 15. Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol 2005;23:901-944.ArticlePubMed
- 16. Bastos KR, Alvarez JM, Marinho CR, Rizzo LV, Lima MR. Macrophages from IL-12p40-deficient mice have a bias toward the M2 activation profile. J Leukoc Biol 2002;71:271-278.ArticlePubMedPDF
- 17. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009;86:1065-1073.ArticlePubMedPDF
- 18. Rezende TM, Vieira LQ, Cardoso FP, Oliveira RR, de Oliveira Mendes ST, Jorge ML, Ribeiro Sobrinho AP. The effect of mineral trioxide aggregate on phagocytic activity and production of reactive oxygen, nitrogen species and arginase activity by M1 and M2 macrophages. Int Endod J 2007;40:603-611.ArticlePubMed
- 19. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958-969.ArticlePubMedPMCPDF
- 20. Abou ElReash A, Hamama H, Grawish M, Saeed M, Zaen El-Din AM, Shahin MA, Zhenhuan W, Xiaoli X. A laboratory study to test the responses of human dental pulp stem cells to extracts from three dental pulp capping biomaterials. Int Endod J 2021;54:1118-1128.ArticlePubMedPDF
- 21. Cintra LT, Benetti F, de Azevedo Queiroz ÍO, de Araújo Lopes JM, Penha de Oliveira SH, Sivieri Araújo G, Gomes-Filho JE. Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA material. J Endod 2017;43:774-778.ArticlePubMed
- 22. Barczak K, Palczewska-Komsa M, Lipski M, Chlubek D, Buczkowska-Radlińska J, Baranowska-Bosiacka I. The influence of new silicate cement mineral trioxide aggregate (MTA Repair HP) on metalloproteinase MMP-2 and MMP-9 expression in cultured THP-1 macrophages. Int J Mol Sci 2020;22:295.ArticlePubMedPMC
- 23. Braga JM, Oliveira RR, de Castro Martins R, Vieira LQ, Sobrinho AP. Assessment of the cytotoxicity of a mineral trioxide aggregate-based sealer with respect to macrophage activity. Dent Traumatol 2015;31:390-395.ArticlePubMed
- 24. Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol 1993;54:283-288.ArticlePubMedPDF
- 25. Giaimis J, Lombard Y, Makaya-Kumba M, Fonteneau P, Poindron P. A new and simple method for studying the binding and ingestion steps in the phagocytosis of yeasts. J Immunol Methods 1992;154:185-193.ArticlePubMed
- 26. Trusk MA, Wilson ME, Dyke KV. The generation of chemiluminescence by phagocytic cells. Methods Enzymol 1978;57:462-493.
- 27. Mishra P, Singh U, Pandey CM, Mishra P, Pandey G. Application of student’s t-test, analysis of variance, and covariance. Ann Card Anaesth 2019;22:407-411.ArticlePubMedPMC
- 28. Hazra A, Gogtay N. Biostatistics series module 3: comparing groups: numerical variables. Indian J Dermatol 2016;61:251-260.ArticlePubMedPMC
- 29. Ferreira CM, Sassone LM, Gonçalves AS, de Carvalho JJ, Tomás-Catalá CJ, García-Bernal D, Oñate-Sánchez RE, Rodríguez-Lozano FJ, Silva EJ. Physicochemical, cytotoxicity and in vivo biocompatibility of a high-plasticity calcium-silicate based material. Sci Rep 2019;9:3933.PubMedPMC
- 30. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy 2005;4:281-286.ArticlePubMed
- 31. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000;164:6166-6173.ArticlePubMedPDF
- 32. Collado-González M, López-García S, García-Bernal D, Oñate-Sánchez RE, Tomás-Catalá CJ, Moraleda JM, Lozano A, Forner L, Rodríguez-Lozano FJ. Biological effects of acid-eroded MTA Repair HP and ProRoot MTA on human periodontal ligament stem cells. Clin Oral Investig 2019;23:3915-3924.ArticlePubMedPDF
- 33. Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans 2001;29:345-349.ArticlePubMed
- 34. Xanthoulea S, Pasparakis M, Kousteni S, Brakebusch C, Wallach D, Bauer J, Lassmann H, Kollias G. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J Exp Med 2004;200:367-376.ArticlePubMedPMCPDF
- 35. Maciel KF, Neves de Brito LC, Tavares WL, Moreira G, Nicoli JR, Vieira LQ, Ribeiro Sobrinho AP. Cytokine expression in response to root canal infection in gnotobiotic mice. Int Endod J 2012;45:354-362.ArticlePubMed
- 36. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol 1998;16:137-161.ArticlePubMed
- 37. Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-β - an excellent servant but a bad master. J Transl Med 2012;10:183.ArticlePubMedPMCPDF
- 38. Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 2005;115:66-75.ArticlePubMedPMC
- 39. Marciano MA, Guimarães BM, Amoroso-Silva P, Camilleri J, Hungaro Duarte MA. Physical and chemical properties and subcutaneous implantation of mineral trioxide aggregate mixed with propylene glycol. J Endod 2016;42:474-479.ArticlePubMed
 , Layara Cristine Tomaz Tavares
, Layara Cristine Tomaz Tavares , Danilo Couto da Silva
, Danilo Couto da Silva , Francielen Oliveira Fonseca
, Francielen Oliveira Fonseca , Francine Benetti
, Francine Benetti , Antônio Paulino Ribeiro Sobrinho
, Antônio Paulino Ribeiro Sobrinho , Warley Luciano Fonseca Tavares
, Warley Luciano Fonseca Tavares










 KACD
KACD
 ePub Link
ePub Link Cite
Cite

