-
Incorporation of amoxicillin-loaded microspheres in mineral trioxide aggregate cement: an in vitro study
-
Fábio Rocha Bohns, Vicente Castelo Branco Leitune, Isadora Martini Garcia, Bruna Genari, Nélio Bairros Dornelles, Silvia Stanisçuaski Guterres, Fabrício Aulo Ogliari, Mary Anne Sampaio de Melo, Fabrício Mezzomo Collares
-
Restor Dent Endod 2020;45(4):e50. Published online October 7, 2020
-
DOI: https://doi.org/10.5395/rde.2020.45.e50
-
-
 Abstract Abstract
 PDF PDF PubReader PubReader ePub ePub
- Objectives
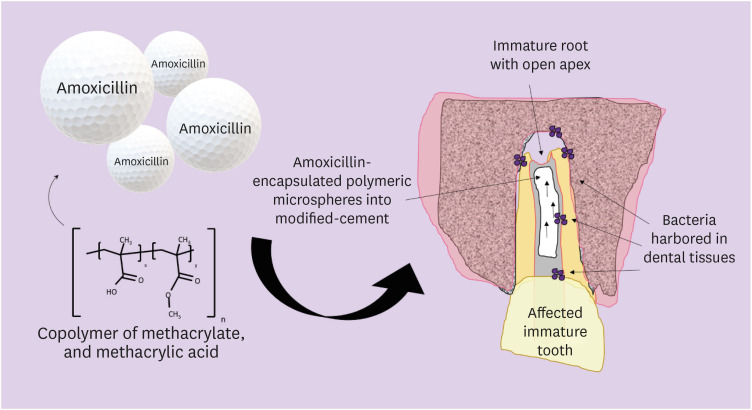
In this study, we investigated the potential of amoxicillin-loaded polymeric microspheres to be delivered to tooth root infection sites via a bioactive reparative cement. Materials and MethodsAmoxicillin-loaded microspheres were synthesized by a spray-dray method and incorporated at 2.5% and 5% into a mineral trioxide aggregate cement clinically used to induce a mineralized barrier at the root tip of young permanent teeth with incomplete root development and necrotic pulp. The formulations were modified in liquid:powder ratios and in composition by the microspheres. The optimized formulations were evaluated in vitro for physical and mechanical eligibility. The morphology of microspheres was observed under scanning electron microscopy. ResultsThe optimized cement formulation containing microspheres at 5% exhibited a delayed-release response and maintained its fundamental functional properties. When mixed with amoxicillin-loaded microspheres, the setting times of both test materials significantly increased. The diametral tensile strength of cement containing microspheres at 5% was similar to control. However, phytic acid had no effect on this outcome (p > 0.05). When mixed with modified liquid:powder ratio, the setting time was significantly longer than that original liquid:powder ratio (p < 0.05). ConclusionsLack of optimal concentrations of antibiotics at anatomical sites of the dental tissues is a hallmark of recurrent endodontic infections. Therefore, targeting the controlled release of broad-spectrum antibiotics may improve the therapeutic outcomes of current treatments. Overall, these results indicate that the carry of amoxicillin by microspheres could provide an alternative strategy for the local delivery of antibiotics for the management of tooth infections.
-
Citations
Citations to this article as recorded by  - Local drug delivery for regeneration and disinfection in endodontics: A narrative review
Anu Elsa Swaroop, Sylvia Mathew, P. Harshini, Shruthi Nagaraja
Journal of Conservative Dentistry and Endodontics.2025; 28(2): 119. CrossRef - Modified Mineral Trioxide Aggregate—A Versatile Dental Material: An Insight on Applications and Newer Advancements
C. Pushpalatha, Vismaya Dhareshwar, S. V. Sowmya, Dominic Augustine, Thilla Sekar Vinothkumar, Apathsakayan Renugalakshmi, Amal Shaiban, Ateet Kakti, Shilpa H. Bhandi, Alok Dubey, Amulya V. Rai, Shankargouda Patil
Frontiers in Bioengineering and Biotechnology.2022;[Epub] CrossRef - Local Drug Delivery Systems for Vital Pulp Therapy: A New Hope
Ardavan Parhizkar, Saeed Asgary, Carlo Galli
International Journal of Biomaterials.2021; 2021: 1. CrossRef
-
1,419
View
-
11
Download
-
3
Crossref
-
Carbohydrate-electrolyte drinks exhibit risks for human enamel surface loss
-
Mary Anne Sampaio de Melo, Vanara Florêncio Passos, Juliana Paiva Marques Lima, Sérgio Lima Santiago, Lidiany Karla Azevedo Rodrigues
-
Restor Dent Endod 2016;41(4):246-254. Published online August 16, 2016
-
DOI: https://doi.org/10.5395/rde.2016.41.4.246
-
-
 Abstract Abstract
 PDF PDF PubReader PubReader ePub ePub
- Objectives
The aim of this investigation was to give insights into the impact of carbohydrate-electrolyte drinks on the likely capacity of enamel surface dissolution and the influence of human saliva exposure as a biological protective factor. Materials and MethodsThe pH, titratable acidity (TA) to pH 7.0, and buffer capacity (β) of common beverages ingested by patients under physical activity were analyzed. Then, we randomly distributed 50 specimens of human enamel into 5 groups. Processed and natural coconut water served as controls for testing three carbohydrate-electrolyte drinks. In all specimens, we measured surface microhardness (Knoop hardness numbers) and enamel loss (profilometry, µm) for baseline and after simulated intake cycling exposure model. We also prepared areas of specimens to be exposed to human saliva overnight prior to the simulated intake cycling exposure. The cycles were performed by alternated immersions in beverages and artificial saliva. ANOVA two-way and Tukey HDS tests were used. ResultsThe range of pH, TA, and β were 2.85 - 4.81, 8.33 - 46.66 mM/L and 3.48 - 10.25 mM/L × pH, respectively. The highest capacity of enamel surface dissolution was found for commercially available sports drinks for all variables. Single time human saliva exposure failed to significantly promote protective effect for the acidic attack of beverages. ConclusionsIn this study, carbohydrate-electrolyte drinks usually consumed during endurance training may have a greater capacity of dissolution of enamel surface depending on their physicochemical proprieties associated with pH and titratable acidity.
-
Citations
Citations to this article as recorded by  - Evaluation of developmentally hypomineralised enamel after surface pretreatment with Papacarie Duo gel and different etching modes: an in vitro SEM and AFM study
Y.-L. Lee, K. C. Li, C. K. Y. Yiu, D. H. Boyd, M. Ekambaram
European Archives of Paediatric Dentistry.2022; 23(1): 117. CrossRef - Is the consumption of beverages and food associated to dental erosion? A cross-sectional study in Portuguese athletes
M.-R.G. Silva, M.-A. Chetti, H. Neves, M.-C. Manso
Science & Sports.2021; 36(6): 477.e1. CrossRef - Assessment of surface roughness changes on orthodontic acrylic resin by all-in-one spray disinfectant solutions
Kuei-ling Hsu, Abdulrahman A. Balhaddad, Isadora Martini Garcia, Fabricio Mezzomo Collares, Louis DePaola, Mary Anne Melo
Journal of Dental Research, Dental Clinics, Dental Prospects.2020; 14(2): 77. CrossRef - Nitrate-rich beetroot juice offsets salivary acidity following carbohydrate ingestion before and after endurance exercise in healthy male runners
Mia C. Burleigh, Nicholas Sculthorpe, Fiona L. Henriquez, Chris Easton, Yi-Hung Liao
PLOS ONE.2020; 15(12): e0243755. CrossRef - Dental erosion’ prevalence and its relation to isotonic drinks in athletes: a systematic review and meta-analysis
Pedro Henrique Pereira de Queiroz Gonçalves, Ludmila Silva Guimarães, Fellipe Navarro Azevedo de Azeredo, Letícia Maira Wambier, Lívia Azeredo A. Antunes, Leonardo Santos Antunes
Sport Sciences for Health.2020; 16(2): 207. CrossRef - Atomic force microscopy analysis of enamel nanotopography after interproximal reduction
Shadi Mohebi, Nazila Ameli
American Journal of Orthodontics and Dentofacial Orthopedics.2017; 152(3): 295. CrossRef
-
1,536
View
-
14
Download
-
6
Crossref
|