Abstract
-
Objectives
This study compared the Biodentine, MTA Repair HP, and Bio-C Repair bioceramics in terms of bond strength to dentin, failure mode, and compression.
-
Materials and Methods
Fifty-four slices obtained from the cervical third of 18 single-rooted human mandibular premolars were randomly distributed (n = 18). After insertion of the bioceramic materials, the push-out test was performed. The failure mode was analyzed using stereomicroscopy. Another set of cylindrically-shaped bioceramic samples (n = 10) was prepared for compressive strength testing. The normality of data distribution was analyzed using the Shapiro-Wilk test. The Kruskal-Wallis and Friedman tests were used for the push-out test data, while compressive strength was analyzed with analysis of variance and the Tukey test, considering a significance level of 0.05.
-
Results
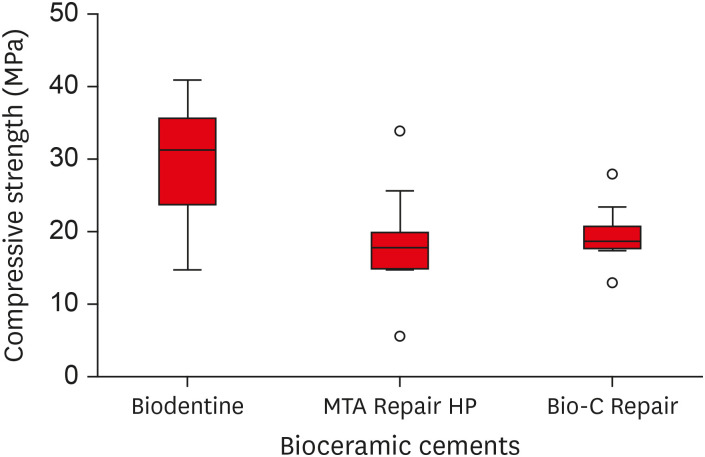
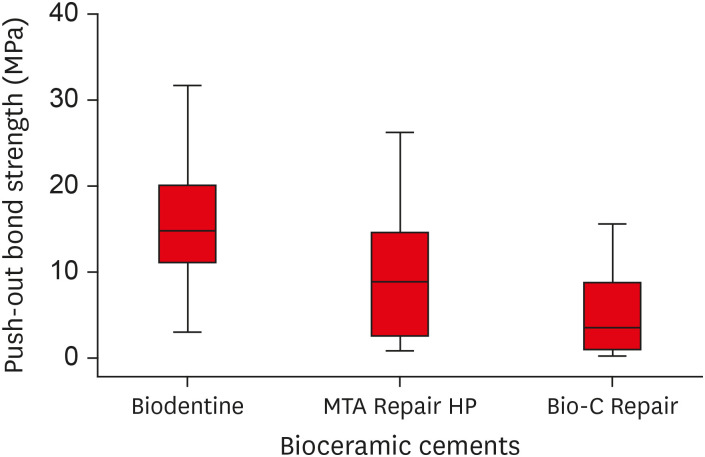
Biodentine presented a higher median bond strength value (14.79 MPa) than MTA Repair HP (8.84 MPa) and Bio-C Repair (3.48 MPa), with a significant difference only between Biodentine and Bio-C Repair. In the Biodentine group, the most frequent failure mode was mixed (61%), while in the MTA Repair HP and Bio-C Repair groups, it was adhesive (94% and 72%, respectively). Biodentine showed greater resistance to compression (29.59 ± 8.47 MPa) than MTA Repair HP (18.68 ± 7.40 MPa) and Bio-C Repair (19.96 ± 3.96 MPa) (p < 0.05).
-
Conclusions
Biodentine showed greater compressive strength than MTA Repair HP and Bio-C Repair, and greater bond strength than Bio-C Repair. The most frequent failure mode of Biodentine was mixed, while that of MTA Repair HP and Bio-C Repair was adhesive.
-
Keywords: Calcium silicate cement; Compressive strength; Dentin bond strength; Regenerative endodontics
INTRODUCTION
The endodontic treatment of permanent necrotic teeth with incomplete root development is complex, with limitations inherent to the preparation and filling of the root canal. The presence of a wide canal with parallel walls makes it difficult to clean and disinfect. An open apex of the canal can also cause the filling material to leak into the periapex when performing conventional filling techniques. In addition, there is a high risk of root fractures, since dentinal walls are thin and fragile [
1]. Regenerative endodontics is a promising alternative to treat necrotic permanent teeth with incomplete root development, because it allows both maturation of root development and apical closure [
2]. Consequently, regenerative treatment gives the tooth greater resistance and longevity [
3].
This contemporary form of treatment is based on 3 fundamental pillars: effective root canal decontamination, migration and differentiation of mesenchymal stem cells into the root canal, and an adequate cervical seal. The last of these pillars is provided by the placement of a cervical barrier, followed by restoration [
4]. Bioceramic cements are the materials of choice for creating this cervical barrier, not only because of their excellent sealing ability, but especially because they allow the differentiation of mesenchymal stem cells, which are essential for tissue regeneration process [
5,
6].
Mineral trioxide aggregate (MTA) is widely used for this purpose because of its biocompatibility, sealing and radiopacity adjustment, effective antimicrobial action, low solubility, ability to be inserted in the presence of moisture, and bioactivity with osteoinductive action, which allows cells to grow and proliferate on its surface [
7,
8,
9]. However, some MTA formulations have bismuth oxide as a radiopacifier, which causes discoloration of the tooth and marginal gum, compromising the patient's aesthetic outcomes [
10,
11]. In addition, MTA is difficult to spatulate and insert owing to its sandy texture [
12]. Seeking to improve the MTA product, MTA Repair HP was released to the market (Angelus, Londrina, PR, Brazil). This alternative uses calcium tungstate as a radiopacifier, thus avoiding dental staining; furthermore, it is a thicker liquid, resulting in greater plasticity and better handling than its previous version.
Biodentine cement (Septodont, Saint Maurdes Fossés, France) is marketed as a bioactive dental substitute with the same clinical indications as MTA. According to Han and Okiji [
13], it has the ability to induce pulp cell differentiation and
in vitro biomineralization due to the release and penetration of calcium ions into the dentinal tubules.
Bio-C Repair cement (Angelus) was recently made available on the market. According to the manufacturer, it not only has all the benefits of a bioceramic formulation, but its qualities surpass previous products, especially because it does not require manipulation, thus making it easy to use and to insert into the cavity, ultimately saving time.
It is well known that a safe barrier is needed to prevent direct contact with restorative material in order to avoid harmful effects on mesenchymal stem cells [
4]. Thus, cements must have adequate mechanical strength to withstand the displacement forces resulting from the occlusion and condensation of restorative materials, as well as adequate bonding to root dentin [
14,
15,
16].
The mechanical push-out test is commonly used to determine the bond strength of a dental material to dentin by testing sample failure from shear stress [
17]. Its advantages are that it is easy to reproduce and interpret, and provides a reliable assessment, even at low levels [
18]. Complementarily, the compressive strength test is indicated to measure the ability of a material to resist vertical forces; this is a relevant mechanical property because masticatory forces can give rise to fractures, causing clinical and technique-related failures [
19,
20].
Given the importance of adequate cervical sealing for the success of regenerative endodontics, further studies should be performed to provide well-grounded information on the mechanical properties of bioceramic cements, especially those recently released to the market. Keeping dental specialists well-informed can improve the success rates of treatment and the survival of dental elements. Therefore, the aim of the present study was to compare the Biodentine, MTA Repair HP, and Bio-C Repair bioceramic cements in terms of their bond strength to root dentin and compressive strength. The null hypothesis was that the bond strength to root dentin and the compressive strength of these cements would not be influenced by different commercial brands.
MATERIALS AND METHODS
Sample calculation
The sample calculation was performed using the WINPEPI 11.65 Program [
21]. Based on data from a previous study evaluating the resistance to displacement of MTA, the sample size needed to detect a difference of 0.87 MPa was 17 specimens for each group, considering a standard deviation between 0.71 and 0.84, a power of 90%, and a significance of 5% [
22].
This study was approved by the Research Ethics Committee of the Federal University of Goiás (CAAE, #16505619.4.0000.5083). In total, 153 single-rooted mandibular premolars requiring orthodontic or periodontal extraction from patients of the Federal University of Goiás School of Dentistry were collected. After extraction, the teeth were cleaned with periodontal curettes, subjected to prophylaxis with a pumice stone and water, and then stored in 0.1% thymol until sample preparation.
The teeth were radiographed with a digital sensor (Acteon, Indaiatuba, SP, Brazil) in the lingual-vestibular (LV) and mesiodistal (MD) directions. This study included teeth that had roots with a single circular channel and a diameter less than or equal to 1.7 mm. Teeth with resorption, calcification, root caries, and non-carious cervical lesions were excluded. Of the total teeth evaluated, 20 were selected that met the described criteria.
The root length was standardized at 5.0 mm from the cementoenamel junction, so that only the cervical third of the root would be used. This was done to simulate teeth with incomplete root development, and highlight the region intended for insertion of the bioceramics. For this purpose, a mark was made with a mechanical pencil and a 150 mm digital caliper (MTX, Schio, Vicenza, Italy). The teeth were fixed on an acrylic plate with a low-melting-point compound, and cut using a diamond cutting disc (Erios, São Paulo, SP, Brazil) in a cutting machine (Labcut 1010; Erios) programmed at a speed of 250 revolutions per minute (rpm), under constant irrigation and a 100-g load.
Immediately after sectioning the roots, the pulp chamber of the teeth was accessed using long-stemmed spherical diamond tips #1012 (KG Sorensen, Cotia, SP, Brazil) driven at high rotation under abundant water cooling. The pulp chamber roof was removed, and the final finishing was performed using a diamond bur with a cone-shaped tip #3082 (KG Sorensen).
In order to obtain a standard internal diameter of 1.7 mm and to simulate teeth with incomplete root development and with parallel walls, the root canal was explored with Kerr #15 files (Dentsply Maillefer, Ballaigues, Switzerland) and prepared with Largo #1, #2, #3, #4, #5 and #6 drills (Microdont, São Paulo, SP, Brazil) activated at low rotation in the entire root length (5.0 mm). At each instrument change, the root canals were irrigated with 3 mL of 2.5% sodium hypochlorite (NaOCl) (Asfer Indústria Química, São Caetano do Sul, SP, Brazil). The teeth were apically sealed with wax 7 (Lysanda, Dental Cremer, Blumenau, SC, Brazil), and the final irrigation was performed using 5 mL of 2.5% NaOCl, followed by flooding the root canal with 17% ethylenediaminetetraacetic acid (EDTA) solution (Biodinâmica Química e Farmacêutica, Ibiporã, PR, Brazil) and 10 mL of distilled water.
Standardization of the internal diameter of the root canals after preparation was checked by radiographing the teeth again in the LV and MD directions, using measurements made with the Dental Master Image software program version 2.0.2.1 (Acteon). The teeth were also examined using an INALH stereomicroscope (MSZ-300) to exclude those with complete cracks. Nineteen of the 20 teeth had no visible root cracks and constituted the sample.
To enhance the reproducibility of the clinical routine, double antibiotic paste (Manipularte-Fármácia de Manipulação, Goiânia, GO, Brazil) was used as intracanal medication (minimum inhibitory concentration; MIC), prior to insertion of the bioceramics. This medication was obtained from a compounding pharmacy, and consisted of 5 mg/mL of ciprofloxacin and metronidazole, diluted in sterile saline.
Prior to inserting the paste, the irrigation protocol recommended by the American Association of Endodontics for regenerative endodontic procedures was carried out. This protocol calls for irrigation of the root canals with 20 mL of 2.5% NaOCl, followed by 5 mL of 0.9% saline solution (Eurofarma, São Paulo, SP, Brazil). The root canals were dried with #80 absorbent paper tips (Tanari, Manaus, AM, Brazil), and the double antibiotic paste was inserted with a syringe and an aspiration tip (Angelus). The entrance to the root canals was sealed with a cotton ball and the coronal sealing of the teeth was performed using temporary filling cement (Villevie, Joinville, SC, Brazil). The specimens were then stored in an incubator at 37°C in 100% humidity for 21 days. The MIC was removed with 20 mL of NaOCl at 2.5%, followed by 5 mL of 0.9% saline and 10 mL of 17% EDTA, using a plastic syringe (Ultradent, South Jordan, UT, USA) and an Endo-Eze Irrigator Tip 27-gauge needle (Ultradent).
Insertion of bioceramic cements
After the MIC was removed, each root was sectioned into 3 slices, 1 ± 0.2 mm thick, extracted from its cervical portions with a diamond cutting disc (Erios), using a cutting machine (Isomet 1000; Buhler, Lake Bluff, NY, USA), programmed at a speed of 250 to 350 rpm, under abundant irrigation. The cutting procedure involved fixing the teeth on an acrylic plate with a low-fusion adhesive and subsequent cutting. The first slice, which was the most apical, was used to correct the inclination errors. Irregular discs and those with thicknesses other than 1 ± 0.2 mm were discarded. At this stage, 1 tooth was discarded due to fracture and 18 remained. Each tooth resulted in 3 slabs, totaling 54 radicular dentin discs.
The slices were divided into 3 groups (
n = 18) according to the bioceramic cement to be used in the cervical seal, as follows: group 1 - Biodentine; group 2 - MTA Repair HP; and group 3 - Bio-C Repair, using 1 slice (specimen) of each tooth for each of the bioceramic cements evaluated, so that all the cements could be tested in a dental element (
Table 1).
Table 1Bioceramics, chemical composition, manufacturer, and number
|
Bioceramics |
Chemical composition |
Manufacturer |
Number |
|
Biodentine |
Powder: Tricalcium silicate, zirconium oxide, calcium oxide, calcium carbonate, yellow pigment, red pigment, and brown iron oxide |
Septodont |
B25217 |
|
Liquid: Calcium chloride dihydrate, sand, and purified water |
|
MTA Repair HP |
Powder: Tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium oxide, and calcium tungstate |
Angelus |
843 |
|
Liquid: Water and plasticizer |
|
Bio-C Repair |
Powder: Calcium silicates, calcium aluminate, calcium oxide, zirconium oxide, iron oxide, and silicon dioxide |
Angelus |
50727 |
|
Liquid: Dispersing agent |
The cements were handled according to the instructions of each manufacturer, and then placed inside the root canal of each slice (
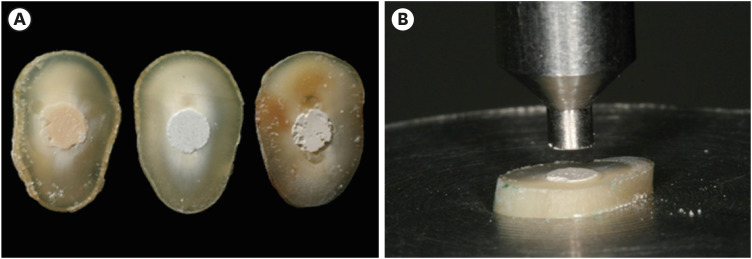
Figure 1A) and positioned on a glass board with condensers. The excess material was removed with a plastic spatula. The specimens were stored in an incubator at 37ºC and 100% relative humidity for 7 days.
Figure 1Sample preparation for the push-out test: (A) Slices of Biodentine, MTA Repair HP, and Bio-C Repair with experimental cements after a storage period of 7 days; (B) Push-out test: specimen positioned on the metallic platform of a universal testing machine.
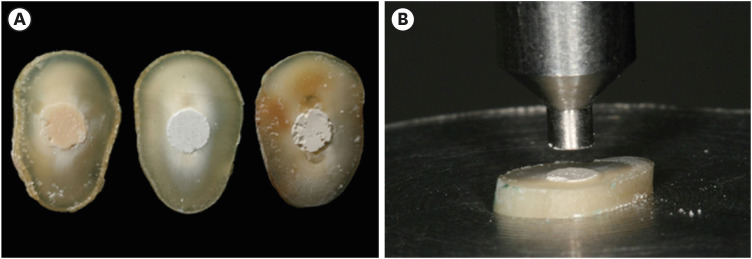
Push-out test
Following storage, the slices were taken to a universal testing machine (Model 5965; Instron, Norwood, MA, USA) with a 2 kN load cell to perform the mechanical push-out test. The specimens were placed on a stainless steel platform with a 2.60 mm hole. A metal rod with a 1.5 mm tip was used, compatible with the diameter of the root canal. The rod was fixed to the upper portion of the machine and securely centralized in relation to the bioceramic cement, so that it would not come into contact with the dentin when the material was pressed and displaced (
Figure 1B). The machine was driven at a constant speed of 0.5 mm/min until it reached maximum tension and provided material displacement. The maximum force in newtons (N) required for the displacement of the cement was measured by a 2 KGF load cell and recorded using the Bluehill software (Illinois Tool Works, Glenview, IL, USA).
The adhesive strength (MPa) was calculated by dividing the maximum force (N) by the area of adhered surface (mm2). Formula 1 was used to determine the area of adhesive strength:
where: π is a constant (3.14), r is the radius (mm) and h is the thickness of the specimen slice.
The adhesive strength (Formula 2) was calculated by dividing the maximum strength value by the calculated area, expressed in (MPa):
where: F is the maximum force (N), and A is the adhered surface area (mm2).
Assessment of the mode of failure
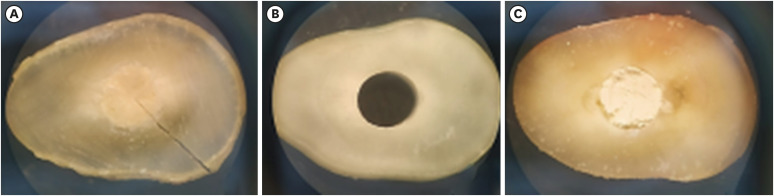
After the mechanical test was performed, both sides of the samples were analyzed under an INALH stereomicroscope (MSZ-300), with ×3 magnification, to determine the failure pattern. The classification was performed according to Shahi
et al. [
16], who defined cohesive failure as a rupture in the material, adhesive failure as disruption of the bond at the dentin/material interface, and mixed failure as a cohesive break in the material and an adhesive break at the dentin interface.
The samples were analyzed by 2 examiners, who were previously trained by a third examiner with extensive experience in push-out tests and in evaluating dental material failure patterns. In cases where there was no consensus between the examiners, a third examiner analyzed the sample.
Compressive strength test
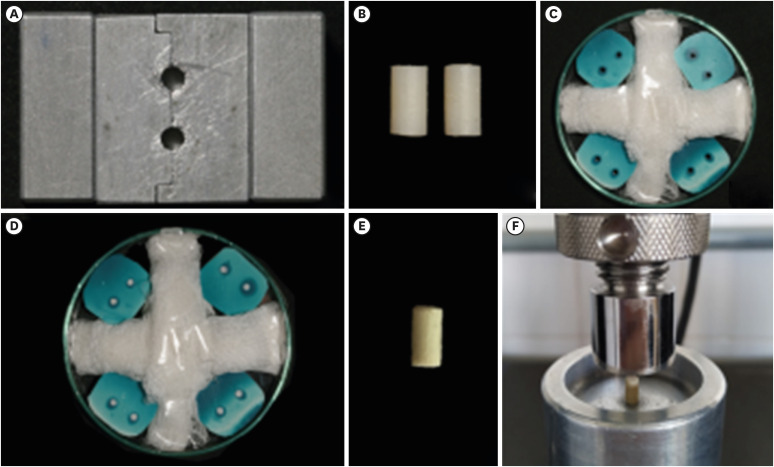
The compressive strength test was performed initially with 2 model specimens made from a bipartite metallic matrix with 2 cylindrical holes 6 mm in height × 3 mm in diameter, as described by Rosatto
et al. [
23] (
Figure 2A). The specimens were made of composite resin (Charisma Classic; Kulzer, Barra Funda, SP, Brazil) by condensing the material in the metallic matrix in approximately 2 mm increments (
Figure 2B). Photoactivation was performed for 40 seconds at each increment, with a light-curing agent at 1,200 mW/cm
2 (Radii-cal; SDI, Bayswater, VIC, Australia). Before photoactivation of the last increment, a polyester strip was superimposed to give the resin a flat, smooth surface. The excess material from specimen removal was scraped off the metallic matrix manually with a #15 scalpel blade and thin piece of paper.
Figure 2Compressive strength test: (A) Split matrix. (B) Model specimens made of composite resin. (C) Matrices made of condensed silicone and placed on a Petri dish or insertion of bioceramic cements. (D) Matrices after the insertion of MTA Repair HP cement. (E) Biodentine cement specimen. (F) Biodentine cement specimen positioned at the center of the compressive strength test device.
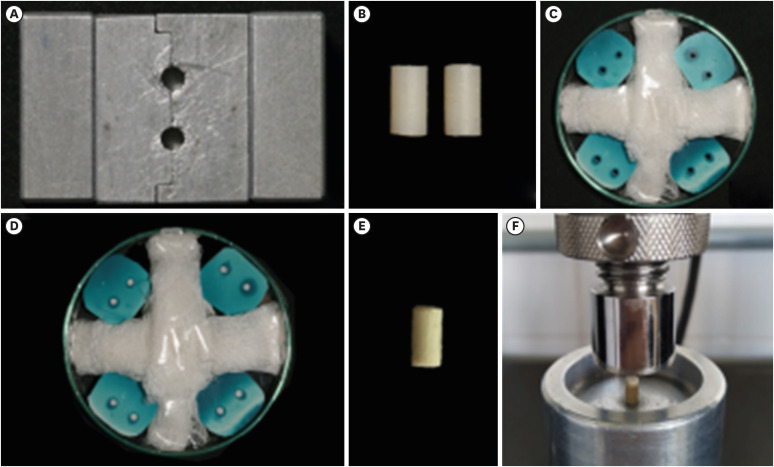
Additional matrices were produced in condensation silicone (Speedex; Coltene, Rio de Janeiro, RJ, Brazil) from the composite resin model specimens (
Figure 2C), in order to have more matrices to store the bioceramic cement specimens during the incubation period. After obtaining the matrices, the Biodentine, MTA Repair HP, and Bio-C Repair bioceramic cements were manipulated according to the manufacturers' instructions and inserted into the holes (
n = 10) with Paiva condensers (Golgran, São Caetano do Sul, SP, Brazil). They were then stored in an incubator at 37°C and 100% relative humidity for 7 days to allow complete setting of the materials (
Figure 2D).
Following the storage period, the matrices were sectioned with a #15 scalpel blade, from the free end to the center, where the sample was located, to allow removal of the bioceramic cement specimens without being damaged. As explained above, the excess cement was removed with a #15 scalpel blade and a thin piece of paper (
Figure 2E).
The sample was characterized previously as an auxiliary step in determining the standardization of the specimens and description of the data. Diameter and height were measured in all samples, using a 150-mm digital caliper (MTX), and weight was measured using a high-precision scale (HR-200; A&E, Tokyo, Japan).
The compressive strength test was performed by positioning each specimen at the center of the universal testing machine test device (model 5965; Instron) with a 2 kN load cell (
Figure 2F). The machine was operated at a constant speed of 0.5 mm/min on the upper face of the cylindrical specimens, up to maximum tension and rupture of the material, recorded in (N).
The compressive strength (δc) was obtained in MPa, according to the Formula 3 below:
where δc is the compressive strength (MPa), F(max) is the maximum stress obtained in the test (N), r is the radius of the specimen (mm), and π is a constant (3.14).
Statistical analysis
The data were analyzed using the Shapiro-Wilk normality test. In the mechanical push-out test, a non-normal distribution was detected; therefore, the data were analyzed using the Kruskal-Wallis non-parametric test and multiple comparisons were performed using the Friedman test. The failure mode of the bioceramic cements after the mechanical push-out test was presented as a percentage. The data from the compressive strength test showed a normal distribution; therefore, analysis of variance (ANOVA) was used and multiple comparisons were performed using the Tukey test. The level of significance was set at 0.05. SPSS version 22.0 for Mac (IBM Corp., Armonk, NY, USA) was used.
RESULTS
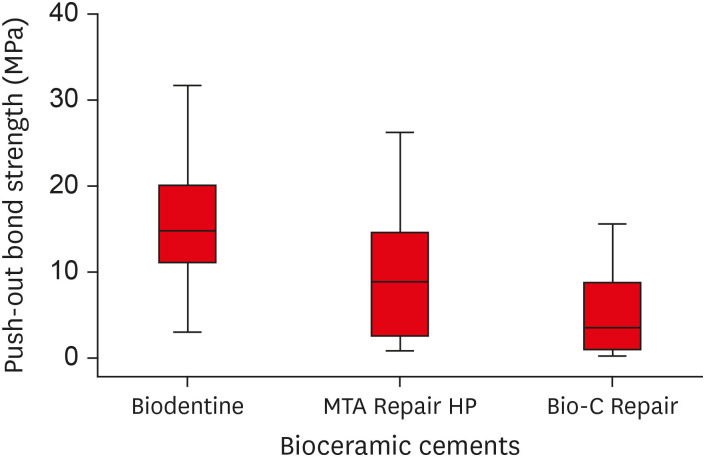
The Kruskal-Wallis test indicated that the Biodentine bioceramic cement had a greater push-out bond strength value than MTA Repair HP and Bio-C Repair cements; however, the difference was only statistically significant between the Biodentine and Bio-C Repair groups (
p = 0.0001) (
Table 2). A boxplot graph was constructed to illustrate the variations in the bond strength data for each experimental group (
Figure 3).
Table 2Median, minimum, and maximum bond strength to root dentin (MPa) of bioceramic cements
|
Bioceramic |
No. of samples |
Bond strength (MPa) |
|
Biodentine |
18 |
14.79 (2.97–31.61)a
|
|
MTA Repair HP |
18 |
8.84 (0.82–26.18)ac
|
|
Bio-C Repair |
18 |
3.48 (0.22–15.55)bc
|
Figure 3Box plot with the median, interquartile range, and maximum and minimum values of bond strength for the tested materials.
The failure mode of bioceramic cements after the mechanical push-out test was visualized in a stereomicroscope with ×3 magnification (
Figure 4). The results were expressed as a percentage (
Table 3). In the Biodentine group, the most frequent failure mode of bioceramic cements after the mechanical push-out test was mixed (61%), followed by cohesive (33%) and adhesive (6%) failures. In contrast, the most frequently observed failure mode in the MTA Repair HP group was adhesive (94%), followed by mixed failure in only 6% of cases and no instances of cohesive failure. Likewise, the Bio-C Repair group did not have cohesive failure, but did show predominantly adhesive failure (72%) and a higher frequency of mixed failure (28%) than the MTA Repair HP group.
Figure 4Illustrative photograph of the classification of the failure mode of bioceramic cements observed after the mechanical push-out test: (A) cohesive; (B) adhesive; (C) mixed.
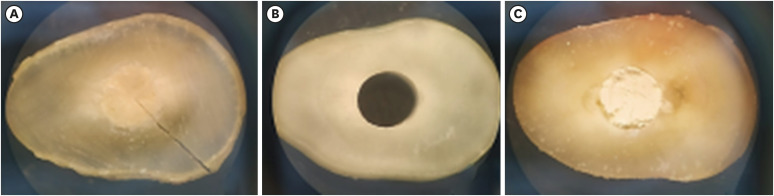
Table 3Frequency (%) of failure modes in the experimental groups
|
Bioceramic |
No. of samples |
Mode of failure |
|
Adhesive |
Cohesive |
Mixed |
|
Biodentine |
18 |
6% |
33% |
61% |
|
MTA Repair HP |
18 |
94% |
- |
6% |
|
Bio-C Repair |
18 |
72% |
- |
28% |
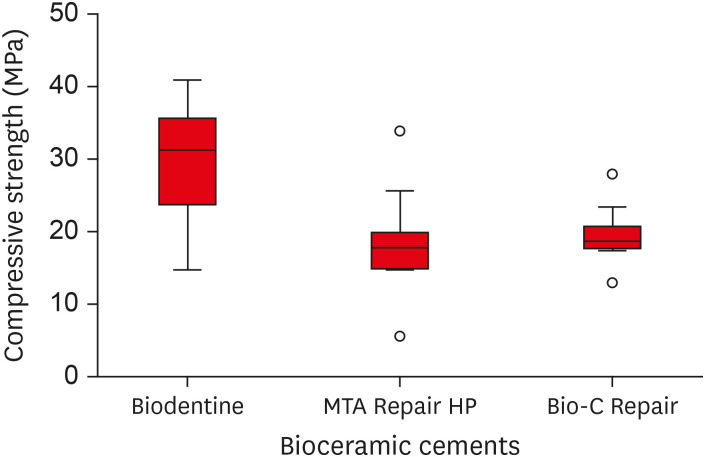
Regarding compressive strength, ANOVA indicated that the Biodentine bioceramic cement presented greater compressive strength than MTA Repair HP and Bio-C Repair, with a statistically significant difference (
p < 0.05). There was no significant difference between the MTA Repair HP and Bio-C Repair cements (
p > 0.05) (
Table 4). A boxplot graph illustrates the variation in the data for each experimental group (
Figure 5).
Table 4Mean values of compressive strength (MPa) collected in each experimental group
|
Bioceramic |
No. of samples |
Mean ± SD |
|
Biodentine |
10 |
29.59a ± 8.47 |
|
MTA Repair HP |
10 |
18.68b ± 7.40 |
|
Bio-C Repair |
10 |
19.59b ± 3.96 |
Figure 5Box plot with the median, interquartile range, and maximum and minimum values of compressive strength for the tested materials.
DISCUSSION
This study evaluated the mechanical properties of 3 bioceramic cements (Biodentine, MTA Repair HP, and Bio-C Repair) concerning bond strength to root dentin and compressive strength. The null hypothesis tested was rejected, since the 2 mechanical tests performed showed statistically significant differences between the groups.
The bond strength test to root dentin was performed by selecting single-rooted lower premolars with complete root development, as indicated in regenerative endodontic treatments. The premolars were then made into open apex samples, according to a study by Aguiar
et al. [
24]. The root canal was prepared with Largo drills, producing cavities with parallel walls and a standardized diameter of 1.7 mm, a procedure adopted to eliminate a possible confounding factor in the mechanical push-out test.
Double antibiotic paste was used as the intracanal medication of choice to reproduce the clinical routine. This paste is recommended by the American Association of Endodontics because of its efficiency in eliminating bacteria commonly found in the root canal and infected dentine, without staining the dental crown [
25,
26,
27,
28].
Root dentin is a non-uniform structure that can differ widely during endodontic treatment, depending on inherent variations in location, the presence of carious lesions, and patient age [
29,
30]. For this reason, all bioceramic cements were tested on the same dental element and only on the root cervical third, using 1 slice of the element for each cement evaluated.
There is a consensus in the scientific literature that one of the critical aspects of mechanical push-out tests is the lack of standardization. Variability exists in the parameters of the test itself (the diameter of the orifice of the base/applicator tip and speed), as well as in the method of sample preparation (specimen thickness, incubator time and root canal diameter) [
31]. These factors can lead to changes in the stress distribution patterns, which explain the large discrepancy in numerical results obtained with the same materials.
Zanatta
et al. [
32] used the finite element method to show that the diameter of the load applicator tip and the base orifice directly influence the stress distribution and the fracture profile. Bases with holes much larger than the diameter of the root canal tend to cause the sample to flex, thus producing greater tension in dentin, and leading to premature failure before it occurs at the adhesive interface. In contrast, small-diameter load applicator tips mainly cause cohesive failure, since they generate greater stresses in the filling material. The cited authors emphasized that the comparison of values reported in different studies must be regarded with caution and take into account the different methodologies used during the experimental test.
In the present study, the results showed that Biodentine cement presented higher bond strength values to root dentin, and that these values were significantly higher than those of Bio-C Repair. The median resistance to displacement corresponded to 14.79 MPa, with a maximum value of 31.61 MPa and a minimum value of 2.97 MPa. This result is in accordance with those of several published studies, which presented values ranging from 2.16–56.7 MPa, thus highlighting the superior performance of Biodentine cement over other bioceramic formulations [
24,
33,
34,
35]. This performance can be explained by the biomineralization capability of this material. Han and Okiji [
13] demonstrated that Biodentine releases calcium ions, forms calcium- and phosphorus-rich surface precipitates, and causes the uptake of calcium and silicon into human root canal dentin. The formation of dentin bridges can favor greater micromechanical retention.
The MTA Repair HP cement presented intermediate displacement resistance values with no significant differences from the other evaluated cements. Silva
et al. [
36] found different results, namely, that MTA Repair HP showed greater bond strength to root dentin than its predecessor, White MTA; however, Biodentine showed greater resistance to displacement than the 2 MTA formulations. It is worth mentioning that methodological differences in sample preparation and test configuration may explain the results found in the literature. In addition, Ørstavik [
37] reported that the flow of cement is closely related to the powder/liquid ratio. Later, Çelik
et al. [
38] also found that different mixing and compaction techniques can affect the chemical, physical, and biological properties of calcium silicate cements; hence, different results can already be expected, because MTA Repair HP is an operator-dependent bioceramic cement.
This study was the first to assess the bond strength to root dentin values of Bio-C Repair cement, launched in the second half of 2019. Low displacement resistance values were found, especially when compared with Biodentine. A study carried out by Benetti
et al. [
39] investigated the cytotoxicity, biocompatibility, and mineralization-inducing ability of this cement, in comparison with MTA Repair HP and its precursor, White MTA, in the subcutaneous tissue of rats. They concluded that the cement has cytotoxicity similar to that of MTA-based materials, is biocompatible, and induces biomineralization. Currently, no other studies merit further discussion. An important observation noted during the preparation of the samples was the difference in material consistency throughout the length of the storage syringe, as the cement was harder in the upper portion. This influence on the results may be a relevant question, as well as the implications of a ready-to-use formulation on the mechanical properties of cement. Considering this possibility, further investigations are needed.
The mechanical push-out test causes samples to fail from shear stress [
17,
40]. Therefore, it can be expected that there will be failures in the bioceramic/root dentin interface, and that these may vary depending on the characteristics of the cement and its interaction with the dentin substrate. For this reason, it is essential that the test devices be selected correctly in regard to sample characteristics. In this study, the Biodentine group had a low prevalence of adhesive failures (6%), and the most frequent failure mode was mixed (61%), possibly due to chemical interactions and the formation of dentinal bridges. In contrast, in both the MTA Repair HP and the Bio-C Repair (72%), groups, adhesive failure was observed more frequently (94%). This may explain the lower values of resistance to displacement, with less adhesion to the dentin of the root canal.
One of the limitations of this study was the number of samples, although calculations were performed based on studies evaluating the adhesive strength of bioceramic cements using the push-out test. In the present study, a large dispersion of results was observed. A high standard deviation is usually attributed to imprecision of the method, sample number, or the nature of the materials. It is worth mentioning that the last 2 factors may have influenced the results of the present study, and that the Biodentine exhibited less data variability than the other tested cements.
The results of the compressive strength test indicate that the Biodentine cement had greater compressive strength (29.59 ± 8.47 MPa) than MTA Repair HP (18.68 ± 7.40 MPa) and Bio-C Repair (19.59 ± 3.96 MPa); this difference was statistically significant (
p < 0.05). Similar results were found by Lucas
et al. [
20], who reported that Biodentine showed greater resistance to compression (37.22 ± 5.27 MPa) and greater bond strength to dentin (11.2 ± 2.16 MPa) than White MTA, which had a resistance to compression of 27.68 ± 3.56 MPa and a bond strength of 2.98 ± 0.64 MPa. These findings are assumed to reflect the chemical composition of the cement. In Biodentine liquid, there are water-soluble polymers containing polycarboxylate, which acts as a water reducer, promoting a less porous material that consequently has greater resistance to compression [
20].
Additional studies are warranted. It is known that high bond strength to root dentin and compression resistance are fundamental requirements for the success of regenerative endodontic procedures, because they minimize the clinical failures of root canal recontamination by displacing materials from forces arising from occlusion or condensation restorative materials [
4,
14,
15,
16]. However, no data exist regarding the minimum shear or tensile force required for displacement to occur; therefore, an international standard must be established to determine the ideal values of the mechanical properties of endodontic cements, based on laboratory and clinical research.
The Biodentine bioceramic cement showed greater resistance to compression than both MTA Repair HP and Bio-C Repair, and superior bond strength to root dentin only to Bio-C Repair cement. The most frequent failure mode of Biodentine was mixed, while adhesive failure was most commonly observed for MTA Repair HP and Bio-C Repair.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Alencar AHG, Estrela C.
Data curation: Siqueira PC.
Formal analysis: Bruno KF, Silva JDS.
Investigation: Rodrigues MNM, Bruno KF, Siqueira PC.
Methodology: Rodrigues MNM, Decurcio DA, Silva JDS.
Project administration: Alencar AHG.
Supervision: Alencar AHG, Estrela C.
Validation: Decurcio DA, Bruno KF, Siqueira PC.
Visualization: Rodrigues MNM, Silva JDS.
Writing - original draft: Rodrigues MNM, Bruno KF, Silva JDS, Siqueira PC.
Writing - review & editing: Alencar AHG, Estrela C, Decurcio DA.
REFERENCES
- 1. Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol 1992;8:45-55.ArticlePubMed
- 2. Nosrat A, Seifi A, Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. J Endod 2011;37:562-567.ArticlePubMed
- 3. Bansal R, Bansal R. Regenerative endodontics: a state of the art. Indian J Dent Res 2011;22:122-131.ArticlePubMed
- 4. Diogenes A, Ruparel NB. Regenerative endodontic procedures: clinical outcomes. Dent Clin North Am 2017;61:111-125.PubMed
- 5. Téclès O, Laurent P, Aubut V, About I. Human tooth culture: a study model for reparative dentinogenesis and direct pulp capping materials biocompatibility. J Biomed Mater Res B Appl Biomater 2008;85:180-187.PubMed
- 6. Zhao X, He W, Song Z, Tong Z, Li S, Ni L. Mineral trioxide aggregate promotes odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp stem cells. Mol Biol Rep 2012;39:215-220.ArticlePubMedPDF
- 7. Zhu Q, Haglund R, Safavi KE, Spangberg LS. Adhesion of human osteoblasts on root-end filling materials. J Endod 2000;26:404-406.ArticlePubMed
- 8. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part I: chemical, physical, and antibacterial properties. J Endod 2010;36:16-27.ArticlePubMed
- 9. Neha K, Kansal R, Garg P, Joshi R, Garg D, Grover HS. Management of immature teeth by dentin-pulp regeneration: a recent approach. Med Oral Patol Oral Cir Bucal 2011;16:e997-ee1004.PubMed
- 10. Bortoluzzi EA, Araújo GS, Guerreiro Tanomaru JM, Tanomaru-Filho M. Marginal gingiva discoloration by gray MTA: a case report. J Endod 2007;33:325-327.ArticlePubMed
- 11. Marconyak LJ Jr, Kirkpatrick TC, Roberts HW, Roberts MD, Aparicio A, Himel VT, Sabey KA. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J Endod 2016;42:470-473.ArticlePubMed
- 12. Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials 2004;25:787-793.ArticlePubMed
- 13. Han L, Okiji T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int Endod J 2013;46:808-814.ArticlePubMed
- 14. Shokouhinejad N, Nekoofar MH, Iravani A, Kharrazifard MJ, Dummer PM. Effect of acidic environment on the push-out bond strength of mineral trioxide aggregate. J Endod 2010;36:871-874.ArticlePubMed
- 15. Hashem AA, Wanees Amin SA. The effect of acidity on dislodgment resistance of mineral trioxide aggregate and bioaggregate in furcation perforations: an in vitro comparative study. J Endod 2012;38:245-249.PubMed
- 16. Shahi S, Rahimi S, Yavari HR, Samiei M, Janani M, Bahari M, Abdolrahimi M, Pakdel F, Aghbali A. Effects of various mixing techniques on push-out bond strengths of white mineral trioxide aggregate. J Endod 2012;38:501-504.ArticlePubMed
- 17. Kurtz JS, Perdigão J, Geraldeli S, Hodges JS, Bowles WR. Bond strengths of tooth-colored posts, effect of sealer, dentin adhesive, and root region. Am J Dent 2003;16:31A-36A.PubMed
- 18. Fisher MA, Berzins DW, Bahcall JK. An in vitro comparison of bond strength of various obturation materials to root canal dentin using a push-out test design. J Endod 2007;33:856-858.PubMed
- 19. Tagger M, Tagger E, Tjan AH, Bakland LK. Measurement of adhesion of endodontic sealers to dentin. J Endod 2002;28:351-354.ArticlePubMed
- 20. Lucas CP, Viapiana R, Bosso-Martelo R, Guerreiro-Tanomaru JM, Camilleri J, Tanomaru-Filho M. Physicochemical properties and dentin bond strength of a tricalcium silicate-based retrograde material. Braz Dent J 2017;28:51-56.ArticlePubMed
- 21. Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov 2011;8:1-9.PubMedPMC
- 22. Topçuoğlu HS, Arslan H, Akçay M, Saygili G, Çakici F, Topçuoğlu G. The effect of medicaments used in endodontic regeneration technique on the dislocation resistance of mineral trioxide aggregate to root canal dentin. J Endod 2014;40:2041-2044.ArticlePubMed
- 23. Rosatto CM, Bicalho AA, Veríssimo C, Bragança GF, Rodrigues MP, Tantbirojn D, Versluis A, Soares CJ. Mechanical properties, shrinkage stress, cuspal strain and fracture resistance of molars restored with bulk-fill composites and incremental filling technique. J Dent 2015;43:1519-1528.ArticlePubMed
- 24. Aguiar BA, Frota LM, Taguatinga DT, Vivan RR, Camilleri J, Duarte MA, de Vasconcelos BC. Influence of ultrasonic agitation on bond strength, marginal adaptation, and tooth discoloration provided by three coronary barrier endodontic materials. Clin Oral Investig 2019;23:4113-4122.ArticlePubMedPDF
- 25. American Association of Endodontists. AAE Clinical Considerations for a Regenerative Procedure Revised 4/1/2018 [Internet]. updated 2020 Oct 20]. Available from: https://www.aae.org/specialty/wp-content/uploads/sites/2/2018/06/ConsiderationsForRegEndo_AsOfApril2018.pdf.
- 26. Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol 2001;17:185-187.ArticlePubMedPDF
- 27. Akcay M, Arslan H, Yasa B, Kavrık F, Yasa E. Spectrophotometric analysis of crown discoloration induced by various antibiotic pastes used in revascularization. J Endod 2014;40:845-848.ArticlePubMed
- 28. Tagelsir A, Yassen GH, Gomez GF, Gregory RL. Effect of antimicrobials used in regenerative endodontic procedures on 3-week-old Enterococcus faecalis biofilm. J Endod 2016;42:258-262.PubMed
- 29. Arnold WH, Konopka S, Gaengler P. Qualitative and quantitative assessment of intratubular dentin formation in human natural carious lesions. Calcif Tissue Int 2001;69:268-273.ArticlePubMedPDF
- 30. Lo Giudice G, Cutroneo G, Centofanti A, Artemisia A, Bramanti E, Militi A, Rizzo G, Favaloro A, Irrera A, Lo Giudice R, Cicciù M. Dentin morphology of root canal surface: a quantitative evaluation based on a scanning electronic microscopy study. BioMed Res Int 2015;2015:164065.PubMedPMC
- 31. Brichko J, Burrow MF, Parashos P. Design variability of the push-out bond test in endodontic research: a systematic review. J Endod 2018;44:1237-1245.ArticlePubMed
- 32. Zanatta RF, Barreto BC, Xavier TA, Versluis A, Soares CJ. Effect of punch and orifice base sizes in different push-out test setups: stress distribution analysis. J Adhes Dent 2015;17:45-50.PubMed
- 33. Nagas E, Cehreli ZC, Uyanik MO, Vallittu PK, Lassila LV. Effect of several intracanal medicaments on the push-out bond strength of ProRoot MTA and Biodentine. Int Endod J 2016;49:184-188.PubMed
- 34. Turk T, Fidler A. Effect of medicaments used in endodontic regeneration technique on push-out bond strength of MTA and Biodentine. Biotechnol Biotechnol Equip 2016;30:140-144.Article
- 35. Majeed A, AlShwaimi E. Push-out bond strength and surface microhardness of calcium silicate-based biomaterials: an in vitro study. Med Princ Pract 2017;26:139-145.ArticlePubMedPDF
- 36. Silva EJ, Carvalho NK, Zanon M, Senna PM, DE-Deus G, Zuolo ML, Zaia AA. Push-out bond strength of MTA HP, a new high-plasticity calcium silicate-based cement. Braz Oral Res 2016;30:1-5.Article
- 37. Ørstavik D. Physical properties of root canal sealers: measurement of flow, working time, and compressive strength. Int Endod J 1983;16:99-107.ArticlePubMed
- 38. Çelik D, Er K, Serper A, Taşdemir T, Ceyhanlı KT. Push-out bond strength of three calcium silicate cements to root canal dentine after two different irrigation regimes. Clin Oral Investig 2014;18:1141-1146.ArticlePubMedPDF
- 39. Benetti F, Queiroz ÍO, Cosme-Silva L, Conti LC, Oliveira SH, Cintra LT. Cytotoxicity, biocompatibility and biomineralization of a new ready-for-use bioceramic repair material. Braz Dent J 2019;30:325-332.ArticlePubMed
- 40. Soares CJ, Santana FR, Castro CG, Santos-Filho PC, Soares PV, Qian F, Armstrong SR. Finite element analysis and bond strength of a glass post to intraradicular dentin: comparison between microtensile and push-out tests. Dent Mater 2008;24:1405-1411.ArticlePubMed
 , Kely Firmino Bruno2
, Kely Firmino Bruno2 , Ana Helena Gonçalves de Alencar1
, Ana Helena Gonçalves de Alencar1 , Julyana Dumas Santos Silva1
, Julyana Dumas Santos Silva1 , Patrícia Correia de Siqueira1
, Patrícia Correia de Siqueira1 , Daniel de Almeida Decurcio1
, Daniel de Almeida Decurcio1 , Carlos Estrela1
, Carlos Estrela1







 KACD
KACD
 ePub Link
ePub Link Cite
Cite

