Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(4); 2021 > Article
- Research Article Push-out bond strength and marginal adaptation of apical plugs with bioactive endodontic cements in simulated immature teeth
-
Maria Aparecida Barbosa de Sá1
 , Eduardo Nunes1
, Eduardo Nunes1 , Alberto Nogueira da Gama Antunes1
, Alberto Nogueira da Gama Antunes1 , Manoel Brito Júnior2
, Manoel Brito Júnior2 , Martinho Campolina Rebello Horta1
, Martinho Campolina Rebello Horta1 , Rodrigo Rodrigues Amaral1
, Rodrigo Rodrigues Amaral1 , Stephen Cohen3
, Stephen Cohen3 , Frank Ferreira Silveira1
, Frank Ferreira Silveira1
-
Restor Dent Endod 2021;46(4):e53.
DOI: https://doi.org/10.5395/rde.2021.46.e53
Published online: October 20, 2021
1Department of Dentistry, Pontifical Catholic University of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
2Department Dentistry, Montes Claros, Minas Gerais, Brazil.
3Department of Endodontics, Arthur School of Dentistry, University of the Pacific, San Francisco, CA, USA.
- Correspondence to Frank Ferreira Silveira, DDS, MSc, PhD. Adjunct Professor, Department of Dentistry, Pontifical Catholic University of Minas Gerais, Avenida Dom José Gaspar, 500, Minas Gerais, Belo Horizonte 3035610, Brazil. frankfoui@uol.com.br
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives This study evaluates the bond strength and marginal adaptation of mineral trioxide aggregate (MTA) Repair HP and Biodentine used as apical plugs; MTA was used as reference material for comparison.
-
Materials and Methods A total of 30 single-rooted teeth with standardized, artificially created open apices were randomly divided into 3 groups (n = 10 per group), according to the material used to form 6-mm-thick apical plugs: group 1 (MTA Repair HP); group 2 (Biodentine); and group 3 (white MTA). Subsequently, the specimens were transversely sectioned to obtain 2 (cervical and apical) 2.5-mm-thick slices per root. Epoxy resin replicas were observed under a scanning electron microscope to measure the gap size at the material/dentin interface (the largest and smaller gaps were recorded for each replica). The bond strength of the investigated materials to dentin was determined using the push-out test. The variable bond strengths and gap sizes were evaluated independently at the apical and cervical root dentin slices. Data were analyzed using descriptive and analytic statistics.
-
Results The comparison between the groups regarding the variables' bond strengths and gap sizes showed no statistical difference (p > 0.05) except for a single difference in the smallest gap at the cervical root dentin slice, which was higher in group 3 than in group 1 (p < 0.05).
-
Conclusions The bond strength and marginal adaptation to root canal walls of MTA HP and Biodentine cement were comparable to white MTA.
INTRODUCTION
MATERIALS AND METHODS
Composition of calcium silicate cements used, along with the manufacture instruction of its manipulation
RESULTS
Means and standard deviations of the bond strength values and comparisons among the investigated groups
Median, minimum and maximum gap values and comparisons among groups
Scanning electron microscope resin replica photomicrographies of HP MTA, Biodentine and white MTA cements. Plug circumference (A); Largest gap (B); Smallest gap (C). Arrows indicate small defects seen at 60× magnification. The largest and smallest gaps are shown at 700×.
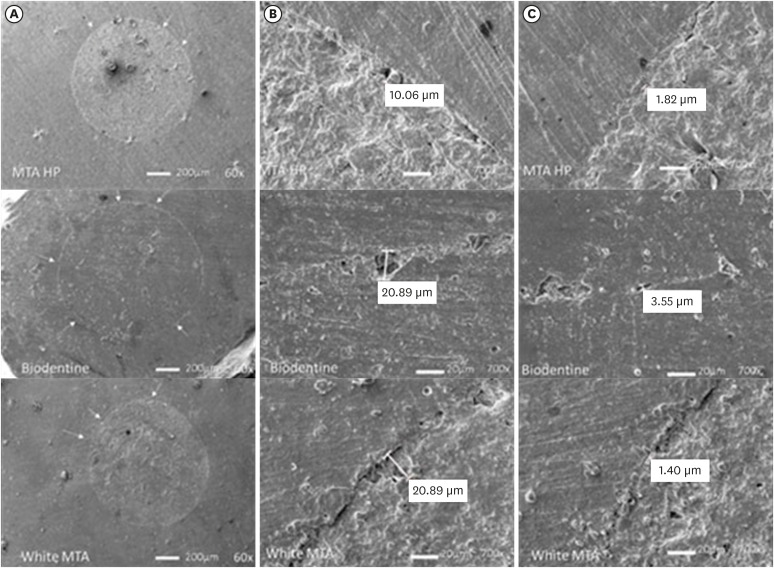
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
-
Funding: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brasil (CAPES) - Finance Code 001. MCRH is a research fellow of Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG CDS-PPM-00653-16).
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Sá MAB, Nunes E, Brito Jr M, Silveira FF.
Data curation: Sá MAB, Nunes E, Brito Jr M, Amaral RR, Silveira FF, Horta MCR, Antunes ANG, Cohen S.
formal analysis: Sá MAB, Horta MCR, Silveira FF.
Funding acquisition: Sá MAB, Brito Jr M, Silveira FF.
Investigation: Sá MAB, Silveira FF.
Methodology: Sá MAB, Brito Jr M, Silveira FF, Antunes ANG.
Project administration: Sá MAB, Brito Jr M, Silveira FF.
resources: Sá MAB, Silveira FF.
Software: Silveira FF, Horta MCR.
Supervision: Sá MAB, Nunes E, Brito Jr M, Amaral RR, Silveira FF, Horta MCR, Antunes ANG, Cohen S.
Validation: Sá MAB, Nunes E, Brito Jr M, Amaral RR, Silveira FF, Horta MCR, Antunes ANG, Cohen S.
Visualization: Sá MAB, Nunes E, Brito Jr M, Amaral RR, Silveira FF, Horta MCR, Antunes ANG, Cohen S.
Writing original draft: Sá MAB, Silveira FF.
Writing review and editing: Sá MAB, Nunes E, Brito Jr M, Amaral RR, Silveira FF, Horta MCR, Antunes ANG, Cohen S.
- 1. Sisli SN, Ozbas H. Comparative micro-computed tomographic evaluation of the sealing quality of ProRoot MTA and MTA Angelus apical plugs placed with various techniques. J Endod 2017;43:147-151.ArticlePubMed
- 2. Torabinejad M, Parirokh M, Dummer PM. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part II: other clinical applications and complications. Int Endod J 2018;51:284-317.ArticlePubMedPDF
- 3. Ber BS, Hatton JF, Stewart GP. Chemical modification of ProRoot MTA to improve handling characteristics and decrease setting time. J Endod 2007;33:1231-1234.ArticlePubMed
- 4. Guimarães BM, Prati C, Duarte MA, Bramante CM, Gandolfi MG. Physicochemical properties of calcium silicate-based formulations MTA Repair HP and MTA Vitalcem. J Appl Oral Sci 2018;26:e2017115.PubMedPMC
- 5. Marciano MA, Costa RM, Camilleri J, Mondelli RF, Guimarães BM, Duarte MA. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J Endod 2014;40:1235-1240.ArticlePubMed
- 6. Możyńska J, Metlerski M, Lipski M, Nowicka A. Tooth discoloration induced by different calcium silicate-based cements: a systematic review of in vitro studies. J Endod 2017;43:1593-1601.PubMed
- 7. Rajasekharan S, Martens LC, Cauwels RG, Anthonappa RP, Verbeeck RM. Biodentine™ material characteristics and clinical applications: a 3 year literature review and update. Eur Arch Paediatr Dent 2018;19:1-22.ArticlePubMedPDF
- 8. Vidal K, Martin G, Lozano O, Salas M, Trigueros J, Aguilar G. Apical closure in apexification: a review and case report of apexification treatment of an immature permanent tooth with Biodentine. J Endod 2016;42:730-734.ArticlePubMed
- 9. Hachmeister DR, Schindler WG, Walker WA 3rd, Thomas DD. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod 2002;28:386-390.ArticlePubMed
- 10. Yilmaz Z, Küçükkaya Eren S, Uzunoğlu E, Görduysus M, Görduysus MÖ. Interaction of backfilling techniques and MTA plugs with additives: Fracture strength and adaptation analyses. Dent Mater J 2017;36:809-815.ArticlePubMed
- 11. Kadić S, Baraba A, Miletić I, Ionescu A, Brambilla E, Ivanišević Malčić A, Gabrić D. Push-out bond strength of three different calcium silicate-based root-end filling materials after ultrasonic retrograde cavity preparation. Clin Oral Investig 2018;22:1559-1565.ArticlePubMedPDF
- 12. Tran D, He J, Glickman GN, Woodmansey KF. Comparative analysis of calcium silicate-based root filling materials using an open apex model. J Endod 2016;42:654-658.ArticlePubMed
- 13. Araújo AC, Nunes E, Fonseca AA, Cortes MI, Horta MC, Silveira FF. Influence of smear layer removal and application mode of MTA on the marginal adaptation in immature teeth: a SEM analysis. Dent Traumatol 2013;29:212-217.PubMed
- 14. Bidar M, Disfani R, Gharagozloo S, Khoynezhad S, Rouhani A. Medication with calcium hydroxide improved marginal adaptation of mineral trioxide aggregate apical barrier. J Endod 2010;36:1679-1682.ArticlePubMed
- 15. López-García S, Pecci-Lloret MP, Pecci-Lloret MR, Oñate-Sánchez RE, García-Bernal D, Castelo-Baz P, Rodríguez-Lozano FJ, Guerrero-Gironés J. In vitro evaluation of the biological effects of ACTIVA Kids BioACTIVE Restorative, Ionolux, and Riva Light Cure on human dental pulp stem cells. Materials (Basel) 2019;12:3694-3705.PubMedPMC
- 16. Ertas H, Kucukyilmaz E, Ok E, Uysal B. Push-out bond strength of different mineral trioxide aggregates. Eur J Dent 2014;8:348-352.ArticlePubMedPMC
- 17. Shokouhinejad N, Yazdi KA, Nekoofar MH, Matmir S, Khoshkhounejad M. Effect of acidic environment on dislocation resistance of endosequence root repair material and mineral trioxide aggregate. J Dent (Tehran) 2014;11:161-166.PubMedPMC
- 18. Thompson JI, Gregson PJ, Revell PA. Analysis of push-out test data based on interfacial fracture energy. J Mater Sci Mater Med 1999;10:863-868.ArticlePubMedPDF
- 19. Saghiri MA, Garcia-Godoy F, Gutmann JL, Lotfi M, Asatourian A, Ahmadi H. Push-out bond strength of a nano-modified mineral trioxide aggregate. Dent Traumatol 2013;29:323-327.ArticlePubMed
- 20. Shokouhinejad N, Nekoofar MH, Iravani A, Kharrazifard MJ, Dummer PM. Effect of acidic environment on the push-out bond strength of mineral trioxide aggregate. J Endod 2010;36:871-874.ArticlePubMed
- 21. Camilleri J. The chemical composition of mineral trioxide aggregate. J Conserv Dent 2008;11:141-143.ArticlePubMedPMC
- 22. Stefaneli Marques JH, Silva-Sousa YT, Rached-Júnior FJ, Macedo LM, Mazzi-Chaves JF, Camilleri J, Sousa-Neto MD. Push-out bond strength of different tricalcium silicate-based filling materials to root dentin. Braz Oral Res 2018;32:e18.PubMed
- 23. Silva EJ, Carvalho NK, Zanon M, Senna PM, De-Deus G, Zuolo ML, Zaia AA. Push-out bond strength of MTA HP, a new high-plasticity calcium silicate-based cement. Braz Oral Res 2016;30:S1806-83242016000100269.ArticlePubMed
- 24. Bodanezi A, Carvalho N, Silva D, Bernardineli N, Bramante CM, Garcia RB, de Moraes IG. Immediate and delayed solubility of mineral trioxide aggregate and Portland cement. J Appl Oral Sci 2008;16:127-131.ArticlePubMedPMC
- 25. Bachoo IK, Seymour D, Brunton P. A biocompatible and bioactive replacement for dentine: is this a reality? The properties and uses of a novel calcium-based cement. Br Dent J 2013;214:E5.ArticlePubMedPDF
- 26. Stabholz A, Friedman S, Abed J. Marginal adaptation of retrograde fillings and its correlation with sealability. J Endod 1985;11:218-223.ArticlePubMed
- 27. Johnson BR. Considerations in the selection of a root-end filling material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:398-404.ArticlePubMed
- 28. Zafar M, Iravani M, Eghbal MJ, Asgary S. Coronal and apical sealing ability of a new endodontic cement. Iran Endod J 2009;4:15-19.PubMedPMC
- 29. Bolhari B, Ashofteh Yazdi K, Sharifi F, Pirmoazen S. Comparative scanning electron microscopic study of the marginal adaptation of four root-end filling materials in presence and absence of blood. J Dent (Tehran) 2015;12:226-234.PubMedPMC
- 30. Shokouhinejad N, Nekoofar MH, Ashoftehyazdi K, Zahraee S, Khoshkhounejad M. Marginal adaptation of new bioceramic materials and mineral trioxide aggregate: a scanning electron microscopy study. Iran Endod J 2014;9:144-148.PubMedPMC
- 31. Costa AT, Konrath F, Dedavid B, Weber JB, de Oliveira MG. Marginal adaptation of root-end filling materials: an in vitro study with teeth and replicas. J Contemp Dent Pract 2009;10:75-82.
- 32. Badr AE. Marginal adaptation and cytotoxicity of bone cement compared with amalgam and mineral trioxide aggregate as root-end filling materials. J Endod 2010;36:1056-1060.ArticlePubMed
- 33. Gondim E Jr, Zaia AA, Gomes BP, Ferraz CC, Teixeira FB, Souza-Filho FJ. Investigation of the marginal adaptation of root-end filling materials in root-end cavities prepared with ultrasonic tips. Int Endod J 2003;36:491-499.ArticlePubMedPDF
- 34. Teaford MF, Oyen OJ. Live primates and dental replication: new problems and new techniques. Am J Phys Anthropol 1989;80:73-81.ArticlePubMed
- 35. Gondim E Jr, Gomes BP, Ferraz CC, Teixeira FB, Souza-Filho FJ. Effect of sonic and ultrasonic retrograde cavity preparation on the integrity of root apices of freshly extracted human teeth: scanning electron microscopy analysis. J Endod 2002;28:646-650.ArticlePubMed
- 36. Bocker C, Kracker M, Rüssel C. Replica extraction method on nanostructured gold coatings and orientation determination combining SEM and TEM techniques. Microsc Microanal 2014;20:1654-1661.ArticlePubMed
- 37. Küçükkaya Eren S, Görduysus MÖ, Şahin C. Sealing ability and adaptation of root-end filling materials in cavities prepared with different techniques. Microsc Res Tech 2017;80:756-762.PubMed
- 38. Bolhari B, Yazdi KA, Sharifi F, Pirmoazen S. Comparative scanning electron microscopic study of the marginal adaptation of four root-end filling materials in presence and absence of blood. J Dent (Tehran) 2015;12:226-234.PubMedPMC
- 39. Torres FFE, Jacobs R, EzEldeen M, Guerreiro-Tanomaru JM, Dos Santos BC, Lucas-Oliveira É, Bonagamba TJ, Tanomaru-Filho M. Micro-computed tomography high resolution evaluation of dimensional and morphological changes of 3 root-end filling materials in simulated physiological conditions. J Mater Sci Mater Med 2020;31:14.ArticlePubMedPDF
- 40. Küçükkaya Eren S, Aksel H, Askerbeyli Örs S, Serper A, Koçak Y, Ocak M, Çelik HH. Obturation quality of calcium silicate-based cements placed with different techniques in teeth with perforating internal root resorption: a micro-computed tomographic study. Clin Oral Investig 2019;23:805-811.ArticlePubMedPDF
REFERENCES
Tables & Figures
REFERENCES
Citations

- Evaluation of marginal adaptation and bond strength of apical root canal plugs using different bioceramic cements
Michel Sena Fernandes Faria Lima, Alberto Nogueira da Gama Antunes, Kênia Maria Pereira Soares de Toubes, Fábio Fernandes Borém Bruzinga, Camila de Sousa Caneschi, Luís Fernando dos Santos Alves Morgan, Frank Ferreira Silveira
BMC Oral Health.2026;[Epub] CrossRef - Application of Biodentine for Apexification of Immature Teeth of Children: A Scoping Review
Liz M Gerard, Sumit Gaur
International Journal of Clinical Pediatric Dentistry.2025; 18(5): 573. CrossRef - Evaluation of the root dentin bond strength and intratubular biomineralization of a premixed calcium aluminate-based hydraulic bioceramic endodontic sealer
Yu-Na Lee, Min-Kyeong Kim, Hee-Jin Kim, Mi-Kyung Yu, Kwang-Won Lee, Kyung-San Min
Journal of Oral Science.2024; 66(2): 96. CrossRef - Managing Cracked Teeth with Root Extension: A Prospective Preliminary Study Using Biodentine™ Material
Kênia Maria Soares de Toubes, Isabella Sousa Corrêa, Regina Célia Lopes Valadares, Stephanie Quadros Tonelli, Fábio Fernandes Borém Bruzinga, Frank Ferreira Silveira, Dr Karthikeyan Ramalingam
International Journal of Dentistry.2024;[Epub] CrossRef - Marginal adaptation of customized gutta percha cone with calcium silicate based sealer versus MTA and biodentine apical plugs in simulated immature permanent teeth (an in vitro study)
Mary M. Mina, Sybel M. Moussa, Mahmoud R. Aboelseoud
BMC Oral Health.2024;[Epub] CrossRef - Comparative Evaluation of Push-Out Bond Strength of Conventional Mineral Trioxide Aggregate, Biodentine, a Modified Mineral Trioxide Aggregate, and Two Novel Antibacterial-Enhanced Mineral Trioxide Aggregates
Arokia Rajkumar Shancy Merlin, Vignesh Ravindran, Ganesh Jeevanandan, Rajalakshmanan Eswaramoorthy, Abirami Arthanari
Cureus.2024;[Epub] CrossRef - Push out bond strength of hydraulic cements used at different thicknesses
C. Ruiz Durán, Dra L. Gancedo-Caravia, V. Vera González, C. González Losada
BMC Oral Health.2023;[Epub] CrossRef - Effects of different calcium-silicate based materials on fracture resistance of immature permanent teeth with replacement root resorption and osteoclastogenesis
Gabriela Leite de Souza, Gabrielle Alves Nunes Freitas, Maria Tereza Hordones Ribeiro, Nelly Xiomara Alvarado Lemus, Carlos José Soares, Camilla Christian Gomes Moura
Restorative Dentistry & Endodontics.2023;[Epub] CrossRef
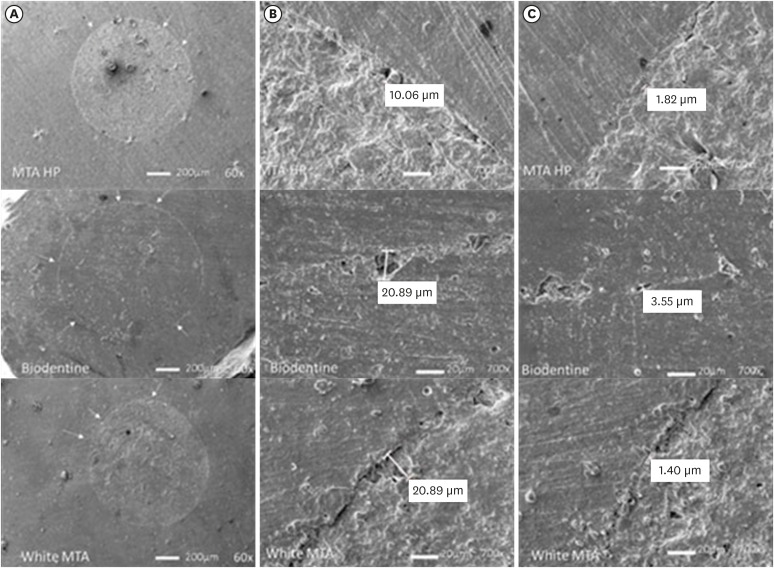
Figure 1
Composition of calcium silicate cements used, along with the manufacture instruction of its manipulation
| Calcium silicate cement | Composition | Manufacture instruction of its manipulation |
|---|---|---|
| MTA Repair HP | Powder: Tricalcium silicate 3CaO, SiO2; Dicalcium silicate 2CaO.SiO2; Tricalcium aluminate 3CaO.Al2O3; Oxide Calcium CaO; Calcium Tungsate CaWO4 | 1) Dispense the contents of 1 pack of MTA Repair HP and 2 drops of liquid on the glass plate; 2) Mix for 40 seconds. until complete homogenization of the powder and liquid. The cement obtained is similar to a modeling clay; 3) Take the MTA Repair HP to the desired location with an MTA carrier condensing it with appropriate instruments for this purpose. |
| Liquid: Water and Plasticizer | ||
| Biodentine | Powder: Tricalcium silicate, zirconium oxide, calcium oxide, yellow pigment, red pigment, brown iron oxide | 1) Open a capsule and place it in the capsule holder; 2) Detach an ampoule containing the liquid and gently tap the cap to force all the liquid to flow into the flacon; 3) Rotate the lid to open; 4) Place 5 drops of liquid in the capsule; 5) Close the capsule. Place it and an amalgamator at a speed of 4,000–4,200 revolutions min; 6) Let it stir for 30 seconds; 7) Open the capsule and check the consistency of the material; 8) Remove the Biodentine with the help of the spatula provided. Depending on the desired application, it is possible to apply Biodentine with an amalgam holder, a spatula or an MTA carrier. |
| Liquid: Calcium chloride dihydrate, air, purified water | ||
| White MTA | Tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium oxide, bismuth oxide | 1) Mix for 30 seconds the content of 1 sachet of (or 1 spoon) with 1 drop of distilled water. The mixture should be homogeneous and with a consistency similar to wet sand; 2) Place the cement on the selected site with a sterilized amalgam carrier or other appropriate instrument; 3) Condense the cement with instruments such as amalgam condensers, a number 1 spatula or absorbent paper points moistened with distilled water. |
MTA, mineral trioxide aggregate.
Means and standard deviations of the bond strength values and comparisons among the investigated groups
| Root dentin slices | MTA Repair HP (group 1) | Biodentine (group 2) | White MTA (group 3) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | 1 × 2 | 1 × 3 | 2 × 3 | |
| Apical | 17.69 ± 8.00 | 12.97 ± 10.89 | 16.60 ± 7.80 | ns | ns | ns |
| Cervical | 27.53 ± 6.49 | 17.68 ± 9.51 | 23.42 ± 11.17 | ns | ns | ns |
| Mean values of both slices | 22.61 ± 5.07 | 15.32 ± 8.47 | 20.01 ± 7.09 | ns | ns | ns |
MTA, mineral trioxide aggregate; SD, standard deviation; ns, not significant (
*
Median, minimum and maximum gap values and comparisons among groups
| Root dentin slices | MTA Repair HP (group 1) | Biodentine (group 2) | White MTA (group 3) | |||
|---|---|---|---|---|---|---|
| Median (minimum–maximum) | Median (minimum–maximum) | Median (minimum–maximum) | 1 × 2 | 1 × 3 | 2 × 3 | |
| Apical (largest gap) | 15.78 (0.00–86.03) | 18.68 (5.94–55.07) | 29.86 (1.33–71.62) | ns | ns | ns |
| Cervical (largest gap) | 7.86 (0.00–411.0) | 19.72 (5.02–145.5) | 45.68 (4.13–208.4) | ns | ns | ns |
| Apical (smallest gap) | 1.48 (0.00–25.96) | 2.37 (0.82–8.87) | 1.52 (0.58–87.54) | ns | ns | ns |
| Cervical (smallest gap) | 1.11 (0.00–6.82) | 2.62 (0.00–12.48) | 6.26 (0.75–21.20) | ns | < 0.05 | ns |
MTA, mineral trioxide aggregate; ns, not significant (
*
MTA, mineral trioxide aggregate.
MTA, mineral trioxide aggregate; SD, standard deviation; ns, not significant (
*
MTA, mineral trioxide aggregate; ns, not significant (
*

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

