Abstract
-
Objectives
The aim of this study was to evaluate the apical pressure generated by 2 endodontic irrigation needles and the GentleWave system in mandibular molars.
-
Materials and Methods
The mesial and distal root canals of 12 mandibular molars were irrigated with a 30-gauge close-end needle or with a 30-gauge open-end needle. Procedures were performed in the mesial and distal canals. The GentleWave procedure and irrigation at 1 mm from the apex in the distal roots using an open-end needle were used, respectively, as negative and positive controls. The apical pressure was measured using a data acquisition pressure setup. Apical pressure exerted by the different needles in the 2 different canal types was statistically compared using 2-way analysis of variance.
-
Results
Significant differences were found in the apical pressure for both needles and the canal type. The lowest values were obtained with close-end needles and in mesial canals. Negative apical pressure values were obtained using GentleWave.
-
Conclusions
The needle and the canal type influenced the apical pressure. The GentleWave procedure produced negative apical pressure.
-
Keywords: Apical pressure; Irrigation needles; Irrigation technique
INTRODUCTION
The goal of endodontic therapy is to restore and maintain the health of apical tissues so patients can achieve functional and asymptomatic dentition. The removal of infected tissue and bacteria appears to increase the success rate of endodontic treatment [
1,
2]. Due to limitations in mechanical debridement alone and complex anatomy, many different irrigation and activation techniques have been utilized [
3,
4,
5]. Traditionally, irrigants have been delivered to root canal systems with plastic syringes and needles. These needles are made in a variety of sizes and tip designs. However, conventional needle irrigation alone is ineffective in debriding lateral anatomies such as accessory canals, isthmuses, or the apical portions of root canal spaces [
6,
7]. Data obtained from computational fluid dynamics models have demonstrated that side-vented needles deliver irrigants approximately 1–1.5 mm beyond the needle tips, while open-ended needles deliver irrigants 2 mm or more beyond the needle tip [
8]. However, open-ended needles lead to higher pressures at apical foramina than side-vented needles. Another limitation of needle irrigation is the presence of a “vapor lock” and fluid stagnation in the apical portion of canals, which can restrict apical disinfection of canals [
9].
The GentleWave system (Sonendo, Orange, CA, USA) utilizes multisonic technology to deliver sodium hypochlorite, ethylenediaminetetraacetic acid (EDTA), and distilled water from the tip of a procedure instrument into the pulp chamber and canals [
10]. These fluids are degassed to prevent energy loss. The GentleWave induces a negative pressure throughout the procedure, which was measured
in vitro by Haapasalo
et al. [
11], who mounted 4 maxillary molars in an air-tight custom fixture and coupled them to a pressure transducer. The generated apical pressure after no instrumentation, instrumentation to a size 15/0.04, 40/0.04 taper, and after perforating the apical foramen maintained negative values compared to the needle irrigation technique [
11].
Several methodologies have been used to test irrigant extrusion, including chemical methods and the measurement of extruded debris after endodontic procedures [
12]. In order to overcome the limitations of previous methodologies, the apical pressure measurement technique was presented by Park
et al. [
13] and Khan
et al. [
14] who used standardized plastic models (30.06 and 35.06, respectively) to test the influence of the irrigant flow rate and the needle design on the apical pressure. One limitation of using artificial canals is that they remove the inherent variability of the root canal anatomy. Thus, it is unknown whether the results would be similar if the procedure is repeated under different anatomical circumstances. To date, only 1 study compared the apical pressure generated from different irrigation techniques and the GentleWave procedure in posterior teeth (4 maxillary molars) [
11]. This study aimed to evaluate the apical pressure generated by 2 types of endodontic irrigation needles and the GentleWave system in mandibular molars. The negative apical pressure of the GentleWave system was also verified as a control. The null hypothesis of this study was that the apical pressure generated in mesial and distal canals by open-end needles would not be significantly different from that of teeth irrigated with closed-end side-vented needles.
MATERIALS AND METHODS
Twelve mandibular first molars extracted for causes not related to this study were stored in 0.1% thymol (Institutional Review Board study 0004735). The sample size was determined using power calculation with the G*Power software (Heinrich-Heine-Universitat, Dusseldorf, Germany). A pilot study for each group was performed to calculate the mean and standard deviation (SD) of the apical pressure. The minimum sample size per group was determined to be between 5 and 7 with a power of 80% and a type I error rate of 5%.
Teeth were evaluated radiographically and visually for suitability and discarded if resorption, root cracks, open apices, previous root canal therapy, or existing crowns were present. Conventional endodontic access cavities were prepared in each of the molars and #10 FlexoFiles (Dentsply, Charlotte, NC, USA) were introduced into the canals. Patency was visually confirmed, and a working length was established at 0.5 mm from the apical foramen. The root canals were then shaped with Vortex Blue instruments (Dentsply) using a crown-down technique to a final preparation size 30/0.04 for mesial canals and size 40/0.04 for distal canals. A total of 10 mL of 6% sodium hypochlorite was used through the instrumentation process. All molars were cleaned with the GentleWave System (Sonendo Inc., Laguna Hills, CA, USA) to ensure complete removal of debris from the apical third of the root canals. The protocol involved the use of 400 mL of liquids, including 3% NaOCl, 8% EDTA, and distilled water.
Apical pressure measurement setup
The apical pressure generated by the irrigation procedure was measured using a data acquisition pressure setup as previously described by Park
et al. [
13]. Teeth were mounted into a special cap and placed into an air-tight custom fixture that was filled with sterile water. The fixture was coupled with a pressure transducer (PXM409-350, Omega, Stamford, CT, USA). The pressure generated at the root apex during irrigation was transferred through the incompressible fluid media to the pressure transducer [
11,
13]. The current (mA) from the pressure transducer was converted to voltage (V) through a resistor connected to the data acquisition board (USB6008, National Instruments, Austin, TX, USA). The voltage readings were converted into pressure readings (mmHg) using the LabVIEW software (National Instruments, considering the sensitivity of the calibrated pressure transducer used [
11,
13]. The setup was calibrated by connecting a tooth mount with a luer fitting and a calibrated analog pressure gauge to the air-tight custom fixture [
11,
13].
A syringe filled with sterile water was used to generate pressure within the air-tight custom fixture via the tooth mount [
11,
13]. The pressure read from the in-house setup using the LabVIEW program and the pressure read from the calibrated analog pressure gauge were compared, as previously reported [
11,
13]. Apical pressure readings in the mesial and distal canals were evaluated using only distilled water in order to avoid damaging the pressure transducer. Apical pressure measurements were performed for each individual root separately. After confirming apical patency, the apical pressure was acquired by placing the irrigation needle 2-3 mm from the working length at a constant flow rate of 10 mL/min using a digitally controlled peristaltic pump (Reglo Digital MS-2/8, Ismatec, Wertheim, Germany) over a 30-second data acquisition time frame in the mesiobuccal and the distal canal independently. The irrigants were expressed over a period of 30 seconds with constant movement of the irrigation needle coronally and apically. The readings were recorded 3 times for each root canal. The average apical pressure was calculated for each irrigation method tested. In total, each of the 12 specimens underwent 4 irrigation protocols: A) irrigation with distilled water with a 30-gauge close-end needle (ProRinse Probes, Dentsply) in the mesial canal, B) the same procedure repeated in the distal canal, C) irrigation with distilled water with a 30-gauge open-end needle (Navitip, Ultradent, South Jordan, UT, USA) in the mesial and D) distal canals. The GentleWave procedure and irrigation at 1 mm from the apex in the distal roots using an open-end needle were used as negative and positive controls, respectively. For the GentleWave procedure (Sonendo Inc.), data were acquired simultaneously from both roots, since this device can irrigate all canals present in a molar at the same time. Data acquisition for this group was performed over 60 seconds because apical pressure was generated from both roots at the same time when the procedure instrument was placed inside the pulp chamber. For the positive control, a 30-gauge open-ended needle was locked into the last apical 1 mm of the distal canals to induce the maximum positive pressure possible with syringe irrigation. New needles were used for each root canal in each group. During these 30 seconds, 6,000 data points were acquired (200 readings per second). A 1-second moving average was calculated for each of the 3 runs performed on the individual root canal, and the average of all 3 was calculated and recoded as the final apical pressure average for that root [
11,
13]. It is important to mention that the first few seconds of each run were excluded and only stable apical pressure (30 seconds) data were included in this analysis. The apical pressure values were plotted as a function of time to further understand the results. The apical pressure exerted by the different needles in the 2 different canal types was statistically compared at a
p < 0.05 significance level, using 2-way analysis of variance after verification of compatibility with a normal distribution (SPSS 22.0 Inc., Chicago, IL, USA).
RESULTS
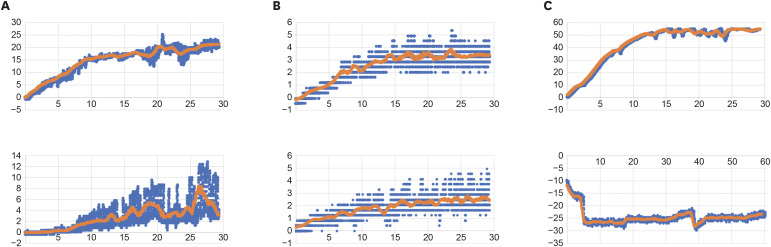
The mean positive apical pressure generated by the positive control was 63.29 mmHg, with values ranging from 18.47 to 170.2 mmHg. The GentleWave generated negative apical pressure (mean = −30.79 mmHg; with values ranging from −19.34 to −41.94 mmHg). The open-ended needles generated mean (SD) apical pressure of 3.62 mmHg (2.44) and 21.95 mmHg (9.83) for mesial and distal root canals, respectively. The close-ended needles generated significant lower apical pressures for both mesial and distal canals: 0.77 (0.71) and 5.25 (3.87) mmHg, respectively. Apical pressure differed significantly both according to the needle used (p = 1.6 × 10
−7) and the type of root canal (p = 4.7 × 10
−9). In fact, the type of root canal had a higher effect size for the apical pressure exerted (partial η
2 = 0.54). A variation of 54% in the apical pressure was produced by the type of root, while a variation of 30.7% was due to the needle × canal interaction. The lowest values, with statistical significance, were obtained with close-ended needles in mesial canals (
Figure 1).
Figure 1 Representative apical pressures (mmHg) generated by the different methods. (A) Open-end needle, distal canal (top) and mesial canal (bottom); (B) Close-end needle, distal canal (top) and mesial canal (bottom); (C) Positive control (top), GentleWave procedure (bottom). Needle irrigation was recorded for a period of 30 seconds, GentleWave apical pressure was recorded for 60 seconds. The X axis represents time, and the Y axis represents pressure in mmHg. Moving average (orange), raw data (blue).
DISCUSSION
Irrigant extrusion, especially the extrusion of sodium hypochlorite beyond the apical foramen, can be a serious complication during root canal therapy [
15,
16]. A sodium hypochlorite accident can result in severe pain, inflammation, swelling, infection, paresthesia, and ecchymosis [
15,
16]. Thus, the apical pressure generated during irrigation should be taken into account when using different needle designs or new machine-assisted irrigation systems [
11,
17]. Some
in vitro studies hypothesized the possibility of irrigant extrusion if the apical pressure exceeds 10-15 mmHg of the capillary pressure [
13]. It has also been reported that a high positive apical pressure (higher than 5.88 mmHg of central venous pressure) may cause air embolism in some cases [
14]. Many factors are considered to be relevant to the apical pressure, including the anatomy of the apical foramen, irrigation depth, irrigant flow rate, and needle type [
8,
12,
18,
19]. In particular, the effects of needle designs, the method of delivery, and the root canal taper have been investigated in previous studies.
Positive apical pressure is generated through conventional irrigation by open-ended or close-ended syringe needles. In the present study, open-ended syringe needles induced greater positive pressure than close-ended ones, which showed a good agreement with previous studies [
11,
13,
18]. According to a computational fluid dynamics study, a close-ended syringe needle presented lower velocity jets of irrigant and a different flow pattern compared to an open-ended one [
18,
20]. However, as the apical pressure increases, higher cleaning efficiency and better irrigant replenishment could be acquired using open-ended syringe needles than side-vented ones [
13,
18]. Thus, the balance between safety and effectiveness should be considered when choosing an irrigation instrument. The range of apical pressure generated by the syringe irrigation was between 0.5 and 50 mmHg, in accordance with the above studies using a similar irrigant flow rate.
Canal size or shape could also affect the apical pressure during irrigation. In our study, the apical pressure of the distal canal of a mandibular molar was significantly higher than the mesial canal, especially when an open-end needle was used. The distal canal is wider, more oval, and has a larger foramen, while the mesial canal is narrower. In a similar study, the apical pressure of canals with different sizes and shapes in 4 maxillary molars were compared [
11]. It was shown in round canals such as the distobuccal canal of maxillary molars, that a minimally instrumented canal (size 15/0.04) presented higher apical pressure than wider canals (size 40/0.04). In contrast, in the palatal canals of the same tooth, the pressures of canals with different instrumentation sizes were similar. A previous study showed that irrigation with a close-end needle demonstrated lower irrigation velocities toward the apex [
18]. Clinically, irrigant extrusion is not often contemplated to be a concern in minimally instrumented canals, which are even narrower than the syringe needle diameter (30-gauge). Thus, the present study instrumented the canals up to sizes 30/0.04 and 40/0.04, both of which are common canal sizes used during conventional root canal preparation. Thus, the apical pressure induced in these canals is clinically relevant. According to the results of the present study, clinicians should be cautious when irrigating wide canals such as the distal canals of mandibular molars, especially with open-end needles.
In comparison to irrigation with syringe needles, the EndoVac and GentleWave are able to produce negative apical pressure during the cleaning of the root canal system [
11,
14,
19]. In many studies of the EndoVac, the generated negative apical pressure was able to avoid irrigant extrusion beyond the apical foramen [
19,
21]. The GentleWave system appears to effectively debride root canal systems; however, there is limited evidence for the safety of this equipment in irrigant extrusion [
22]. To date, only 1 study on apical pressure and 1 study on apical extrusion of GentleWave have been reported [
11,
19]. In the first study, the apical pressure in maxillary molars ranged from −11 mmHg to −20 mmHg, which was slightly higher than the values in our study (−20 to −30 mmHg). This slight difference may be attributed to the tooth type or pressure measurement used. Charara
et al. [
19] measured the apical extrusion during irrigation of mandibular molars and found that no extrusion occurred using the GentleWave. Thus, it can be seen that the GentleWave system could produce negative apical pressure that prevents irrigant extrusion beyond the apical foramen.
CONCLUSIONS
Both the needle and the canal type influenced the apical pressure generated. Close-ended needles generated less apical pressure than open-ended needles. Irrigation induced less apical pressure in mesial than in distal root canals. The GentleWave procedure produced negative apical pressure.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Ordinola-Zapata R, Crepps JT.
Data curation: Ordinola-Zapata R.
Formal analysis: Arias A.
Investigation: Ordinola-Zapata R, Crepps JT.
Methodology: Ordinola-Zapata R.
Project administration: Ordinola-Zapata R.
Software: Ordinola-Zapata R.
Supervision: Ordinola-Zapata R.
Validation: Ordinola-Zapata R.
Writing - original draft: Ordinola-Zapata R, Crepps JT, Lin F.
Writing - review & editing: Ordinola-Zapata R, Arias A, Lin F.
REFERENCES
- 1. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J 1997;30:297-306.ArticlePubMed
- 2. Vera J, Siqueira JF Jr, Ricucci D, Loghin S, Fernández N, Flores B, Cruz AG. One- versus two-visit endodontic treatment of teeth with apical periodontitis: a histobacteriologic study. J Endod 2012;38:1040-1052.ArticlePubMed
- 3. Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod 2009;35:791-804.ArticlePubMed
- 4. Susin L, Liu Y, Yoon JC, Parente JM, Loushine RJ, Ricucci D, Bryan T, Weller RN, Pashley DH, Tay FR. Canal and isthmus debridement efficacies of two irrigant agitation techniques in a closed system. Int Endod J 2010;43:1077-1090.ArticlePubMedPMC
- 5. Burleson A, Nusstein J, Reader A, Beck M. The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. J Endod 2007;33:782-787.ArticlePubMed
- 6. Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am 2010;54:291-312.ArticlePubMed
- 7. Nair PN, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:231-252.ArticlePubMed
- 8. Boutsioukis C, Gogos C, Verhaagen B, Versluis M, Kastrinakis E, Van der Sluis LW. The effect of root canal taper on the irrigant flow: evaluation using an unsteady Computational Fluid Dynamics model. Int Endod J 2010;43:909-916.ArticlePubMed
- 9. Tay FR, Gu LS, Schoeffel GJ, Wimmer C, Susin L, Zhang K, Arun SN, Kim J, Looney SW, Pashley DH. Effect of vapor lock on root canal debridement by using a side-vented needle for positive-pressure irrigant delivery. J Endod 2010;36:745-750.ArticlePubMedPMC
- 10. Shon WJ. Introducing the GentleWave system. Restor Dent Endod 2016;41:235.ArticlePubMedPMCPDF
- 11. Haapasalo M, Shen Y, Wang Z, Park E, Curtis A, Patel P, Vandrangi P. Apical pressure created during irrigation with the GentleWave™ system compared to conventional syringe irrigation. Clin Oral Investig 2016;20:1525-1534.ArticlePubMedPDF
- 12. Boutsioukis C, Psimma Z, van der Sluis LW. Factors affecting irrigant extrusion during root canal irrigation: a systematic review. Int Endod J 2013;46:599-618.ArticlePubMed
- 13. Park E, Shen Y, Khakpour M, Haapasalo M. Apical pressure and extent of irrigant flow beyond the needle tip during positive-pressure irrigation in an in vitro root canal model. J Endod 2013;39:511-515.ArticlePubMed
- 14. Khan S, Niu LN, Eid AA, Looney SW, Didato A, Roberts S, Pashley DH, Tay FR. Periapical pressures developed by nonbinding irrigation needles at various irrigation delivery rates. J Endod 2013;39:529-533.ArticlePubMed
- 15. Hales JJ, Jackson CR, Everett AP, Moore SH. Treatment protocol for the management of a sodium hypochlorite accident during endodontic therapy. Gen Dent 2001;49:278-281.PubMed
- 16. Zhu WC, Gyamfi J, Niu LN, Schoeffel GJ, Liu SY, Santarcangelo F, Khan S, Tay KC, Pashley DH, Tay FR. Anatomy of sodium hypochlorite accidents involving facial ecchymosis - a review. J Dent 2013;41:935-948.ArticlePubMed
- 17. Desai P, Himel V. Comparative safety of various intracanal irrigation systems. J Endod 2009;35:545-549.ArticlePubMed
- 18. Boutsioukis C, Verhaagen B, Versluis M, Kastrinakis E, Wesselink PR, van der Sluis LW. Evaluation of irrigant flow in the root canal using different needle types by an unsteady computational fluid dynamics model. J Endod 2010;36:875-879.ArticlePubMed
- 19. Charara K, Friedman S, Sherman A, Kishen A, Malkhassian G, Khakpour M, Basrani B. Assessment of apical extrusion during root canal irrigation with the novel GentleWave system in a simulated apical environment. J Endod 2016;42:135-139.ArticlePubMed
- 20. Boutsioukis C, Lambrianidis T, Verhaagen B, Versluis M, Kastrinakis E, Wesselink PR, van der Sluis LW. The effect of needle-insertion depth on the irrigant flow in the root canal: evaluation using an unsteady computational fluid dynamics model. J Endod 2010;36:1664-1668.ArticlePubMed
- 21. Azim AA, Aksel H, Margaret Jefferson M, Huang GT. Comparison of sodium hypochlorite extrusion by five irrigation systems using an artificial root socket model and a quantitative chemical method. Clin Oral Investig 2018;22:1055-1061.ArticlePubMedPDF
- 22. Molina B, Glickman G, Vandrangi P, Khakpour M. Evaluation of root canal debridement of human molars using the GentleWave system. J Endod 2015;41:1701-1705.ArticlePubMed
 , Joseph T. Crepps1
, Joseph T. Crepps1 , Ana Arias2
, Ana Arias2 , Fei Lin1,3
, Fei Lin1,3



 KACD
KACD
 ePub Link
ePub Link Cite
Cite

