Articles
- Page Path
- HOME > Restor Dent Endod > Volume 44(1); 2019 > Article
-
Research Article
Inhibition of nicotine-induced
Streptococcus mutans biofilm formation by salts solutions intended for mouthrinses -
Abdulrahman A. Balhaddad1,2,4
 , Mary Anne S. Melo2,3
, Mary Anne S. Melo2,3 , Richard L. Gregory4
, Richard L. Gregory4
-
Restor Dent Endod 2019;44(1):e4.
DOI: https://doi.org/10.5395/rde.2019.44.e4
Published online: January 16, 2019
1Department of Restorative Dental Sciences, College of Dentistry, Imam Abdulrahman bin Faisal University, Dammam, Saudi Arabia.
2PhD Program in Biomedical Sciences, University of Maryland School of Dentistry, Baltimore, MD, USA.
3Operative Dentistry Division, Department of General Dentistry, University of Maryland School of Dentistry, Baltimore, MD, USA.
4Department of Biomedical and Applied Sciences, Indiana University School of Dentistry, Indianapolis, IN, USA.
- Correspondence to Abdulrahman A. Balhaddad, BDS, MSD. PhD student, PhD Program in Biomedical Sciences, University of Maryland School of Dentistry, 650 W Baltimore Street, Baltimore, MD, 21201, USA. aabalhaddad@umaryland.edu
Copyright © 2019. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,780 Views
- 13 Download
- 16 Crossref
Abstract
-
Objectives Biofilm formation is critical to dental caries initiation and development. The aim of this study was to investigate the effects of nicotine exposure on Streptococcus mutans (S. mutans) biofilm formation concomitantly with the inhibitory effects of sodium chloride (NaCl), potassium chloride (KCl) and potassium iodide (KI) salts. This study examined bacterial growth with varying concentrations of NaCl, KCl, and KI salts and nicotine levels consistent with primary levels of nicotine exposure.
-
Materials and Methods A preliminary screening experiment was performed to investigate the appropriate concentrations of NaCl, KCl, and KI to use with nicotine. With the data, a S. mutans biofilm growth assay was conducted using nicotine (0–32 mg/mL) in Tryptic Soy broth supplemented with 1% sucrose with and without 0.45 M of NaCl, 0.23 M of KCl, and 0.113 M of KI. The biofilm was stained with crystal violet dye and the absorbance measured to determine biofilm formation.
-
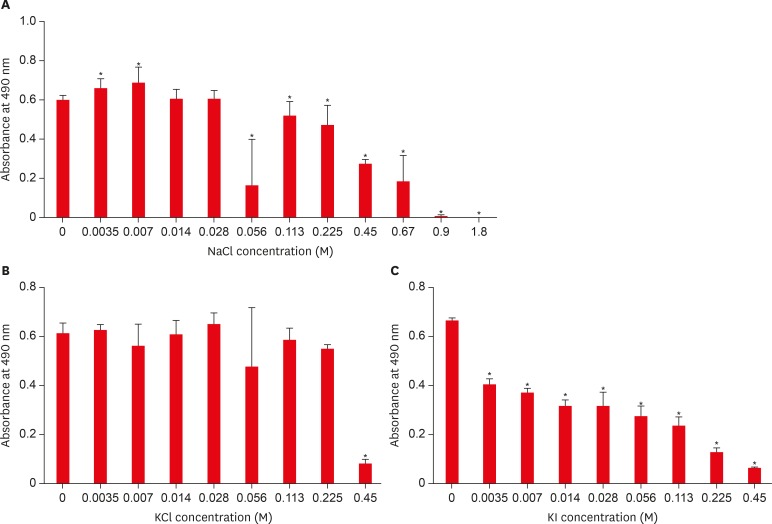
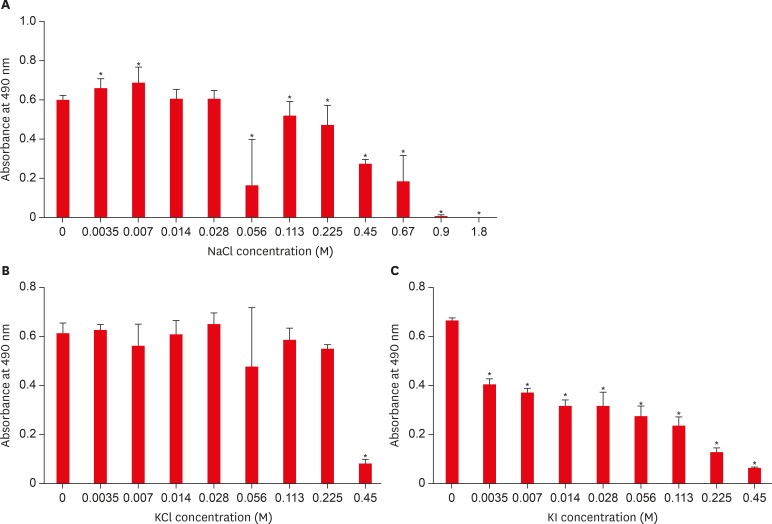
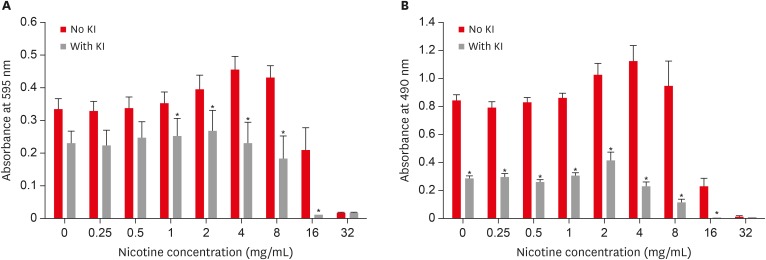
Results The presence of 0.45 M of NaCl, 0.23 M of KCl, and 0.113 M of KI significantly inhibited (p < 0.05) nicotine-induced S. mutans biofilm formation by 52%, 79.7%, and 64.1%, respectively.
-
Conclusions The results provide additional evidence regarding the biofilm-enhancing effects of nicotine and demonstrate the inhibitory influence of these salts in reducing the nicotine-induced biofilm formation. A short-term exposure to these salts may inhibit S. mutans biofilm formation.
INTRODUCTION
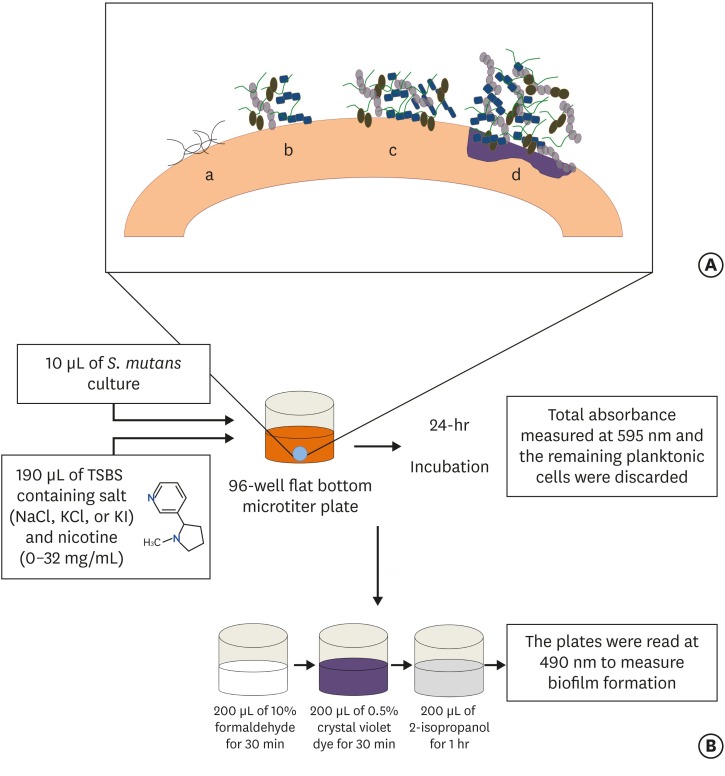
(A) Stages of biofilm formation: (a) Pellicle formation; (b) Binding of single organisms and multiplication; (c) Continued growth; (d) Biofilm maturation that triggers surface demineralization. (B) Schematic diagram of initiation of the biofilm using the 96-well microtiter plate crystal violet staining assay.
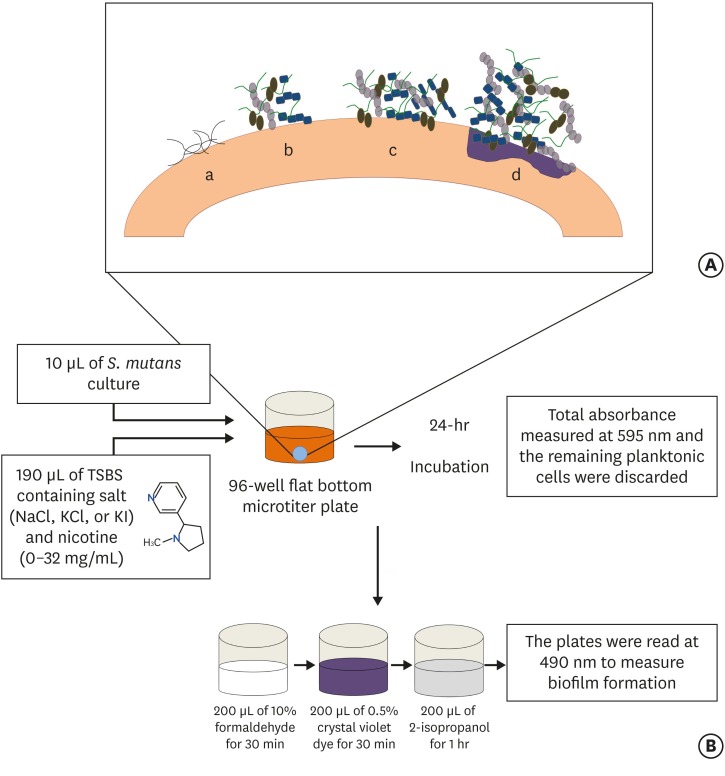
MATERIALS AND METHODS
RESULTS
Effect of sodium chloride (NaCl), potassium chloride (KCl), and potassium iodide (KI) on S. mutans biofilm formation. (A) Effect of NaCl on S. mutans biofilm formation; (B) Effect of KCl on S. mutans biofilm formation; (C) Effect of KI on S. mutans biofilm formation.
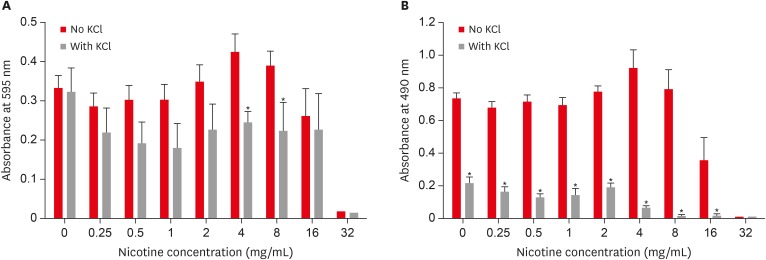
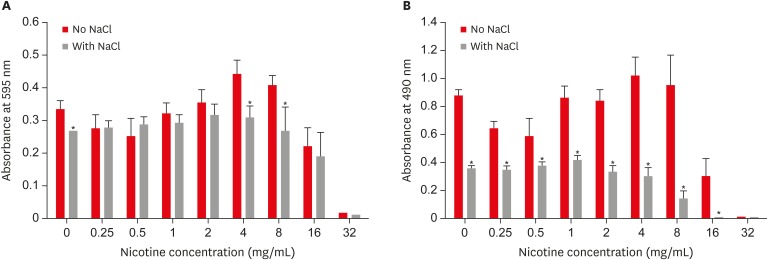
Combined effect of sodium chloride (NaCl) and nicotine on S. mutans total absorbance (A) and biofilm formation (B).

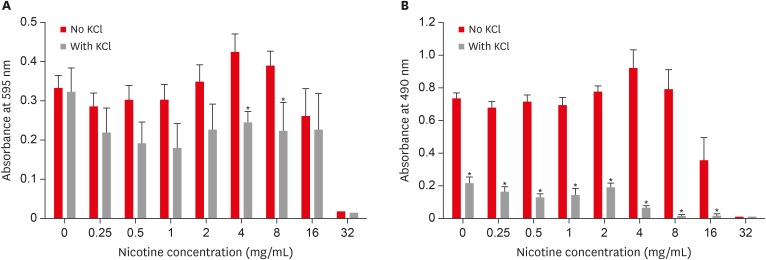
Combined effect of potassium chloride (KCl) and nicotine on S. mutans total absorbance (A) and biofilm formation (B).
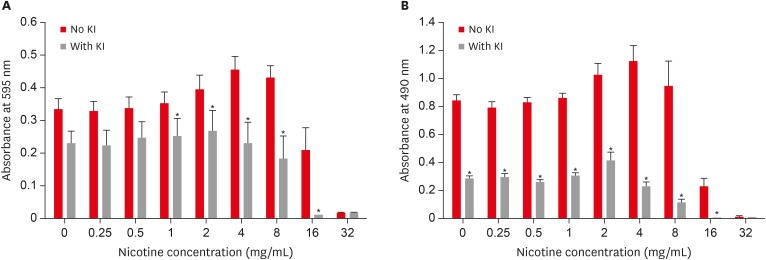
Combined effect of potassium iodide (KI) and nicotine on S. mutans total absorbance (A) and biofilm formation (B).
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Balhaddad AA, Gregory RL.
Data curation: Balhaddad AA, Gregory RL.
Formal analysis: Balhaddad AA, Gregory RL.
Funding acquisition: Gregory RL.
Investigation: Balhaddad AA, Melo MAS, Gregory RL.
Methodology: Gregory RL.
Project administration: Balhaddad AA, Gregory RL.
Resources: Balhaddad AA, Melo MAS, Gregory RL.
Software: Balhaddad AA, Melo MAS, Gregory RL.
Supervision: Melo MAS, Gregory RL.
Validation: Balhaddad AA, Melo MAS, Gregory RL.
Visualization: Balhaddad AA, Melo MAS, Gregory RL.
Writing - original draft: Balhaddad AA.
Writing - review & editing: Balhaddad AA, Melo MAS, Gregory RL.
- 1. Aguilar-Zinser V, Irigoyen ME, Rivera G, Maupomé G, Sánchez-Pérez L, Velázquez C. Cigarette smoking and dental caries among professional truck drivers in Mexico. Caries Res 2008;42:255-262.ArticlePubMedPDF
- 2. Avşar A, Darka O, Topaloğlu B, Bek Y. Association of passive smoking with caries and related salivary biomarkers in young children. Arch Oral Biol 2008;53:969-974.ArticlePubMed
- 3. Campus G, Cagetti MG, Senna A, Blasi G, Mascolo A, Demarchi P, Strohmenger L. Does smoking increase risk for caries? A cross-sectional study in an Italian military academy. Caries Res 2011;45:40-46.ArticlePubMedPDF
- 4. Jacob N, Golmard JL, Berlin I. Relationships between nicotine and cotinine concentrations in maternal milk and saliva. Acta Paediatr 2015;104:e360-e366.ArticlePubMed
- 5. Papaseit E, Farré M, Graziano S, Pacifici R, Pérez-Mañá C, García-Algar O, Pichini S. Monitoring nicotine intake from e-cigarettes: measurement of parent drug and metabolites in oral fluid and plasma. Clin Chem Lab Med 2017;55:415-423.ArticlePubMed
- 6. Shafagoj YA, Mohammed FI, Hadidi KA. Hubble-bubble (water pipe) smoking: levels of nicotine and cotinine in plasma, saliva and urine. Int J Clin Pharmacol Ther 2002;40:249-255.ArticlePubMed
- 7. Hoffmann D, Adams JD. Carcinogenic tobacco-specific N-nitrosamines in snuff and in the saliva of snuff dippers. Cancer Res 1981;41:4305-4308.PubMed
- 8. Feyerabend C, Higenbottam T, Russell MA. Nicotine concentrations in urine and saliva of smokers and non-smokers. Br Med J (Clin Res Ed) 1982;284:1002-1004.ArticlePubMedPMC
- 9. Dhar P. Measuring tobacco smoke exposure: quantifying nicotine/cotinine concentration in biological samples by colorimetry, chromatography and immunoassay methods. J Pharm Biomed Anal 2004;35:155-168.ArticlePubMed
- 10. Huang R, Li M, Gregory RL. Effect of nicotine on growth and metabolism of Streptococcus mutans . Eur J Oral Sci 2012;120:319-325.ArticlePubMed
- 11. Li M, Huang R, Zhou X, Zhang K, Zheng X, Gregory RL. Effect of nicotine on dual-species biofilms of Streptococcus mutans and Streptococcus sanguinis . FEMS Microbiol Lett 2014;350:125-132.ArticlePubMed
- 12. Huang R, Li M, Gregory RL. Nicotine promotes Streptococcus mutans extracellular polysaccharide synthesis, cell aggregation and overall lactate dehydrogenase activity. Arch Oral Biol 2015;60:1083-1090.ArticlePubMed
- 13. Li M, Huang R, Zhou X, Qiu W, Xu X, Gregory RL. Effect of nicotine on cariogenic virulence of Streptococcus mutans . Folia Microbiol (Praha) 2016;61:505-512.ArticlePubMedPDF
- 14. Balhaddad A, Gregory RL. In-vitro model of Scardovia wiggsiae biofilm formation and effect of nicotine. J Dent Res 2018;97(Special Issue A):Abstract #0593.Article
- 15. Tanaka K, Miyake Y, Nagata C, Furukawa S, Arakawa M. Association of prenatal exposure to maternal smoking and postnatal exposure to household smoking with dental caries in 3-year-old Japanese children. Environ Res 2015;143:148-153.ArticlePubMed
- 16. Wagenknecht DR, BalHaddad AR, Gregory RL. Effects of nicotine on oral microorganisms, human tissues, and the interactions between them. Curr Oral Health Rep 2018;5:78-87.ArticlePDF
- 17. Hirasawa M, Takada K. Susceptibility of Streptococcus mutans and Streptococcus sobrinus to cell wall inhibitors and development of a novel selective medium for S. sobrinus . Caries Res 2002;36:155-160.ArticlePubMedPDF
- 18. Twetman S, Linder L, Modéer T. Influence of bacterial cell concentration and inorganic anions on lysis of Streptococcus mutans BHT by salivary lysozyme. Scand J Dent Res 1984;92:533-538.ArticlePubMed
- 19. Pollock JJ, Goodman H, Elsey PK, Iacono VJ. Synergism of lysozyme, proteases and inorganic monovalent anions in the bacteriolysis of oral Streptococcus mutans GS5. Arch Oral Biol 1983;28:865-871.ArticlePubMed
- 20. Hamada S, Torii M, Kotani S, Masuda N, Ooshima T, Yokogawa K, Kawata S. Lysis of Streptococcus mutans cells with mutanolysin, a lytic enzyme prepared from a culture liquor of Streptomyces globisporus 1829. Arch Oral Biol 1978;23:543-549.ArticlePubMed
- 21. Ligtenberg AJ, Veerman EC, Nieuw Amerongen AV. A role for Lewis a antigens on salivary agglutinin in binding to Streptococcus mutans . Antonie van Leeuwenhoek 2000;77:21-30.ArticlePubMedPDF
- 22. Dashper SG, Reynolds EC. Effects of organic acid anions on growth, glycolysis, and intracellular pH of oral streptococci . J Dent Res 2000;79:90-96.ArticlePubMedPDF
- 23. Radcliffe CE, Akram NC, Hurrell F, Drucker DB. Effects of nitrite and nitrate on the growth and acidogenicity of Streptococcus mutans . J Dent 2002;30:325-331.ArticlePubMed
- 24. Skjörland K, Gjermo P, Rölla G. Effect of some polyvalent cations on plaque formation in vivo . Scand J Dent Res 1978;86:103-107.ArticlePubMed
- 25. Hamama HH, Yiu CK, Burrow MF. Effect of silver diamine fluoride and potassium iodide on residual bacteria in dentinal tubules. Aust Dent J 2015;60:80-87.ArticlePubMed
- 26. Knight GM, McIntyre JM, Craig GG, Mulyani , Zilm PS, Gully NJ. Differences between normal and demineralized dentine pretreated with silver fluoride and potassium iodide after an in vitro challenge by Streptococcus mutans . Aust Dent J 2007;52:16-21.ArticlePubMed
- 27. Marsh PD, Williamson MI, Keevil CW, McDermid AS, Ellwood DC. Influence of sodium and potassium ions on acid production by washed cells of Streptococcus mutans ingbritt and Streptococcus sanguis NCTC 7865 grown in a chemostat. Infect Immun 1982;36:476-483.ArticlePubMedPMCPDF
- 28. Goodman H, Pollock JJ, Katona LI, Iacono VJ, Cho MI, Thomas E. Lysis of Streptococcus mutans by hen egg white lysozyme and inorganic sodium salts. J Bacteriol 1981;146:764-774.ArticlePubMedPMCPDF
- 29. Greger JE, Eisenberg AD. Adenosine 5′-triphosphate content of Streptococcus mutans GS-5 during starvation in a buffered salt medium. Caries Res 1985;19:314-319.ArticlePubMed
- 30. Sanui T, Gregory RL. Analysis of Streptococcus mutans biofilm proteins recognized by salivary immunoglobulin A. Oral Microbiol Immunol 2009;24:361-368.ArticlePubMed
- 31. Schreiber S, Ronfani L, Ghirardo S, Minen F, Taddio A, Jaber M, Rizzello E, Barbi E. Nasal irrigation with saline solution significantly improves oxygen saturation in infants with bronchiolitis. Acta Paediatr 2016;105:292-296.ArticlePubMedPDF
- 32. Mahajan VK. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract 2014;2014:272376.ArticlePubMedPMCPDF
- 33. Kardalas E, Paschou SA, Anagnostis P, Muscogiuri G, Siasos G, Vryonidou A. Hypokalemia: a clinical update. Endocr Connect 2018;7:R135-R146.ArticlePubMedPMC
- 34. Demirhan H, Yıldız M, Çelebi ÖÖ, Baz Ş, İnal BB, Yiğit Ö. The role of fetuin-A and electrolytes in the etiology of sialolithiasis. Otolaryngol Head Neck Surg 2017;156:840-843.ArticlePubMedPDF
- 35. Gulaboglu M, Yildiz L, Gul M, Celebi F, Peker K. Blood and urine iodine levels in patients with gastric cancer. Biol Trace Elem Res 2006;113:261-271.ArticlePubMed
- 36. Myant NB. Iodine metabolism of salivary glands. Ann N Y Acad Sci 1960;85:208-214.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Biofilm forming and swarming activities of Bacillus cereus modulated by multiclass compounds
Abdul Rafay Rafiq, Mohsin Tariq, Syeda Tahseen Zahra, Temoor Ahmed
The Microbe.2026; 10: 100644. CrossRef - The Influence of Nicotine on Collagen Binding in Streptococcus mutans Serotype C Strains: An In Vitro Study
Naif N Abogazalah, Richard L Gregory, Mohammed M Al Moaleem
World Journal of Dentistry.2026; 16(11): 967. CrossRef - The Inhibition of Streptococcus mutans Biofilms following Exposure to Different Chocolate Ingredients
Hadi A. Almoabid, Leen Saleh Almutairi, Abdul Samad Khan, Mohammed A. Aljaffary, Rasha AlSheikh, Khalid S. Almulhim, Abdulrahman A. Balhaddad
European Journal of Dentistry.2025;[Epub] CrossRef - The impact of Caralluma munbyana extracts on Streptococcus mutans biofilm formation
Turki Alshehri, Israa Alkhalifah, Areeb Alotaibi, Alaa F. Alsulaiman, Abdullah Al Madani, Basil Almutairi, Abdulrahman A. Balhaddad
Frontiers in Dental Medicine.2025;[Epub] CrossRef - Tobacco‐enhanced biofilm formation by Porphyromonas gingivalis and other oral microbes
Jinlian Tan, Gwyneth J. Lamont, David A. Scott
Molecular Oral Microbiology.2024; 39(5): 270. CrossRef - Nicotine is a potent extracellular polysaccharide inducer in Fusobacterium nucleatum biofilms
Adaias Oliveira Matos, Valentim Adelino Ricardo Barão, Richard Lee Gregory
Brazilian Journal of Oral Sciences.2023;[Epub] CrossRef - Effect of eucalyptus oil on Streptococcus mutans and Enterococcus faecalis growth
Abdulrahman A. Balhaddad, Rasha N. AlSheikh
BDJ Open.2023;[Epub] CrossRef - Microorganisms: crucial players of smokeless tobacco for several health attributes
Akanksha Vishwakarma, Digvijay Verma
Applied Microbiology and Biotechnology.2021; 105(16-17): 6123. CrossRef - Microbiology of the American Smokeless Tobacco
A. J. Rivera, R. E. Tyx
Applied Microbiology and Biotechnology.2021; 105(12): 4843. CrossRef - The Impact of Photosensitizer Selection on Bactericidal Efficacy Of PDT against Cariogenic Biofilms: A Systematic Review and Meta-Analysis
Maurício Ítalo Silva Teófilo, Teresa Maria Amorim Zaranza de Carvalho Russi, Paulo Goberlanio de Barros Silva, Abdulrahman A. Balhaddad, Mary Anne S. Melo, Juliana P.M.L. Rolim
Photodiagnosis and Photodynamic Therapy.2021; 33: 102046. CrossRef - Antibacterial Activities of Methanol and Aqueous Extracts of Salvadora persica against Streptococcus mutans Biofilms: An In Vitro Study
Abdulrahman A. Balhaddad, Lamia Mokeem, Mary Anne S. Melo, Richard L. Gregory
Dentistry Journal.2021; 9(12): 143. CrossRef - The burden of root caries: Updated perspectives and advances on management strategies
Mohammed S. AlQranei, Abdulrahman A. Balhaddad, Mary A.S. Melo
Gerodontology.2021; 38(2): 136. CrossRef - Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials
Heba Mitwalli, Rashed Alsahafi, Abdulrahman A. Balhaddad, Michael D. Weir, Hockin H. K. Xu, Mary Anne S. Melo
Bioengineering.2020; 7(3): 83. CrossRef - In-Vitro Model of Scardovia wiggsiae Biofilm Formation and Effect of Nicotine
Abdulrahman A. Balhaddad, Hadeel M. Ayoub, Richard L. Gregory
Brazilian Dental Journal.2020; 31(5): 471. CrossRef - Antibacterial efficacy and remineralization capacity of glycyrrhizic acid added casein phosphopeptide‐amorphous calcium phosphate
Feride Sahin, Fatih Oznurhan
Microscopy Research and Technique.2020; 83(7): 744. CrossRef - Concentration dependence of quaternary ammonium monomer on the design of high-performance bioactive composite for root caries restorations
Abdulrahman A. Balhaddad, Maria S. Ibrahim, Michael D. Weir, Hockin H.K. Xu, Mary Anne S. Melo
Dental Materials.2020; 36(8): e266. CrossRef

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

