Articles
- Page Path
- HOME > Restor Dent Endod > Volume 44(2); 2019 > Article
- Case Report Oral manifestation and root canal therapy of the patient with mucopolysaccharidosis
-
Ji-Hye Yoon1
 , Hyo-Il Lee1
, Hyo-Il Lee1 , Ji-Hyun Jang2
, Ji-Hyun Jang2 , Sung-Hyeon Choi1
, Sung-Hyeon Choi1 , Hoon-Sang Chang1
, Hoon-Sang Chang1 , Yun-Chan Hwang1
, Yun-Chan Hwang1 , In-Nam Hwang1
, In-Nam Hwang1 , Bin-Na Lee1
, Bin-Na Lee1 , Won-Mann Oh1
, Won-Mann Oh1
-
Restor Dent Endod 2019;44(2):e14.
DOI: https://doi.org/10.5395/rde.2019.44.e14
Published online: April 4, 2019
1Department of Conservative Dentistry, School of Dentistry, Dental Science Research Institute, Chonnam National University, Gwangju, Korea.
2Department of Conservative Dentistry, School of Dentistry, Kyung Hee University, Seoul, Korea.
- Correspondence to Bin-Na Lee, DDS, MSD, PhD. Associate Professor, Department of Conservative Dentistry, School of Dentistry, Dental Science Research Institute, Chonnam National University, 77 Youngbong-ro, Buk-gu, Gwangju 61186, Korea. bnlee13@jnu.ac.kr
- Correspondence to Won-Mann Oh, DDS, MSD, PhD. Professor, Department of Conservative Dentistry, School of Dentistry, Dental Science Research Institute, Chonnam National University, 77 Youngbong-ro, Buk-gu, Gwangju 61186, Korea. wmoh@chonnam.ac.kr
Copyright © 2019. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,630 Views
- 7 Download
- 5 Crossref
Abstract
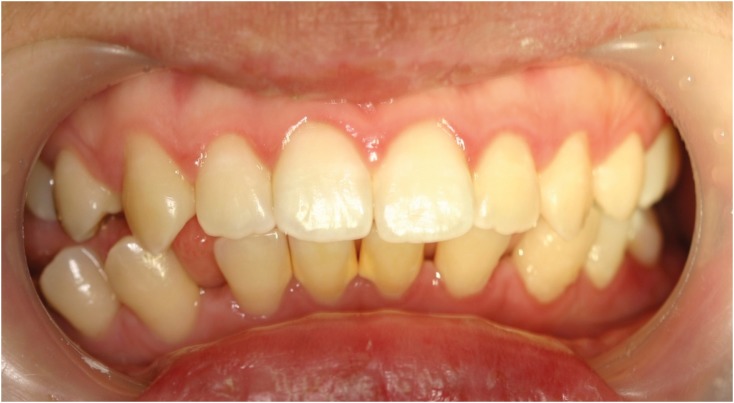
- Mucopolysaccharidosis (MPS) is an inherited metabolic disorder caused by a deficiency in enzymes that participate in the degradation of glycosaminoglycans (GAGs) such as heparin sulfate and dermatan sulfate. Left untreated, patients show progressive mental and physical deterioration due to deposition of GAGs in organs. Death often occurs due to cardiac or respiratory failure before patients reach their early twenties. MPS has several oral and dental manifestations. An enlarged head, short neck, and open mouth associated with a large tongue are major characteristics of MPS patients. Dental complications can be severe, including unerupted dentition, dentigerous cyst-like follicles, malocclusions, condylar defects, and gingival hyperplasia. A 21-year-old female patient with MPS was described in this article, with special emphasis on oral manifestations and dental treatment.
INTRODUCTION
CASE REPORT
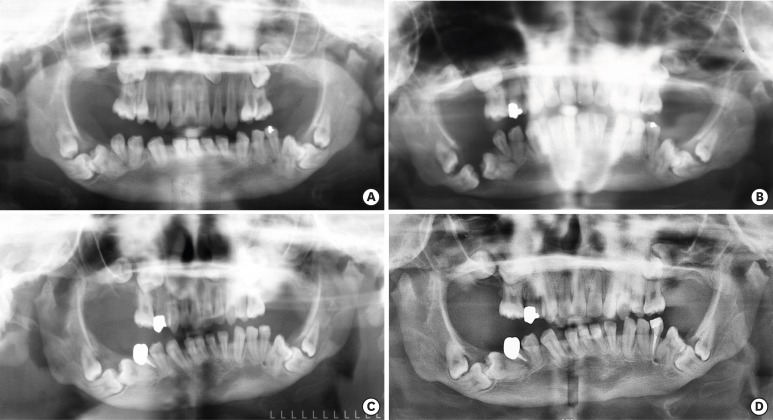
A preoperative panoramic view (A). A panoramic view of 2nd visit (B). A panoramic view after marsupialization of dentigerous cyst on teeth #46 and #47 (C). A panoramic view after 6 years of marsupialization on teeth #46 and #47 (D).
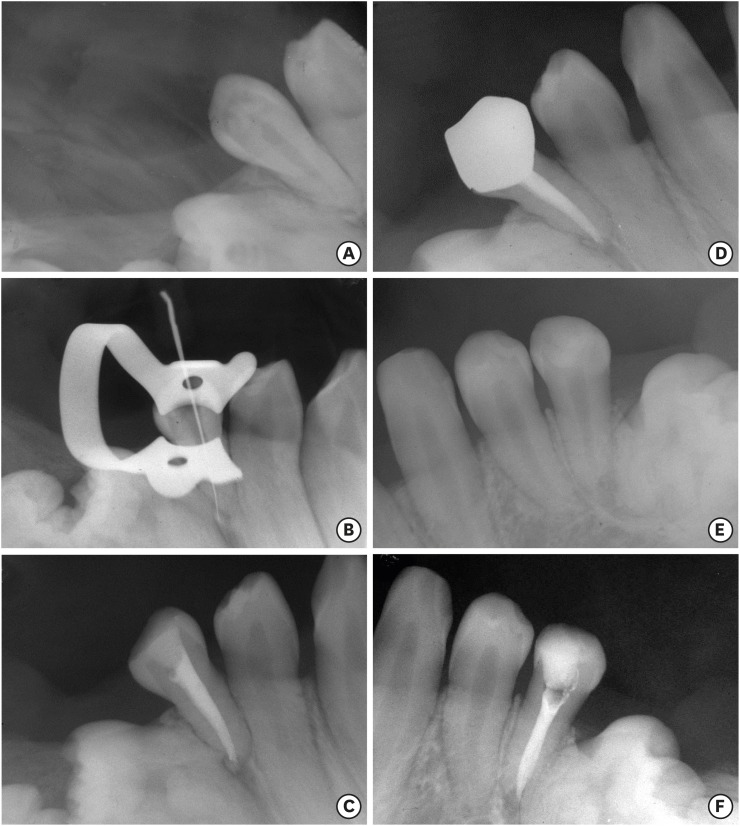
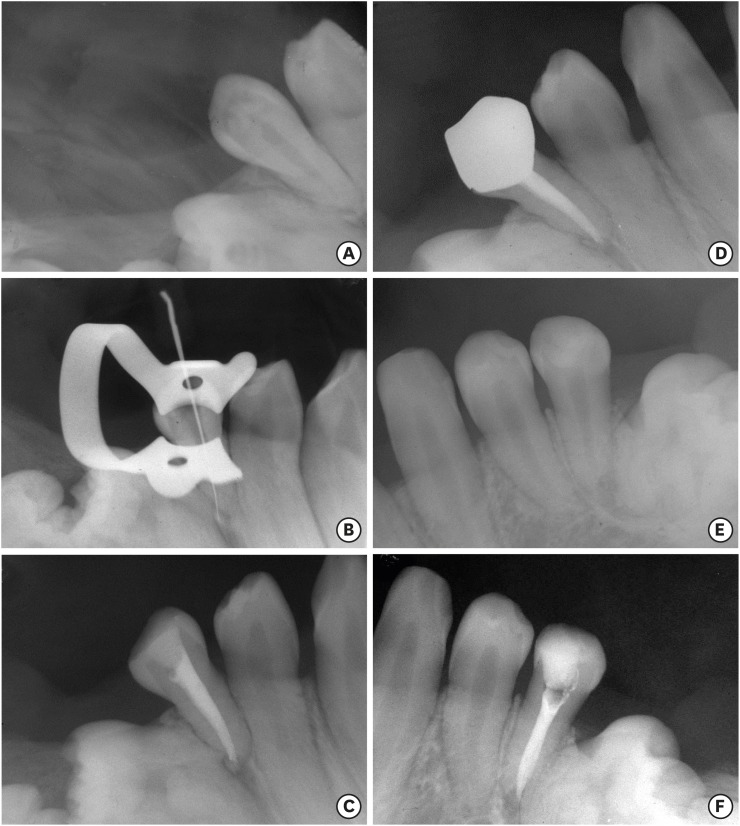
A preoperative radiograph of the tooth #45 (A). A radiograph of the tooth #45 with initial apical file (B). A radiograph of the tooth #45 after filling (C). One year and 5 months follow-up radiograph of the tooth #45 (D). A preoperative radiograph of the tooth #35 (E). A radiograph of the tooth #35 after filling (F).
DISCUSSION
CONCLUSIONS
-
Funding: This study was supported by a grant (CRI 18029-1) of Chonnam National University Hospital Biomedical Research Institute and a grant (2016R1C1B1012703) of the National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT and Future Planning (MSIP), Republic of Korea.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Oh WM.
Data curation: Yoon JH, Lee HI.
Formal analysis: Lee BN.
Funding acquisition: Lee BN.
Investigation: Choi SH.
Methodology: Chang HS.
Project administration: Hwang YC.
Resources: Hwang IN.
Software: Yoon JH, Lee BN.
Supervision: Oh WM, Lee BN.
Validation: Jang JH.
Visualization: Yoon JH, Lee HI.
Writing - original draft: Yoon JH, Lee HI.
Writing - review & editing: Lee BN, Oh WM.
- 1. Lehman TJ, Miller N, Norquist B, Underhill L, Keutzer J. Diagnosis of the mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl 5):v41-v48.ArticlePubMed
- 2. Baehner F, Schmiedeskamp C, Krummenauer F, Miebach E, Bajbouj M, Whybra C, Kohlschütter A, Kampmann C, Beck M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis 2005;28:1011-1017.ArticlePubMedPDF
- 3. Nelson J, Crowhurst J, Carey B, Greed L. Incidence of the mucopolysaccharidoses in Western Australia. Am J Med Genet A 2003;123A:310-313.ArticlePubMed
- 4. Vieira T, Schwartz I, Muñoz V, Pinto L, Steiner C, Ribeiro M, Boy R, Ferraz V, de Paula A, Kim C, Acosta A, Giugliani R. Mucopolysaccharidoses in Brazil: what happens from birth to biochemical diagnosis? Am J Med Genet A 2008;146A:1741-1747.ArticlePubMed
- 5. Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet 1997;101:355-358.ArticlePubMedPDF
- 6. Wraith JE. The first 5 years of clinical experience with laronidase enzyme replacement therapy for mucopolysaccharidosis I. Expert Opin Pharmacother 2005;6:489-506.ArticlePubMed
- 7. Keith O, Scully C, Weidmann GM. Orofacial features of Scheie (Hurler-Scheie) syndrome (α-L-iduronidase deficiency). Oral Surg Oral Med Oral Pathol 1990;70:70-74.ArticlePubMed
- 8. Kahler SG. Atlas of clinical syndromes: a visual aid to diagnosis for clinicians and practicing physicians. JAMA 1993;269:1050.Article
- 9. Simmons MA, Bruce IA, Penney S, Wraith E, Rothera MP. Otorhinolaryngological manifestations of the mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol 2005;69:589-595.ArticlePubMed
- 10. de Santana Sarmento DJ, de Carvalho SH, Melo SL, Fonseca FR, Diniz DN, Bento PM, Mesquita GQ, de Melo DP. Mucopolysaccharidosis: radiographic findings in a series of 16 cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:e240-e246.ArticlePubMed
- 11. Guven G, Cehreli ZC, Altun C, Sençimen M, Ide S, Bayari SH, Karaçay S. Mucopolysaccharidosis type I (Hurler syndrome): oral and radiographic findings and ultrastructural/chemical features of enamel and dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;105:72-78.ArticlePubMed
- 12. Hingston EJ, Hunter ML, Hunter B, Drage N. Hurler's syndrome: dental findings in a case treated with bone marrow transplantation in infancy. Int J Paediatr Dent 2006;16:207-212.ArticlePubMed
- 13. Kantaputra PN, Kayserili H, Güven Y, Kantaputra W, Balci MC, Tanpaiboon P, Uttarilli A, Dalal A. Oral manifestations of 17 patients affected with mucopolysaccharidosis type VI. J Inherit Metab Dis 2014;37:263-268.ArticlePubMed
- 14. Hobbs JR, Hugh-Jones K, Barrett AJ, Byrom N, Chambers D, Henry K, James DC, Lucas CF, Rogers TR, Benson PF, Tansley LR, Patrick AD, Mossman J, Young EP. Reversal of clinical features of Hurler's disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet 1981;2:709-712.ArticlePubMed
- 15. Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood S, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med 2004;350:1960-1969.ArticlePubMed
- 16. Fonseca FR, de Santana Sarmento DJ, Vasconcelos Medeiros PF, Diniz DN, dos Santos MT. Patients with mucopolysaccharidosis have tendencies towards vertical facial growth. J Oral Maxillofac Surg 2014;72:2539-2546.ArticlePubMed
- 17. Oussoren E, Brands MM, Ruijter GJ, der Ploeg AT, Reuser AJ. Bone, joint and tooth development in mucopolysaccharidoses: relevance to therapeutic options. Biochim Biophys Acta 2011;1812:1542-1556.ArticlePubMed
- 18. Alpöz AR, Çoker M, Çelen E, Ersin NK, Gökçen D, van Diggelenc OP, Huijmansc JG. The oral manifestations of Maroteaux-Lamy syndrome (mucopolysaccharidosis VI): a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:632-637.ArticlePubMed
- 19. Nakamura T, Miwa K, Kanda S, Nonaka K, Anan H, Higash S, Beppu K. Rosette formation of impacted molar teeth in mucopolysaccharidoses and related disorders. Dentomaxillofac Radiol 1992;21:45-49.ArticlePubMed
- 20. Pastores GM, Arn P, Beck M, Clarke JT, Guffon N, Kaplan P, Muenzer J, Norato DY, Shapiro E, Thomas J, Viskochil D, Wraith JE. The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with mucopolysaccharidosis type I. Mol Genet Metab 2007;91:37-47.ArticlePubMed
- 21. Pennock CA, Barnes IC. The mucopolysaccharidoses. J Med Genet 1976;13:169-181.ArticlePubMedPMC
- 22. Thomas S, Tandon S. Hurler syndrome: a case report. J Clin Pediatr Dent 2000;24:335-338.ArticlePubMedPDF
- 23. Glober GA, Tanaka KR, Turner JA, Liu CK. Mucopolysaccharidosis, an unusual cause of cardiac valvular disease. Am J Cardiol 1968;22:133-136.ArticlePubMed
- 24. Wadenya RO, Stout AM, Gupta A, Monge J. Hurler syndrome: a case report of a 5-year follow-up of dental findings after bone marrow transplantation. Spec Care Dentist 2010;30:14-17.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Pediatric Interventions in a Sanfilippo Syndrome Patient Under General Anesthesia: A Case Report
Ahmad Al Malak, Hassan Issawi, Mohammad Hassoun, Mohammad Al Halabi, Darko Macan
Case Reports in Dentistry.2025;[Epub] CrossRef - Behavioural disorders and sleep problems in Sanfilippo syndrome: overlaps with some other conditions and importance indications
Karolina Wiśniewska, Jakub Wolski, Paulina Anikiej-Wiczenbach, Magdalena Żabińska, Grzegorz Węgrzyn, Karolina Pierzynowska
European Child & Adolescent Psychiatry.2025; 34(6): 1795. CrossRef - Hurler syndrome: Oral and radiographic findings of a rare clinical case
W. Kabbassi, H. Hessissen, J. Hammouti
Medical Reports.2025; 14: 100325. CrossRef - Sanfilippo syndrome: consensus guidelines for clinical care
Nicole Muschol, Roberto Giugliani, Simon A. Jones, Joseph Muenzer, Nicholas J. C. Smith, Chester B. Whitley, Megan Donnell, Elise Drake, Kristina Elvidge, Lisa Melton, Cara O’Neill
Orphanet Journal of Rare Diseases.2022;[Epub] CrossRef - Manifestaciones bucales de pacientes con mucopolisacaridosis. Serie de casos
Andrea Verónica Ríos, Mariana Llorensi
Revista de la Asociación Odontológica Argentina.2021;[Epub] CrossRef

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

