Articles
- Page Path
- HOME > Restor Dent Endod > Volume 44(1); 2019 > Article
- Research Article Discoloration of teeth due to different intracanal medicaments
-
Farzaneh Afkhami1
 , Sadaf Elahy2
, Sadaf Elahy2 , Alireza Mahmoudi Nahavandi3
, Alireza Mahmoudi Nahavandi3 , Mohamad Javad Kharazifard4
, Mohamad Javad Kharazifard4 , Aidin Sooratgar1
, Aidin Sooratgar1
-
Restor Dent Endod 2019;44(1):e10.
DOI: https://doi.org/10.5395/rde.2019.44.e10
Published online: February 12, 2019
1Department of Endodontics, Faculty of Dentistry, Tehran University of Medical Sciences, International Campus, Tehran, Iran.
2General Dentist, Private Practice, Tehran, Iran.
3Color Imaging and Color Image Processing Department, Institute for Color Science and Technology (ICST), Tehran, Iran.
4Department of Epidemiology and Biostatistics, Faculty of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
- Correspondence to Aidin Sooratgar, DDS, MS. Assistant Professor, Department of Endodontics, Faculty of Dentistry, Tehran University of Medical Sciences, International Campus, Baradaran Mozaffar Street, Tehran 1856714984, Iran. a-sooratgar@sina.tums.ac.ir, aidin_s66@yahoo.com, f-afkhami@tums.ac.ir
Copyright © 2019. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,822 Views
- 50 Download
- 12 Crossref
Abstract
-
Objectives The objective of this study was to assess coronal discoloration induced by the following intracanal medicaments: calcium hydroxide (CH), a mixture of CH paste and chlorhexidine gel (CH/CHX), and triple antibiotic paste (3Mix).
-
Materials and Methods Seventy extracted single-canal teeth were selected. Access cavities were prepared and each canal was instrumented with a rotary ProTaper system. The specimens were randomly assigned to CH, CH/CHX, and 3Mix paste experimental groups (n = 20 each) or a control group (n = 10). Each experimental group was randomly divided into 2 subgroups (A and B). In subgroup A, medicaments were only applied to the root canals, while in subgroup B, the root canals were completely filled with medicaments and a cotton pellet dipped in medicament was also placed in the pulp chamber. Spectrophotometric readings were obtained from the mid-buccal surface of the tooth crowns immediately after placing the medicaments (T1) and at 1 week (T2), 1 month (T3), and 3 months (T4) after filling. The ∆E was then calculated. Data were analyzed using 2-way analysis of variance (ANOVA), 3-way ANOVA, and the Scheffé post hoc test.
-
Results The greatest color change (ΔE) was observed at 3 months (p < 0.0001) and in 3Mix subgroup B (p = 0.0057). No significant color change occurred in the CH (p = 0.7865) or CH/CHX (p = 0.1367) groups over time, but the 3Mix group showed a significant ΔE (p = 0.0164).
-
Conclusion Intracanal medicaments may induce tooth discoloration. Use of 3Mix must be short and it must be carefully applied only to the root canals; the access cavity should be thoroughly cleaned afterwards.
INTRODUCTION
MATERIALS AND METHODS
Medicaments in the experimental and control groups
RESULTS
Mean ΔE* values ± standard deviation of tooth samples from the different groups and at different time points
Mean ΔL* values ± standard deviation of tooth samples from the different groups and at different time points
Mean Δa* values ± standard deviation of tooth samples from the different groups and at different time points
Mean Δb* values ± standard deviation of tooth samples from the different groups at different time points
DISCUSSION
CONCLUSIONS
-
Funding: This research was supported by Tehran University of Medical Sciences, International Campus (grant No. 92-02-168-22998). The authors report no conflicts of interest.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Afkhami F.
Data curation: Kharazifard MJ.
Formal analysis: Kharazifard MJ.
Funding acquisition: Elahy S.
Investigation: Elahy S.
Methodology: Afkhami F, Mahmoudi Nahavandi A.
Project administration: Afkhami F, Elahy S.
Resources: Elahy S.
Software: Sooratgar A.
Supervision: Mahmoudi Nahavandi A.
Validation: Afkhami F.
Visualization: Elahy S.
Writing - original draft: Afkhmi F.
Writing - review & editing: Sooratgar A.
- 1. DaSilva L, Finer Y, Friedman S, Basrani B, Kishen A. Biofilm formation within the interface of bovine root dentin treated with conjugated chitosan and sealer containing chitosan nanoparticles. J Endod 2013;39:249-253.ArticlePubMed
- 2. Shrestha A, Shi Z, Neoh KG, Kishen A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J Endod 2010;36:1030-1035.ArticlePubMed
- 3. Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod 2008;34:1515-1520.ArticlePubMed
- 4. Safavi K, Nakayama TA. Influence of mixing vehicle on dissociation of calcium hydroxide in solution. J Endod 2000;26:649-651.ArticlePubMed
- 5. Ballal V, Kundabala M, Acharya S, Ballal M. Antimicrobial action of calcium hydroxide, chlorhexidine and their combination on endodontic pathogens. Aust Dent J 2007;52:118-121.ArticlePubMed
- 6. Valera MC, Silva KC, Maekawa LE, Carvalho CA, Koga-Ito CY, Camargo CH, Lima RS. Antimicrobial activity of sodium hypochlorite associated with intracanal medication for Candida albicans and Enterococcus faecalis inoculated in root canals. J Appl Oral Sci 2009;17:555-559.ArticlePubMedPMC
- 7. Ahmed HM, Abbott PV. Discolouration potential of endodontic procedures and materials: a review. Int Endod J 2012;45:883-897.ArticlePubMed
- 8. Thomson AD, Athanassiadis B, Kahler B, Walsh L. Tooth discolouration: staining effects of various sealers and medicaments. Aust Endod J 2012;38:2-9.ArticlePubMed
- 9. Kim ST, Abbott PV, McGinley P. The effects of Ledermix paste on discolouration of immature teeth. Int Endod J 2000;33:233-237.ArticlePubMed
- 10. Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G. Tooth discoloration induced by endodontic materials: a laboratory study. Int Endod J 2012;45:942-949.ArticlePubMed
- 11. Ordinola-Zapata R, Bramante CM, Minotti PG, Cavenago BC, Garcia RB, Bernardineli N, Jaramillo DE, Hungaro Duarte MA. Antimicrobial activity of triantibiotic paste, 2% chlorhexidine gel, and calcium hydroxide on an intraoral-infected dentin biofilm model. J Endod 2013;39:115-118.ArticlePubMed
- 12. Rahimi S, Janani M, Lotfi M, Shahi S, Aghbali A, Vahid Pakdel M, Salem Milani A, Ghasemi N. A review of antibacterial agents in endodontic treatment. Iran Endod J 2014;9:161-168.PubMedPMC
- 13. Almyroudi A, Mackenzie D, McHugh S, Saunders WP. The effectiveness of various disinfectants used as endodontic intracanal medications: an in vitro study. J Endod 2002;28:163-167.ArticlePubMed
- 14. Javidi M, Afkhami F, Zarei M, Ghazvini K, Rajabi O. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis . Aust Endod J 2014;40:61-65.PubMed
- 15. Wu D, Fan W, Kishen A, Gutmann JL, Fan B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J Endod 2014;40:285-290.PubMed
- 16. Hennessey TS. Some antibacterial properties of chlorhexidine. J Periodontal Res Suppl 1973;12:61-67.ArticlePubMed
- 17. Dametto FR, Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, de Souza-Filho FJ. In vitro assessment of the immediate and prolonged antimicrobial action of chlorhexidine gel as an endodontic irrigant against Enterococcus faecalis . Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:768-772.ArticlePubMed
- 18. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 2004;30:196-200.ArticlePubMed
- 19. Akcay M, Arslan H, Yasa B, Kavrık F, Yasa E. Spectrophotometric analysis of crown discoloration induced by various antibiotic pastes used in revascularization. J Endod 2014;40:845-848.ArticlePubMed
- 20. Agahian F, Amirshahi SA, Amirshahi SH. Reconstruction of reflectance spectra using weighted principal component analysis. Color Res Appl 2008;33:360-371.Article
- 21. Lee YK, Lim BS, Powers JM. Color changes of dental resin composites by a salivary enzyme. J Biomed Mater Res B Appl Biomater 2004;70:66-72.ArticlePubMed
- 22. Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J 1991;24:119-125.ArticlePubMed
- 23. Garcia-Godoy F, Murray PE. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent Traumatol 2012;28:33-41.ArticlePubMed
- 24. Feiz A, Barekatain B, Khalesi S, Khalighinejad N, Badrian H, Swift EJ. Effect of several bleaching agents on teeth stained with a resin-based sealer. Int Endod J 2014;47:3-9.ArticlePubMed
- 25. Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod 2008;34:876-887.ArticlePubMed
- 26. Gomes-Filho JE, Duarte PC, de Oliveira CB, Watanabe S, Lodi CS, Cintra LT, Bernabé PF. Tissue reaction to a triantibiotic paste used for endodontic tissue self-regeneration of nonvital immature permanent teeth. J Endod 2012;38:91-94.ArticlePubMed
- 27. Kim JH, Kim Y, Shin SJ, Park JW, Jung IY. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. J Endod 2010;36:1086-1091.ArticlePubMed
- 28. Day PF, Duggal MS, High AS, Robertson A, Gregg TA, Ashley PF, Welbury RR, Cole BO, Westland S. Discoloration of teeth after avulsion and replantation: results from a multicenter randomized controlled trial. J Endod 2011;37:1052-1057.ArticlePubMed
- 29. Afkhami F, Elahy S, Mahmoudi-Nahavandi A. Spectrophotometric analysis of crown discoloration following the use of silver nanoparticles combined with calcium hydroxide as intracanal medicament. J Clin Exp Dent 2017;9:e842-e847.ArticlePubMedPMC
- 30. Kabaktchieva R, Gateva N, Gusiyska A, Stanimirov P, Milcheva N. Dental care for children after replantation of avulsed permanent incisors. J IMAB 2016;22:1392-1402.Article
- 31. van Maanen-Schakel NW, Slot DE, Bakker EW, Van der Weijden GA. The effect of an oxygenating agent on chlorhexidine-induced extrinsic tooth staining: a systematic review. Int J Dent Hyg 2012;10:198-208.ArticlePubMedPDF
- 32. Gomes BP, Souza SF, Ferraz CC, Teixeira FB, Zaia AA, Valdrighi L, Souza-Filho FJ. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J 2003;36:267-275.ArticlePubMedPDF
- 33. Wang CS, Arnold RR, Trope M, Teixeira FB. Clinical efficiency of 2% chlorhexidine gel in reducing intracanal bacteria. J Endod 2007;33:1283-1289.ArticlePubMed
- 34. Windley W 3rd, Teixeira F, Levin L, Sigurdsson A, Trope M. Disinfection of immature teeth with a triple antibiotic paste. J Endod 2005;31:439-443.ArticlePubMed
- 35. Jang JH, Kang M, Ahn S, Kim S, Kim W, Kim Y, Kim E. Tooth discoloration after the use of new pozzolan cement (Endocem) and mineral trioxide aggregate and the effects of internal bleaching. J Endod 2013;39:1598-1602.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Effects of Intra-canal Medicaments on Infrared Light Energy Transmission Through Enamel and Dentin During Photobiomodulation: An In Vitro Study
Sachin Kulkarni, Laurence J. Walsh, Yash Bhurani, Roy George
Journal of Endodontics.2025; 51(5): 616. CrossRef - Tooth discoloration caused by nanographene oxide as an irrigant and intracanal medicament in the endodontic treatment of extracted single-rooted teeth: An ex-vivo study
Abbas Abbaszadegan, Zeinab Rafiee, Bahar Asheghi, Ahmad Gholami, Mohmed Isaqali Karobari
PLOS One.2025; 20(6): e0325430. CrossRef - Investigation of Discoloration of Anterior Teeth With Three Types of Substances Used in Endodontic Treatment
Sahar Soltani, Eshagh Ali Saberi, Nazanin Shahradnia, Pedram Abdollahzade Sangrodi, Elham Majidi
Clinical and Experimental Dental Research.2025;[Epub] CrossRef - A New Disinfection Approach Using a Chitosan-Based Endodontic Irrigant
Alejandra Itzel Lopez-Flores, Ulises Velazquez-Enriquez, Rogelio Jose Scougall-Vilchis, Laura Susana Acosta-Torres, Laura Emma Rodriguez-Vilchis, Rosalía Contreras-Bulnes, Paloma Netzayeli Serrano-Diaz, Rene Garcia-Contreras
Materials.2025; 18(24): 5552. CrossRef - Time-dependent Tooth Color Changes Following Conventional, Silver-based, and Photodynamic Root Canal Irrigants: An In Vitro Study
Laila Mohamed Mohamed Kenawi, Mohamed Fattouh, Khaled Abid Althaqafi, Abla Arafa
The Open Dentistry Journal.2025;[Epub] CrossRef - Spectrophotometric Analysis of Intracoronal Bleaching on Crown Discoloration Induced by Various Antibiotic Pastes: An In Vitro Study
Avneet Kaur, Harshit Srivastava, Deepak Raisingani, Ashwini B Prasad, Dileep Soni, Poorva R Sharma
International Journal of Clinical Pediatric Dentistry.2025; 18(12): 1443. CrossRef - Effect of Calcium Hydroxide Versus Double Antibiotic Paste on Endodontic Treatment Outcomes in Teeth With Large Periapical Lesions: A Triple‐Blind Randomized Clinical Trial
Afsaneh Rahmati, Farshad Seyedein, Omid Dianat, Sara Saedi, Golriz Rostami, Alireza Akbarzadeh Baghban, Shima Sabertahan, Majid Kazem, Kee Y. Kum
International Journal of Dentistry.2024;[Epub] CrossRef - The effect of different intracanal irrigants on the push-out bond strength of dentin in damaged anterior primary teeth
Leila Bassir, Shirin Taravati, Farzad Nouri, Saeide Rahimi
Journal of Medicine and Life.2024; 17(5): 536. CrossRef - In Vıtro Evaluatıon of Dıscoloratıon Caused by Root Canal Sealers and Color Changes after Bleachıng
Emre Bodrumlu, Esma Dinger
Annals of Dental Specialty.2024; 12(1): 77. CrossRef - Assessment of Discoloration Induced by Root Canal Sealers and Color Alterations Post-Bleaching
T.P. Van der Burgt, T.P. Mullaney, A.J.M. Plasschaert
International Journal of Dental Research and Allied Sciences.2024; 4(1): 1. CrossRef - The effect of four different intracanal medicaments on the push-out bond strength of root canal sealers
Shalu Maan, Vijaya Dhar Bhatt, Rohit Singh, Sayak Gupta, Syed Alay Noorain, Aashna Gill, Pradeep Kumar, Sushil Yadav, Preeti Sharma
Journal of Medicine and Life.2022; 15(4): 448. CrossRef - Effect of hydrogel-based antibiotic intracanal medicaments on crown discoloration
Rayan B. Yaghmoor, Jeffrey A. Platt, Kenneth J. Spolnik, Tien Min Gabriel Chu, Ghaeth H. Yassen
Restorative Dentistry & Endodontics.2021;[Epub] CrossRef

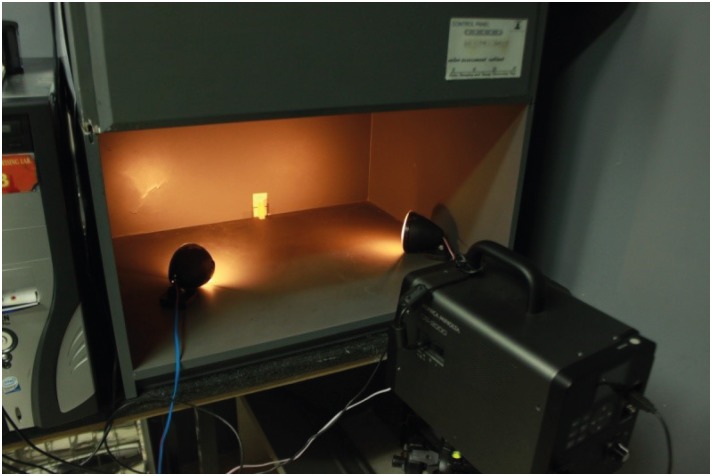
Figure 1
Figure 2
Medicaments in the experimental and control groups
| Group | Material | Composition | Manufacturer |
|---|---|---|---|
| Control (n = 10) | Normal saline | 0.9% sodium chloride | Shahid Ghazi Pharmaceutical Co., Tabriz, Iran |
| CH (n = 20) | Calcium hydroxide | Calcium hydroxide–iodoform | Metapex; Meta Biomed Co., Cheongju, Korea |
| CH/CHX (n = 20) | Calcium hydroxide paste and chlorhexidine gel | Calcium hydroxide was mixed with 2% chlorhexidine gel at a proportion of 1:1 | Metapex; Meta Biomed Co., Cheongju, Korea |
| Gluco-Chex 2% gel; PPH Cerkamed, StalowaWola, Poland | |||
| 3Mix (n = 20) | Ciprofloxacin | Equal portions of metronidazole, ciprofloxacin, and minocycline mixed with distilled water (at a powder/liquid ratio of 3:1) | Bayer, Leverkusen, Germany |
| Metronidazole | Braun, Melsungen, Germany | ||
| Minocycline | Ratiopharm, Ulm, Germany |
Mean ΔE* values ± standard deviation of tooth samples from the different groups and at different time points
| Group (subgroup) | 1 week | 1 month | 3 months |
|---|---|---|---|
| CH (B) | 1.8 ± 0.33aA | 1.6 ± 0.30aA | 2.1 ± 0.34aA |
| CH (A) | 1.9 ± 0.36aA | 2.4 ± 0.54aA | 2.3 ± 0.89aA |
| CH/CHX (B) | 1.8 ± 0.33aA | 2.6 ± 0.68aA | 3.2 ± 0.63aA |
| CH/CHX (A) | 1.4 ± 0.22aA | 1.9 ± 0.43aA | 1.8 ± 0.33aA |
| 3Mix (B) | 5.0 ± 1.40bA | 6.0 ± 1.40bA | 12.0 ± 2.90bA |
| 3Mix (A) | 3.1 ± 0.97aA | 2.5 ± 0.83aA | 5.3 ± 1.50aA |
| Control | 3.9 ± 0.65aA | 5.0 ± 0.72aA | 3.4 ± 0.63aA |
The same uppercase letters in a row and the lowercase letters in a column indicate statistically similar groups (α = 0.05).
CH, alcium hydroxide; CHX, hlorhexidine; 3Mix, triple antibiotic paste (metronidazole, ciprofloxacin, and minocycline); A, subgroup in which medicaments were filled only into the canal (below the cementoenamel junction); B, subgroup in which the canals were completely filled with the materials, and a cotton pellet was dipped in the medicaments and placed in the pulp chamber.
Mean ΔL* values ± standard deviation of tooth samples from the different groups and at different time points
| Group (subgroup) | 1 week | 1 month | 3 months |
|---|---|---|---|
| CH (B) | −0.29 ± 0.50aA | 0.79 ± 0.25aA | −1.50 ± 0.35aA |
| CH (A) | 0.23 ± 0.36aA | 0.98 ± 0.42aA | 0.09 ± 0.52aA |
| CH/CHX (B) | −0.024 ± 0.27aA | 1.20 ± 0.58aA | −1.70 ± 0.65aB |
| CH/CHX (A) | −0.05 ± 0.36aA | 0.78 ± 0.51aA | −0.83 ± 0.50aA |
| 3Mix (B) | −0.81 ± 1.70aA | −3.40 ± 1.70bA | −8.90 ± 2.60bB |
| 3Mix (A) | −0.42 ± 1.20aA | −0.24 ± 1.00aA | −3.50 ± 1.60aA |
| Control | 3.30 ± 0.64aA | 4.50 ± 0.61aA | 1.50 ± 0.60aA |
The same uppercase letters in a row and the same lowercase letters in a column indicate statistically similar groups (α = 0.05).
CH, alcium hydroxide; CHX, hlorhexidine; 3Mix, triple antibiotic paste (metronidazole, ciprofloxacin, and minocycline); A, subgroup in which medicaments were filled only into the canal (below the cementoenamel junction); B, subgroup in which the canals were completely filled with the materials, and a cotton pellet was dipped in the medicaments and placed in the pulp chamber.
Mean Δa* values ± standard deviation of tooth samples from the different groups and at different time points
| Group (subgroup) | 1 week | 1 month | 3 months |
|---|---|---|---|
| CH (B) | −0.21 ± 0.16aA | 0.35 ± 0.09aB | 0.20 ± 0.12aA |
| CH (A) | −0.55 ± 0.19aA | 0.15 ± 0.19aA | −0.05 ± 0.32aA |
| CH/CHX (B) | −0.46 ± 0.08aA | 0.24 ± 0.16aB | 0.12 ± 0.21aA |
| CH/CHX (A) | −0.47 ± 0.09aA | 0.56 ± 0.35aB | 0.35 ± 0.09aB |
| 3Mix (B) | −1.10 ± 0.09bA | −0.031 ± 0.37aA | −0.32 ± 0.45aA |
| 3Mix (A) | −0.52 ± 0.22aA | −0.14 ± 0.26aA | −0.26 ± 0.42aA |
| Control | −0.20 ± 0.09aA | 0.43 ± 0.23aB | 0.71 ± 0.23aB |
The same uppercase letters in a row and the same lowercase letters in a column indicate statistically similar groups (α = 0.05).
CH, alcium hydroxide; CHX, hlorhexidine; 3Mix, triple antibiotic paste (metronidazole, ciprofloxacin, and minocycline); A, subgroup in which medicaments were filled only into the canal (below the cementoenamel junction); B, subgroup in which the canals were completely filled with the materials, and a cotton pellet was dipped in the medicaments and placed in the pulp chamber.
Mean Δb* values ± standard deviation of tooth samples from the different groups at different time points
| Group (subgroup) | 1 week | 1 month | 3 months |
|---|---|---|---|
| CH (B) | −0.21 ± 0.16aA | 0.35 ± 0.09aA | 0.20 ± 0.12aA |
| CH (A) | −0.55 ± 0.19aA | 0.15 ± 0.19aA | −0.05 ± 0.32aA |
| CH/CHX (B) | −0.46 ± 0.08aA | 0.24 ± 0.16aA | 0.12 ± 0.21aA |
| CH/CHX (A) | −0.47 ± 0.09aA | 0.56 ± 0.35aA | 0.35 ± 0.09aA |
| 3Mix (B) | −1.10 ± 0.09aA | −0.03 ± 0.37aA | −0.32 ± 0.45bA |
| 3Mix (A) | −0.52 ± 0.22aA | −0.14 ± 0.26aA | −0.26 ± 0.42aA |
| Control | −0.20 ± 0.09aA | 0.43 ± 0.23aA | 0.71 ± 0.23aA |
The same uppercase letters in a row and the same lowercase letters in a column indicate statistically similar groups (α = 0.05).
CH, alcium hydroxide; CHX, hlorhexidine; 3Mix, triple antibiotic paste (metronidazole, ciprofloxacin, and minocycline); A, subgroup in which medicaments were filled only into the canal (below the cementoenamel junction); B, subgroup in which the canals were completely filled with the materials, and a cotton pellet was dipped in the medicaments and placed in the pulp chamber.
The same uppercase letters in a row and the lowercase letters in a column indicate statistically similar groups (α = 0.05).
CH, alcium hydroxide; CHX, hlorhexidine; 3Mix, triple antibiotic paste (metronidazole, ciprofloxacin, and minocycline); A, subgroup in which medicaments were filled only into the canal (below the cementoenamel junction); B, subgroup in which the canals were completely filled with the materials, and a cotton pellet was dipped in the medicaments and placed in the pulp chamber.
The same uppercase letters in a row and the same lowercase letters in a column indicate statistically similar groups (α = 0.05).
CH, alcium hydroxide; CHX, hlorhexidine; 3Mix, triple antibiotic paste (metronidazole, ciprofloxacin, and minocycline); A, subgroup in which medicaments were filled only into the canal (below the cementoenamel junction); B, subgroup in which the canals were completely filled with the materials, and a cotton pellet was dipped in the medicaments and placed in the pulp chamber.
The same uppercase letters in a row and the same lowercase letters in a column indicate statistically similar groups (α = 0.05).
CH, alcium hydroxide; CHX, hlorhexidine; 3Mix, triple antibiotic paste (metronidazole, ciprofloxacin, and minocycline); A, subgroup in which medicaments were filled only into the canal (below the cementoenamel junction); B, subgroup in which the canals were completely filled with the materials, and a cotton pellet was dipped in the medicaments and placed in the pulp chamber.
The same uppercase letters in a row and the same lowercase letters in a column indicate statistically similar groups (α = 0.05).
CH, alcium hydroxide; CHX, hlorhexidine; 3Mix, triple antibiotic paste (metronidazole, ciprofloxacin, and minocycline); A, subgroup in which medicaments were filled only into the canal (below the cementoenamel junction); B, subgroup in which the canals were completely filled with the materials, and a cotton pellet was dipped in the medicaments and placed in the pulp chamber.

 KACD
KACD

 ePub Link
ePub Link Cite
Cite

