Articles
- Page Path
- HOME > Restor Dent Endod > Volume 43(4); 2018 > Article
- Research Article Improved dentin disinfection by combining different-geometry rotary nickel-titanium files in preparing root canals
-
Marwa M. Bedier1
 , Ahmed Abdel Rahman Hashem2
, Ahmed Abdel Rahman Hashem2 , Yosra M. Hassan3
, Yosra M. Hassan3
-
Restor Dent Endod 2018;43(4):e46.
DOI: https://doi.org/10.5395/rde.2018.43.e46
Published online: November 1, 2018
1Department of Endodontics, Faculty of Dentistry, Cairo University, Cairo, Egypt.
2Department of Endodontics, Faculty of Dentistry, Ain Shams University, Cairo, Egypt.
3Department of Clinical Pathology, Cairo University, Cairo, Egypt.
- Correspondence to Marwa M. Bedier BDS, MSc, MD. Lecturer, Department of Endodontics, Faculty of Dentistry, Cairo University, 12 El Saraya Street, Manial ElRoda, 11451 Cairo, Egypt. m.bedier81@gmail.com
Copyright © 2018. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,475 Views
- 14 Download
- 14 Crossref
Abstract
-
Objectives This study was to evaluate the antibacterial effect of different instrumentation and irrigation techniques using confocal laser scanning microscopy (CLSM) after root canal inoculation with Enterococcus faecalis (E. faecalis).
-
Materials and Methods Mesiobuccal and mesiolingual canals of extracted mandibular molars were apically enlarged up to a size 25 hand K-file, then autoclaved and inoculated with E. faecalis. The samples were randomly divided into 4 main groups according to the system of instrumentation and irrigation: an XP-endo Shaper (XPS) combined with conventional irrigation (XPS/C) or an XP-endo Finisher (XPF) (XPS/XPF), and iRaCe combined with conventional irrigation (iRaCe/C) or combined with an XPF (iRaCe/XPF). A middle-third sample was taken from each group, and then the bacterial reduction was evaluated using CLSM at a depth of 50 µm inside the dentinal tubules. The ratio of red fluorescence (dead cells) to green-and-red fluorescence (live and dead cells) represented the percentage of bacterial reduction. The data were then statistically analyzed using the Kruskal-Wallis test for comparisons across the groups and the Dunn test was used for pairwise comparisons.
-
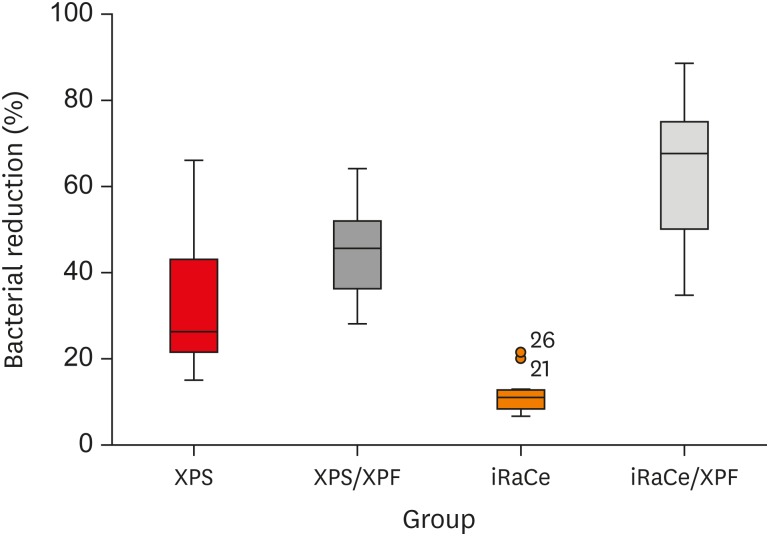
Results The instrumentation and irrigation techniques had a significant effect on bacterial reduction (p < 0.05). The iRaCe/XPF group showed the strongest effect, followed by the XPS/XPF and XPS/C group, while the iRaCe/C group had the weakest effect.
-
Conclusions Combining iRaCe with XPF improved its bacterial reduction effect, while combining XPS with XPF did not yield a significant improvement in its ability to reduce bacteria at a depth of 50 µm in the dentinal tubules.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
The mean and standard deviation (SD) values of the percentage of bacterial reduction after root canal preparation and disinfection in the groups
Box and whisker plot representing the percentage of bacterial reduction in the 4 groups (circles represent outliers).
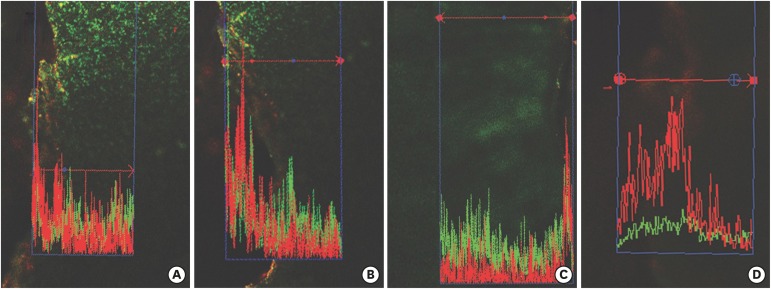
Confocal laser scanning microscopy (CLSM) images after root canal preparation and disinfection in the (A) XP-endo Shaper combined with conventional irrigation (XPS/C), (B) XP-endo Shaper combined with an XP-endo Finisher (XPS/XPF), (C) iRaCe combined with conventional irrigation (iRaCe/C), and (D) iRaCe combined with an XP-endo Finisher (iRaCe/XPF) groups at a depth of 50 µm in the dentinal tubules. The red color denotes dead bacteria, while the green color denotes living bacteria.
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Bedier MM.
Data curation: Hashem AR.
Formal analysis: Hashem AR.
Methodology: Bedier MM, Hassan YM.
Project administration: Bedier MM.
Supervision: Hashem AR.
Validation: Hashem AR, Bedier MM.
Visualization: Bedier MM.
Writing - original draft: Bedier MM, Hassan YM.
Writing - review & editing: Hashem AR.
- 1. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J 1997;30:297-306.ArticlePubMed
- 2. Siqueira JF Jr, Lima KC, Magalhães FA, Lopes HP, de Uzeda M. Mechanical reduction of the bacterial population in the root canal by three instrumentation techniques. J Endod 1999;25:332-335.ArticlePubMed
- 3. Schäfer E, Zapke K. A comparative scanning electron microscopic investigation of the efficacy of manual and automated instrumentation of root canals. J Endod 2000;26:660-664.ArticlePubMed
- 4. Paqué F, Ganahl D, Peters OA. Effects of root canal preparation on apical geometry assessed by micro-computed tomography. J Endod 2009;35:1056-1059.ArticlePubMed
- 5. Paqué F, Balmer M, Attin T, Peters OA. Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instruments: a micro-computed tomography study. J Endod 2010;36:703-707.ArticlePubMed
- 6. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:86-93.ArticlePubMed
- 7. Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J 2003;36:1-11.ArticlePubMedPDF
- 8. Ørstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol 1990;6:142-149.ArticlePubMed
- 9. Peters OA, Arias A, Paqué F. A micro-computed tomographic assessment of root canal preparation with a novel instrument, TRUShape, in mesial roots of mandibular molars. J Endod 2015;41:1545-1550.ArticlePubMed
- 10. Versiani MA, Pécora JD, de Sousa-Neto MD. Flat-oval root canal preparation with self-adjusting file instrument: a micro-computed tomography study. J Endod 2011;37:1002-1007.ArticlePubMed
- 11. Bayram HM, Bayram E, Ocak M, Uygun AD, Celik HH. Effect of ProTaper Gold, self-adjusting File, and XP-endo Shaper instruments on dentinal microcrack formation: a micro-computed tomographic study. J Endod 2017;43:1166-1169.ArticlePubMed
- 12. Elnaghy AM, Elsaka SE. Torsional resistance of XP-endo Shaper at body temperature compared with several nickel-titanium rotary instruments. Int Endod J 2018;51:572-576.ArticlePubMedPDF
- 13. FKG Dentaire SA. The XP-endo Finisher file brochure [Internet]. La Chaux-de-Fonds: FKG Dentaire SA; c2014. updated 2018 Oct 29]. cited 2018 Aug 5]. Available from: https://www.fkg.ch/products/endodontics/canal-shaping-and-cleaning/xp-endo-finisher.
- 14. Silva EJ, Vieira VT, Belladonna FG, Zuolo AS, Antunes HD, Cavalcante DM, Elias CN, De-Deus G. Cyclic and torsional fatigue resistance of XP-endo Shaper and TRUShape instruments. J Endod 2018;44:168-172.ArticlePubMed
- 15. Elnaghy A, Elsaka S. Cyclic fatigue resistance of XP-endo Shaper compared with different nickel-titanium alloy instruments. Clin Oral Investig 2018;22:1433-1437.ArticlePubMedPDF
- 16. Trope M, Debelian G. XP-3D Finisher™ file—the next step in restorative endodontics. Endod Pract US 2015;8:22-24.
- 17. Bao P, Shen Y, Lin J, Haapasalo M. In vitro efficacy of XP-endo Finisher with 2 different protocols on biofilm removal from apical root canals. J Endod 2017;43:321-325.ArticlePubMed
- 18. Leoni GB, Versiani MA, Silva-Sousa YT, Bruniera JF, Pécora JD, Sousa-Neto MD. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int Endod J 2017;50:398-406.ArticlePubMedPDF
- 19. Ozdemir HO, Buzoglu HD, Calt S, Stabholz A, Steinberg D. Effect of ethylenediaminetetraacetic acid and sodium hypochlorite irrigation on Enterococcus faecalis biofilm colonization in young and old human root canal dentin: in vitro study. J Endod 2010;36:842-846.ArticlePubMed
- 20. Wong DT, Cheung GS. Extension of bactericidal effect of sodium hypochlorite into dentinal tubules. J Endod 2014;40:825-829.ArticlePubMed
- 21. Shen Y, Qian W, Chung C, Olsen I, Haapasalo M. Evaluation of the effect of two chlorhexidine preparations on biofilm bacteria in vitro: a three-dimensional quantitative analysis. J Endod 2009;35:981-985.ArticlePubMed
- 22. Ordinola-Zapata R, Bramante CM, de Moraes IG, Bernardineli N, Porto CV, Campanelli AP, Garcia RB, Duarte MH. The use of confocal laser scanning microscopy for the study of dentin infection. In: Mendez-Vilas A, Diaz J, editors. Microscopy: science, technology, applications and education. Badajoz: Formatex; 2010. p. 583-589.
- 23. Silva EJ, Belladonna FG, Zuolo AS, Rodrigues E, Ehrhardt IC, Souza EM, De-Deus G. Effectiveness of XP-endo Finisher and XP-endo Finisher R in removing root filling remnants: a micro-CT study. Int Endod J 2018;51:86-91.ArticlePubMedPDF
- 24. Azim AA, Aksel H, Zhuang T, Mashtare T, Babu JP, Huang GT. Efficacy of 4 irrigation protocols in killing bacteria colonized in dentinal tubules examined by a novel confocal laser scanning microscope analysis. J Endod 2016;42:928-934.ArticlePubMedPMC
- 25. Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol 1971;32:271-275.ArticlePubMed
- 26. George S, Kishen A, Song KP. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis . J Endod 2005;31:867-872.ArticlePubMed
- 27. Siqueira JF Jr. Endodontic infections: concepts, paradigms, and perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:281-293.ArticlePubMed
- 28. Nakamura VC, Candeiro GT, Cai S, Gavini G. Ex vivo evaluation of three instrumentation techniques on E. faecalis biofilm within oval shaped root canals. Braz Oral Res 2015;29:1-7.Article
- 29. Stewart PS. New ways to stop biofilm infections. Lancet 2003;361:97.Article
- 30. Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res 1987;66:1375-1379.ArticlePubMedPDF
- 31. Nair PN, Henry S, Cano V, Vera J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:231-252.ArticlePubMed
- 32. Spratt DA, Pratten J, Wilson M, Gulabivala K. An in vitro evaluation of the antimicrobial efficacy of irrigants on biofilms of root canal isolates. Int Endod J 2001;34:300-307.ArticlePubMedPDF
- 33. Ma J, Wang Z, Shen Y, Haapasalo M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod 2011;37:1380-1385.ArticlePubMed
- 34. Love RM. Regional variation in root dentinal tubule infection by Streptococcus gordonii . J Endod 1996;22:290-293.ArticlePubMed
- 35. Carrigan PJ, Morse DR, Furst ML, Sinai IH. A scanning electron microscopic evaluation of human dentinal tubules according to age and location. J Endod 1984;10:359-363.ArticlePubMed
- 36. Paqué F, Musch U, Hülsmann M. Comparison of root canal preparation using RaCe and ProTaper rotary Ni-Ti instruments. Int Endod J 2005;38:8-16.ArticlePubMed
- 37. Azim AA, Piasecki L, da Silva Neto UX, Cruz AT, Azim KA. XP Shaper, a novel adaptive core rotary instrument: micro-computed tomographic analysis of its shaping abilities. J Endod 2017;43:1532-1538.ArticlePubMed
- 38. Lacerda MF, Marceliano-Alves MF, Pérez AR, Provenzano JC, Neves MA, Pires FR, Gonçalves LS, Rôças IN, Siqueira JF Jr. Cleaning and shaping oval canals with 3 instrumentation systems: a correlative microcomputed tomographic and histoligical study. J Endod 2017;43:1878-1884.PubMed
- 39. Zupanc J, Vahdat-Pajouh N, Schäfer E. New thermomechanically treated NiTi alloys - a review. Int Endod J 2018;51:1088-1103.ArticlePubMedPDF
- 40. FKG Dentaire SA. FKG XP-endo Shaper brochure [Internet]. La Chaux-de-Fonds: FKG Dentaire SA; c2014. updated 2018 Oct 29]. cited 2018 Aug 5]. Available from: https://www.fkg.ch/sites/default/files/201704_fkg_xp_endo_shaper_brochure_v4_fr_web.pdf.
- 41. Alves FR, Andrade-Junior CV, Marceliano-Alves MF, Pérez AR, Rôças IN, Versiani MA, Sousa-Neto MD, Provenzano JC, Siqueira JF Jr. Adjunctive steps for disinfection of the mandibular molar root canal system: a correlative bacteriologic, micro-computed tomography, and cryopulverization approach. J Endod 2016;42:1667-1672.ArticlePubMed
- 42. Elnaghy AM, Mandorah A, Elsaka SE. Effectiveness of XP-endo Finisher, EndoActivator, and File agitation on debris and smear layer removal in curved root canals: a comparative study. Odontology 2017;105:178-183.ArticlePubMedPDF
- 43. Sanabria-Liviac D, Moldauer BI, Garcia-Godoy F, Antonio-Campos A, Casaretto M, Torres-Navarro J, Scalercio JM. Comparison of the XP-Endo Finisher file system and passive ultrasonic irrigation (PUI) on smear layer removal after root canal instrumentation. J Dent Oral Health 2017;4:1-7.
REFERENCES
Tables & Figures
REFERENCES
Citations

- Biofilm-forming activity of Enterococcus faecalis on basic materials of removable dental prosthetic bases
Oksana A. Shuliatnikova, Mikhail V. Yakovlev, Anatoliy P. Godovalov
HERALD of North-Western State Medical University named after I.I. Mechnikov.2025; 17(2): 89. CrossRef - A Short Report on the Effectiveness of Edge Taper Platinum and XP-3D Shaper for the Reduction of Enterococcus faecalis Count in the Root Canal System: An Ex Vivo Study
Hanie Moaveni, Parastou Ghahari, Samira Behrad, Majid Mirmohammadkhani, Sobhan Rashmee, Somayeh Teimoori
Avicenna Journal of Dental Research.2024; 16(2): 77. CrossRef - Shaping ability of non‐adaptive and adaptive core nickel–titanium single‐file systems with supplementary file in ribbon‐shaped canals analysed by micro‐computed tomography
Parichat Chinchiyanont, Kallaya Yanpiset, Danuchit Banomyong, Nathamon Thongbai‐On
Australian Endodontic Journal.2023; 49(1): 38. CrossRef - Impact XP-endo finisher on the 1-year follow-up success of posterior root canal treatments: a randomized clinical trial
Ludmila Smith de Jesus Oliveira, Fabricio Eneas Diniz de Figueiredo, Janaina Araújo Dantas, Maria Amália Gonzaga Ribeiro, Carlos Estrela, Manoel Damião Sousa-Neto, André Luis Faria-e-Silva
Clinical Oral Investigations.2023; 27(12): 7595. CrossRef - In vitro reduction in Enterococcus faecalis count following root canal preparation with Neolix and XP shaper rotary files
Mina Mehrjouei, Somayeh Teimoori, Majid Mirmohammadkhani, Seyed Majed Mortazavi, Maryam Khorasanchi
Saudi Endodontic Journal.2023; 13(3): 236. CrossRef - Antibacterial efficacy of sodium hypochlorite versus apple cider vinegar against Enterococcus faecalis in contracted endodontic cavity
Kaur Supreet, Karkala Venkappa Kishan, Nimisha Chinmay Shah
Endodontology.2022; 34(4): 254. CrossRef - Ex vivo evaluation of the effectiveness of XP-endo Finisher on the removal of smear layer from the root canal
Sângela Maria PEREIRA, Ceci Nunes CARVALHO, Rudys Rodolfo TAVAREZ, Paulo NELSON-FILHO, Léa Assed Bezerra DA SILVA, Etevaldo Matos MAIA FILHO
RGO - Revista Gaúcha de Odontologia.2022;[Epub] CrossRef - Biofilm elimination from infected root canals using four different single files
Sarah A. Hamed, Sarah Shabayek, Hayam Y. Hassan
BMC Oral Health.2022;[Epub] CrossRef - The effectiveness of the supplementary use of the XP-endo Finisher on bacteria content reduction: a systematic review and meta-analysis
Ludmila Smith de Jesus Oliveira, Rafaella Mariana Fontes de Bragança, Rafael Sarkis-Onofre, André Luis Faria-e-Silva
Restorative Dentistry & Endodontics.2021;[Epub] CrossRef - Combination of a new ultrasonic tip with rotary systems for the preparation of flattened root canals
Karina Ines Medina Carita Tavares, Jáder Camilo Pinto, Airton Oliveira Santos-Junior, Fernanda Ferrari Esteves Torres, Juliane Maria Guerreiro-Tanomaru, Mario Tanomaru-Filho
Restorative Dentistry & Endodontics.2021;[Epub] CrossRef - Effect of Adaptive, Rotary, and Manual Root Canal Instrumentation in Primary Molars: A Triple-Armed, Randomized Controlled Clinical Trial
Bhaggyashri A. Pawar, Ajinkya M. Pawar, Anuj Bhardwaj, Dian Agustin Wahjuningrum, Amelia Kristanti Rahardjo, Alexander Maniangat Luke, Zvi Metzger, Anda Kfir
Biology.2021; 10(1): 42. CrossRef - Complete Obturation—Cold Lateral Condensation vs. Thermoplastic Techniques: A Systematic Review of Micro-CT Studies
Shilpa Bhandi, Mohammed Mashyakhy, Abdulaziz S. Abumelha, Mazen F. Alkahtany, Mohamed Jamal, Hitesh Chohan, A. Thirumal Raj, Luca Testarelli, Rodolfo Reda, Shankargouda Patil
Materials.2021; 14(14): 4013. CrossRef - The Effects of Different Endodontic Access Cavity Design and Using XP-endo Finisher on the Reduction of Enterococcus faecalis in the Root Canal System
Pelin Tüfenkçi, Koray Yılmaz
Journal of Endodontics.2020; 46(3): 419. CrossRef - Irrigation in Endodontics: a Review
Sarah Bukhari, Alaa Babaeer
Current Oral Health Reports.2019; 6(4): 367. CrossRef

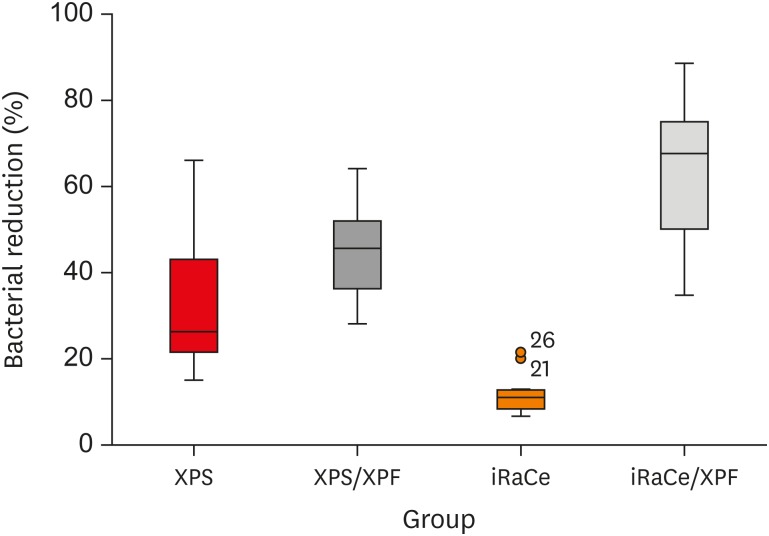
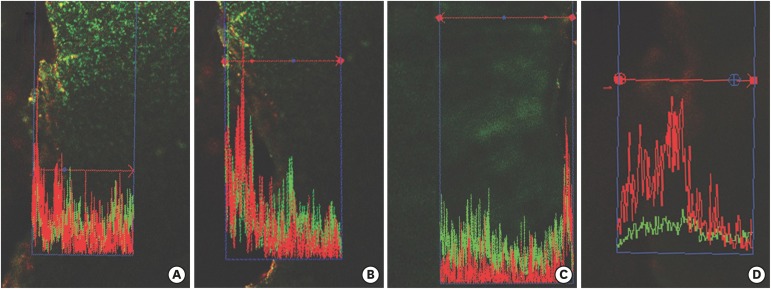
Figure 1
Figure 2
Figure 3
The mean and standard deviation (SD) values of the percentage of bacterial reduction after root canal preparation and disinfection in the groups
| Group | Mean | SD | Median | Minimum | Maximum | 95% CI | p value | |
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| XPS/C | 33.28B | 17.01 | 26.25 | 15.00 | 66.00 | 21.11 | 45.45 | < 0.001* |
| XPS/XPF | 45.17B | 10.25 | 45.58 | 28.00 | 64.08 | 37.83 | 52.50 | |
| IRaCe/C | 11.81C | 5.13 | 10.96 | 6.60 | 21.43 | 8.14 | 15.48 | |
| iRaCe/XPF | 62.91A | 16.96 | 67.58 | 34.78 | 88.57 | 50.78 | 75.04 | |
Different superscripts in the same column indicate statistically significant differences.
CI, confidence interval; XPS/C, XP-endo Shaper combined with conventional irrigation; XPS/XPF, XP-endo Shaper combined with XP-endo Finisher; iRaCe/C, iRaCe combined with conventional irrigation; iRaCe/XPF, iRaCe combined with an XP-endo Finisher.
*Significant at p ≤ 0.05.
Different superscripts in the same column indicate statistically significant differences.
CI, confidence interval; XPS/C, XP-endo Shaper combined with conventional irrigation; XPS/XPF, XP-endo Shaper combined with XP-endo Finisher; iRaCe/C, iRaCe combined with conventional irrigation; iRaCe/XPF, iRaCe combined with an XP-endo Finisher.
*Significant at

 KACD
KACD

 ePub Link
ePub Link Cite
Cite

