Articles
- Page Path
- HOME > Restor Dent Endod > Volume 43(2); 2018 > Article
- Research Article Effect of various bleaching treatments on shear bond strength of different universal adhesives and application modes
-
Fatma Dilsad Oz
 , Zeynep Bilge Kutuk
, Zeynep Bilge Kutuk
-
Restor Dent Endod 2018;43(2):e20.
DOI: https://doi.org/10.5395/rde.2018.43.e20
Published online: April 16, 2018
Department of Restorative Dentistry, School of Dentistry, Hacettepe University, Ankara, Turkey.
- Correspondence to Fatma Dilsad Oz, DDS, PhD. Research Assistant, Department of Restorative Dentistry, School of Dentistry, Hacettepe University, Sihhiye, Ankara, 06100, Turkey. dilsadoz@yahoo.com
Copyright © 2018. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,145 Views
- 21 Download
- 8 Crossref
Abstract
-
Objectives The aim of this in vitro study was to evaluate the bond strength of 2 universal adhesives used in different application modes to bleached enamel.
-
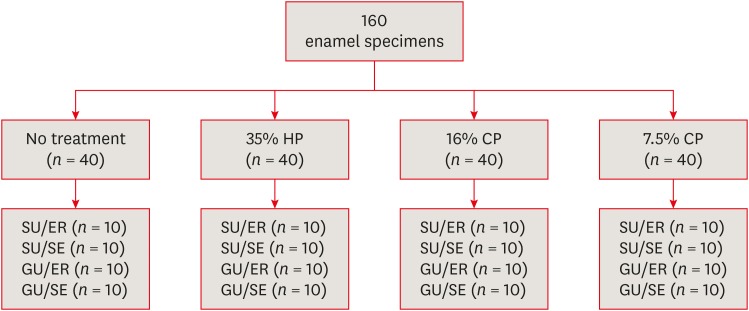
Materials and Methods Extracted 160 sound human incisors were used for the study. Teeth were divided into 4 treatment groups: No treatment, 35% hydrogen peroxide, 16% carbamid peroxide, 7.5% carbamid peroxide. After bleaching treatments, groups were divided into subgroups according to the adhesive systems used and application modes (n = 10): 1) Single Bond Universal, etch and rinse mode; 2) Single Bond Universal, self-etch mode; 3) Gluma Universal, etch and rinse mode; 4) Gluma Universal, self-etch mode. After adhesive procedures nanohybrid composite resin cylinders were bonded to the enamel surfaces. All specimens were subjected to shear bond strength (SBS) test after thermocycling. Data were analyzed using a 3-way analysis of variance (ANOVA) and Tukey post hoc test.
-
Results No significant difference were found among bleaching groups (35% hydrogen peroxide, 16% carbamid peroxide, 7.5% carbamid peroxide, and no treatment groups) in the mean SBS values. There was also no difference in SBS values between Single Bond Universal and Gluma Universal at same application modes, whereas self-etch mode showed significantly lower SBS values than etch and rinse mode (p < 0.05).
-
Conclusions The bonding performance of the universal adhesives was enhanced with the etch and rinse mode application to bleached enamel and non-bleached enamel.
INTRODUCTION
MATERIALS AND METHODS
Materials used in the study
Study design.
RESULTS
Shear bond strength (SBS) values of universal adhesives to enamel according to etch and rinse or self-etch modes
Failure mode distrubutions (%).
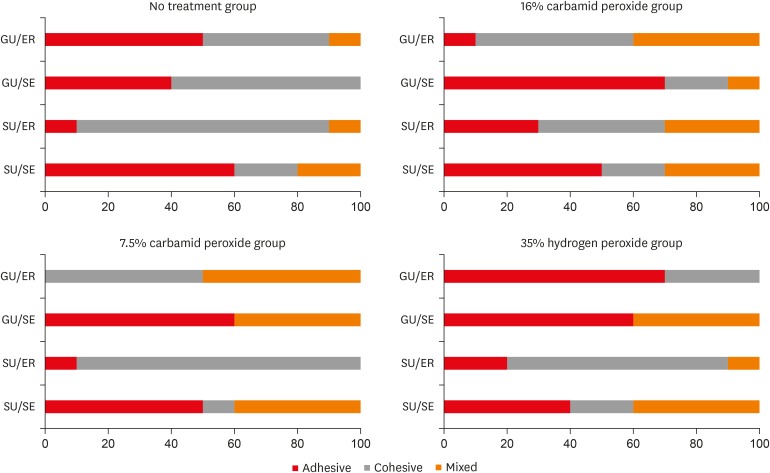
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Oz FD, Kutuk ZB.
Data curation: Oz FD.
Formal analysis: Oz FD.
Investigation: Oz FD.
Methodology: Oz FD, Kutuk ZB.
Project administration: Oz FD.
Resources: Oz FD.
Software: Oz FD.
Supervision: Kutuk ZB.
Validation: Kutuk ZB.
Visualization: Oz FD, Kutuk ZB.
Writing - original draft: Oz FD.
Writing - review & editing: Oz FD, Kutuk ZB.
- 1. Kwon SR, Wertz PW. Review of the mechanism of tooth whitening. J Esthet Restor Dent 2015;27:240-257.ArticlePubMedPDF
- 2. Majeed A, Farooq I, Grobler SR, Rossouw RJ. Tooth-bleaching: a review of the efficacy and adverse effects of various tooth whitening products. J Coll Physicians Surg Pak 2015;25:891-896.PubMed
- 3. Heymann HO. Tooth whitening: facts and fallacies. Br Dent J 2005;198:514-518.ArticlePubMedPDF
- 4. Bernardon JK, Ferrari P, Baratieri LN, Rauber GB. Comparison of treatment time versus patient satisfaction in at-home and in-office tooth bleaching therapy. J Prosthet Dent 2015;114:826-830.ArticlePubMed
- 5. de Geus JL, Wambier LM, Kossatz S, Loguercio AD, Reis A. At-home vs in-office bleaching: a systematic review and meta-analysis. Oper Dent 2016;41:341-356.ArticlePubMedPDF
- 6. Giachetti L, Bertini F, Bambi C, Nieri M, Scaminaci Russo D. A randomized clinical trial comparing at-home and in-office tooth whitening techniques: a nine-month follow-up. J Am Dent Assoc 2010;141:1357-1364.PubMed
- 7. Ritter AV. In-office tooth bleaching. J Esthet Restor Dent 2006;18:168-169.ArticlePubMed
- 8. Unlu N, Cobankara FK, Ozer F. Effect of elapsed time following bleaching on the shear bond strength of composite resin to enamel. J Biomed Mater Res B Appl Biomater 2008;84:363-368.ArticlePubMed
- 9. Attin T, Hannig C, Wiegand A, Attin R. Effect of bleaching on restorative materials and restorations--a systematic review. Dent Mater 2004;20:852-861.ArticlePubMed
- 10. Cavalli V, Reis AF, Giannini M, Ambrosano GM. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent 2001;26:597-602.PubMed
- 11. Potocnik I, Kosec L, Gaspersic D. Effect of 10% carbamide peroxide bleaching gel on enamel microhardness, microstructure, and mineral content. J Endod 2000;26:203-206.ArticlePubMed
- 12. Goldberg M, Grootveld M, Lynch E. Undesirable and adverse effects of tooth-whitening products: a review. Clin Oral Investig 2010;14:1-10.ArticlePubMedPDF
- 13. Bittencourt ME, Trentin MS, Linden MS, de Oliveira Lima Arsati YB, Franca FM, Florio FM, Basting RT. Influence of in situ postbleaching times on shear bond strength of resin-based composite restorations. J Am Dent Assoc 2010;141:300-306.ArticlePubMed
- 14. Nour El-din AK, Miller BH, Griggs JA, Wakefield C. Immediate bonding to bleached enamel. Oper Dent 2006;31:106-114.ArticlePubMedPDF
- 15. Gurgan S, Alpaslan T, Kiremitci A, Cakir FY, Yazici E, Gorucu J. Effect of different adhesive systems and laser treatment on the shear bond strength of bleached enamel. J Dent 2009;37:527-534.ArticlePubMed
- 16. Montalvan E, Vaidyanathan TK, Shey Z, Janal MN, Caceda JH. The shear bond strength of acetone and ethanol-based bonding agents to bleached teeth. Pediatr Dent 2006;28:531-536.PubMed
- 17. Chen C, Niu LN, Xie H, Zhang ZY, Zhou LQ, Jiao K, Chen JH, Pashley DH, Tay FR. Bonding of universal adhesives to dentine--old wine in new bottles? J Dent 2015;43:525-536.ArticlePubMed
- 18. Imai A, Takamizawa T, Sai K, Tsujimoto A, Nojiri K, Endo H, Barkmeier WW, Latta MA, Miyazaki M. Influence of application method on surface free-energy and bond strength of universal adhesive systems to enamel. Eur J Oral Sci 2017;125:385-395.ArticlePubMedPDF
- 19. Suzuki S, Takamizawa T, Imai A, Tsujimoto A, Sai K, Takimoto M, Barkmeier WW, Latta MA, Miyazaki M. Bond durability of universal adhesive to bovine enamel using self-etch mode. Clin Oral Investig 2018;22:1113-1122.ArticlePubMedPDF
- 20. Barghi N, Godwin JM. Reducing the adverse effect of bleaching on composite-enamel bond. J Esthet Dent 1994;6:157-161.ArticlePubMed
- 21. Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater 1994;10:33-36.ArticlePubMed
- 22. Titley KC, Torneck CD, Ruse ND, Krmec D. Adhesion of a resin composite to bleached and unbleached human enamel. J Endod 1993;19:112-115.ArticlePubMed
- 23. Bishara SE, Oonsombat C, Soliman MM, Ajlouni R, Laffoon JF. The effect of tooth bleaching on the shear bond strength of orthodontic brackets. Am J Orthod Dentofacial Orthop 2005;128:755-760.ArticlePubMed
- 24. Rahul M, Kumar PA, Nair AS, Mathew S, Amaladas AS, Ommen A. Effects of at-home and in-office bleaching agents on the shear bond strength of metal, ceramic, and composite brackets to enamel. Indian J Dent Res 2017;28:566-573.ArticlePubMed
- 25. Akin M, Aksakalli S, Basciftci FA, Demir A. The effect of tooth bleaching on the shear bond strength of orthodontic brackets using self-etching primer systems. Eur J Dent 2013;7:55-60.ArticlePubMedPMC
- 26. Gungor AY, Ozcan E, Alkis H, Turkkahraman H. Effects of different bleaching methods on shear bond strengths of orthodontic brackets. Angle Orthod 2013;83:686-690.ArticlePubMedPDF
- 27. McLean DE, Meyers EJ, Guillory VL, Vandewalle KS. Enamel bond strength of new universal adhesive bonding agents. Oper Dent 2015;40:410-417.ArticlePubMedPDF
- 28. Wagner A, Wendler M, Petschelt A, Belli R, Lohbauer U. Bonding performance of universal adhesives in different etching modes. J Dent 2014;42:800-807.ArticlePubMed
- 29. Juneja R, Duhan J, Tewari S, Sangwan P, Bhatnagar N. Effect of blood contamination and decontamination protocols on acetone-based and ethanol-based total etch adhesive systems. J Esthet Restor Dent 2014;26:403-416.ArticlePubMedPDF
- 30. Usha C, Ramarao S, John BM, Rajesh P, Swatha S. Evaluation of the shear bond strength of composite resin to wet and dry enamel using dentin bonding agents containing various solvents. J Clin Diagn Res 2017;11:ZC41-ZC44.Article
- 31. Diniz AC, Bandeca MC, Pinheiro LM, Dos Santosh Almeida LJ Jr, Torres CR, Borges AH, Pinto SC, Tonetto MR, De Jesus Tavarez RR, Firoozmand LM. Influence of different etching modes on bond strength to enamel using universal adhesive systems. J Contemp Dent Pract 2016;17:820-825.ArticlePubMed
- 32. Suzuki T, Takamizawa T, Barkmeier WW, Tsujimoto A, Endo H, Erickson RL, Latta MA, Miyazaki M. Influence of etching mode on enamel bond durability of universal adhesive systems. Oper Dent 2016;41:520-530.ArticlePubMedPDF
- 33. Vermelho PM, Reis AF, Ambrosano GM, Giannini M. Adhesion of multimode adhesives to enamel and dentin after one year of water storage. Clin Oral Investig 2017;21:1707-1715.ArticlePubMedPDF
- 34. Hanabusa M, Mine A, Kuboki T, Momoi Y, Van Ende A, Van Meerbeek B, De Munck J. Bonding effectiveness of a new ‘multi-mode’ adhesive to enamel and dentine. J Dent 2012;40:475-484.ArticlePubMed
- 35. Loguercio AD, Bittencourt DD, Baratieri LN, Reis A. A 36-month evaluation of self-etch and etch-and-rinse adhesives in noncarious cervical lesions. J Am Dent Assoc 2007;138:507-514.ArticlePubMed
- 36. Perdigão J, Kose C, Mena-Serrano AP, De Paula EA, Tay LY, Reis A, Loguercio AD. A new universal simplified adhesive: 18-month clinical evaluation. Oper Dent 2014;39:113-127.ArticlePubMedPDF
- 37. Zhang Z, Wang X, Zhang L, Liang B, Tang T, Fu B, Hannig M. The contribution of chemical bonding to the short- and long-term enamel bond strengths. Dent Mater 2013;29:e103-e112.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Antioxidant effect on shear bond strength of resin composite to in-office versus home bleached enamel surface
Maha Mosaad Mohamed, Magda E. -A. Shalaby, Eman A. E. -G. Shebl
Tanta Dental Journal.2025; 22(3): 409. CrossRef - Effects of Time-Elapsed Bleaching on the Surface and Mechanical Properties of Dentin Substrate Using Hydrogen Peroxide-Free Nanohydroxyapatite Gel
Aftab Khan, Abdulaziz AlKhureif, Manal Almutairi, Abrar Nooh, Saeed Hassan, Yasser Alqahtani
International Journal of Nanomedicine.2024; Volume 19: 10307. CrossRef - Effect of sodium ascorbate on the shear bond strength of orthodontic brackets to bleached enamel using universal dental adhesive
Saeid Sadeghian, Kamyar Fathpour, Mahshid Biglari
Dental Research Journal.2023;[Epub] CrossRef - Quantitative Measurements of the Depth of Enamel Demineralization before and after Bleach: An In Vitro Study
Sara Naim, Gianrico Spagnuolo, Essam Osman, Syed Sarosh Mahdi, Gopi Battineni, Syed Saad B. Qasim, Mariangela Cernera, Hasna Rifai, Nada Jaafar, Elie Maalouf, Carina Mehanna Zogheib, Konstantinos Michalakis
BioMed Research International.2022;[Epub] CrossRef - DİŞ BEYAZLATMA İŞLEMİNİN LİTYUM DİSİLİKAT SERAMİĞİN BAĞLANMA DAYANIMINA ETKİSİ
Merve YILDIRAK, Rıfat GÖZNELİ
Atatürk Üniversitesi Diş Hekimliği Fakültesi Dergisi.2020; : 1. CrossRef - The Effect of Different Bleaching Protocols, Used with and without Sodium Ascorbate, on Bond Strength between Composite and Enamel
Maroun Ghaleb, Giovanna Orsini, Angelo Putignano, Sarah Dabbagh, Georges Haber, Louis Hardan
Materials.2020; 13(12): 2710. CrossRef - Influence of phototherapy on adhesive strength and microleakage of bleached enamel bonded to orthodontic brackets: An in-vitro study
Erum Khan, Ibrahim Alshahrani, Muhammad Abdullah Kamran, Abdulaziz Samran, Ali Alqerban, Saad Abdul Rehman
Photodiagnosis and Photodynamic Therapy.2019; 25: 344. CrossRef - Effect of Er: YAG Laser on Microtensile Bond Strength of Bleached Dentin to Composite
Mohsen Rezaei, Elham Aliasghar, Mohammad Bagher Rezvani, Nasim Chiniforush, Zohreh Moradi
Journal of Lasers in Medical Sciences.2019; 10(2): 117. CrossRef
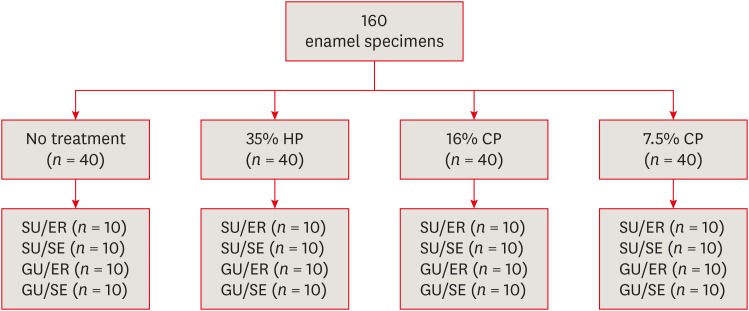
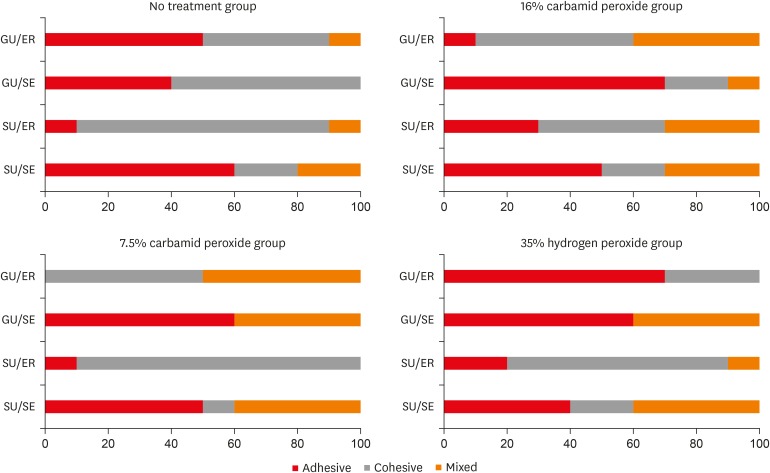
Figure 1
Figure 2
Materials used in the study
| Material/manufacturer | Batch No. | Composition | Application |
|---|---|---|---|
| Total Blanc Office H35/Nova DFL | 12050770 | 35% hydrogen peroxide | A thin layer of gel was applied and left undisturbed for 20 minutes, then removed aith cotton rolls. Afterwards, the gel was applied for 20 more minutes. The enamel surfaces were rinsed with water and air dried. |
| Total Blanc Home C16/Nova DFL | 12050720 | 16% carbamid peroxide | A thin layer of gel was applied and left undisturbed for 8 hours, then removed with cotton rolls. Afterwards, the enamel surfaces were rinsed with water and air dried. The same procedure was applied for 14 days/2 weeks without interruption. |
| Total Blanc Home C7.5/Nova DFL | 12050665 | 7.5% carbamid peroxide | A thin layer of gel was applied and left undisturbed for 8 hours, then removed with cotton rolls. Afterwards, the enamel surfaces were rinsed with water and air dried. The same procedure was applied for 14 days/2 weeks witout interruption |
| Single Bond Universal/3M ESPE | 585360 | MDP phosphate monomers, dimethacrylate resins, HEMA, methacrylate-modified polyalkenoic acid copolymer, fillers, ethanol, water, initiators, silane | Etch and rinse technique |
| Phosphoric acid etching gel (37%) was applied to enamel and left in place for 30 seconds, then rinsed rinse and dried. Bond was applied to the entire cavity wall with the applicator brush and rubbed for 20 seconds. The entire cavity wall was dried sufficiently by blowing mild air for more than 5 seconds until bond does not move. Bond was light-cured with 1,200 mW/cm2 LED for 10 seconds. | |||
| Self-etch technique | |||
| Bond was applied to the entire cavity wall with the applicator brush and rubbed for 20 seconds. The entire cavity wall was dried sufficiently by blowing mild air for more than 5 seconds until bond does not move. Bond was light-cure bond with 1,200 mW/cm2 LED for 10 seconds. | |||
| Gluma Bond Universal/Heraeus Kulzer GmbH | 010022 | MDP phosphate monomers, 4-META, dimethacrylate resins, acetone, fillers, initiators, silane | Etch and rinse technique |
| Phosphoric acid etching gel (37%) was applied to enamel and left in place for 30 seconds, then rinsed rinse and dried. Bond was applied to the entire cavity wall with the applicator brush and rubbed for 20 seconds. The entire cavity wall was dried sufficiently by blowing mild air for more than 5 seconds until bond does not move. Bond was light-cured with 1,200 mW/cm2 LED for 10 seconds. | |||
| Self-etch technique | |||
| Bond was applied to the entire cavity wall with the applicator brush and rubbed for 20 seconds. The entire cavity wall was dried sufficiently by blowing mild air for more than 5 seconds until bond does not move. Bond was light-cure bond with 1,200 mW/cm2 LED for 10 seconds. | |||
| Herculite XRV Ultra Universal Nano-hybrid composite/Kerr Corporation | 5139411 | 3-trimethoxysilylpropyl methacrylate hexamethylene diacrylate | Two mm increments were placed and light cured for 20 seconds with 1,200 mW/cm2 LED |
| 2,2′-ethylenedioxydiethyl dimethacrylate | |||
| 1,6-hexanediyl bismethacrylate | |||
| Bis-GMA |
MDP, 10-methacryloyloxydecyl dihydrogen phosphate; HEMA, 2-hydroxyethyl methacrylate; LED, light-emitting diode; 4-META, 4-methacryloyloxyethy trimellitate; Bis-GMA, bisphenol A-glycidyl methacrylate.
Shear bond strength (SBS) values of universal adhesives to enamel according to etch and rinse or self-etch modes
| Group | No treatment | 16% carbamid peroxide | 7.5% carbamid peroxide | 35% hydrogen peroxide |
|---|---|---|---|---|
| SU/ER | 34.8 ± 7.0a | 32.4 ± 6.4a | 33.2 ± 6.6a | 30.9 ± 7.4a |
| SU/SE | 20.3 ± 6.0b | 18.5 ± 7.4b | 20.6 ± 4.7b | 18.9 ± 6.8b |
| GU/ER | 33.5 ± 8.1a | 31.0 ± 10a | 32.6 ± 8.5a | 29.8 ± 6.7a |
| GU/SE | 22.3 ± 7.1b | 20.4 ± 7.5b | 21.0 ± 7.1b | 20.7 ± 7.0b |
The SBS values are shown as means ± standard deviations.
a,bDifferent superscript letters show significant differences within the column (p < 0.05).
SU, Single Bond Universal; GU, Gluma Universal; ER, etch and rinse; SE, self-etch.
MDP, 10-methacryloyloxydecyl dihydrogen phosphate; HEMA, 2-hydroxyethyl methacrylate; LED, light-emitting diode; 4-META, 4-methacryloyloxyethy trimellitate; Bis-GMA, bisphenol A-glycidyl methacrylate.
The SBS values are shown as means ± standard deviations.
a,bDifferent superscript letters show significant differences within the column (
SU, Single Bond Universal; GU, Gluma Universal; ER, etch and rinse; SE, self-etch.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

