Articles
- Page Path
- HOME > Restor Dent Endod > Volume 42(2); 2017 > Article
- Review Article Recognition and management of palatogingival groove for tooth survival: a literature review
-
Hee-Jin Kim1,2, Yoorina Choi1, Mi-Kyung Yu1,3, Kwang-Won Lee1,3, Kyung-San Min1,3

-
2017;42(2):-86.
DOI: https://doi.org/10.5395/rde.2017.42.2.77
Published online: April 12, 2017
1Department of Conservative Dentistry, School of Dentistry, Chonbuk National University, Jeonju, Korea.
2Department of Dentistry, Kosin University Gospel Hospital, Busan, Korea.
3Biomedical Research Institute of Chonbuk National University Hospital, Jeonju, Korea.
- Correspondence to Kyung-San Min, DDS, MSD, PhD. Professor, Department of Conservative Dentistry, School of Dentistry, Chonbuk National University, 567 Baekje-daero, Deokjin-gu, Jeonju, Korea 54896. TEL, +82-63-250-2764; FAX, +82-63-250-2129; mksdd@jbnu.ac.kr
• Received: August 23, 2016 • Accepted: February 20, 2017
©Copyrights 2017. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 8,094 Views
- 170 Download
- 35 Crossref
Abstract
- Palatogingival groove (PGG) is an anomaly in the maxillary anterior teeth, often accompanied by the area of bony destruction adjacent to the teeth with no carious or traumatic history. The hidden trap in the tooth can harbor plaque and bacteria, resulting in periodontal destruction with or without pulpal pathologic change. Related diseases can involve periodontal destruction, combined endodontic-periodontal lesions, or separate endodontic and periodontal lesions. Disease severity and prognosis related to PGG depend on several factors, including location, range, depth, and type of the groove. Several materials have been used and recommended for cases of extensive periodontal destruction from PGG to remove and block the inflammatory source and recover the health of surrounding periodontal tissues. Even in cases of severe periodontal destruction, several studies have reported favorable treatment outcomes with proper management. With new options in diagnosis and treatment, clinicians need a detailed understanding of the characteristics, treatment, and prognosis of PGG to successfully manage the condition.
Introduction
The maxillary anterior incisors can show various morphologic and anatomic anomalies, including globulomaxillary cysts, cleft palate, congenital absence of tooth, supernumerary tooth, dens invaginatus, Eagle's talon, peg-shaped lateral incisor, germination, fusion, accessory root, and palatal gingival grooved incisors.1,2 Among the abnormalities, palatogingival groove (PGG) is defined as ‘a developmental anomaly in a root that, when present, is usually found on the lingual surface of maxillary incisor teeth’.3 The maxillary lateral incisor is most commonly involved because of the embryological hazard of having its tooth germ locked between those of the central incisor and canine. PGG originates when the central fossa crosses the cingulum and extends to varying distances in an apical direction.4
Frequently, recognizing and managing PGG is complicated and requires an interdisciplinary strategy for combined endodontic-periodontal lesions. However, no controlled investigations have been conducted despite a large number of case reports. Therefore, the aim of this article was to review the literature on the diagnosis and treatment of PGG, thereby helping clinicians to successfully manage PGG.
Literature review
PGG, first described as a radicular groove by Black in 1908, is a developmental anomaly that begins near the tooth cingulum and extends from the cementoenamel junction (CEJ) in apical direction along the root with a wide range of depths and lengths.5 In 1958, Oehlers first reported radicular invagination of a maxillary lateral incisor in a Chinese female.6 In 1968, Lee et al. proposed the term PGG to describe a groove in the lateral incisor with palatal aspects.3 Several terms have been used to describe PGG: distolingual groove, corono-radicular groove, radicular lingual groove, vertical developmental radicular groove, cingulo-radicular groove, developmental radicular anomaly, interruption groove, palatal radicular groove, and palato-radicular groove.3,7,8,9
Several etiologies have been claimed for this developmental anomaly: (i) consequence of an alteration in growth, such as an infolding of the inner enamel epithelium and epithelial sheath of Hertwig,3 (ii) variant of dens invaginatus,3,4 (iii) alteration of a genetic mechanism,10 and (iv) attempt to form another root.7,11
Diverse occurrence rates for PGG have been reported. The investigation by Everett et al. was the first large survey of the prevalence of PGG.8 They reported a prevalence of less than 2% with 0.5% of PGG extending into the apical area in their survey of 625 extracted maxillary lateral incisors.8 Withers et al.4 reported that the prevalence of the PGGs in 531 individuals examined was 8.5%. Among the individuals, 2.33% of the 2,099 maxillary incisor teeth examined had PGG. Most of the PGGs (93.8%) were in maxillary lateral incisor teeth.4 Kogon et al.9 surveyed 3,168 extracted maxillary incisors and reported a prevalence rate of 4.6% total, and 5.6% and 3.4% in maxillary central incisors and lateral incisors, respectively. About half of the grooves terminated on the root, and 58% of those extended more than 5 mm from the CEJ.9
Meanwhile, other researchers have reported higher occurrence rates. Storrer et al. reported a prevalence rate of 9.58% in a survey of 73 extracted maxillary lateral incisors.12 Al-Rasheed et al. reported 10.3% prevalence of the PGG, with 6.5% and 3.8% for coronal and apical grooves, respectively, from clinical examinations of 552 maxillary lateral incisors in 276 Saudi adults.13 In a clinical examination of 200 patients, Iqbal et al.1 reported a prevalence rate of 10%, 6.75% as a coronal groove and 3.25% as an apical groove. They found bilateralism in 57.5% of cases, 63% of coronal and 46.15% of apical grooves.1 Hou et al. reported a prevalence rate of 18.1% in clinical examinations of 404 maxillary incisors in 101 individuals.14
This variance in prevalence rates could be caused by different diagnostic criteria or examination methodologies (e.g., survey of extracted teeth vs. clinical examination) or by ethnic/racial differences, which would suggest a genetic relationship (e.g., relatively high prevalence in Sino-Americans and low prevalence in Sub-Saharan Africans and Sahul-Pacific people).15 Acquiring more precise prevalence rates will require studies with larger samples that represent the entire population.
PGG can be classified by its location as distal, mesial, or central patterns as summarized in Table 1.16 Storrer et al. reported that 9.58% of maxillary lateral incisors showed PGG in an investigation of 73 extracted teeth, with the groove always located in the center of the lingual area.12 On the contrary, Albaricci et al. showed that, among maxillary lateral incisors with PGG, 62.8% of the grooves were proximally located.17 If the groove is located proximally, bacterial plaque accumulation can occur more rapidly and cause greater difficulty in removal, resulting in destruction of periodontal tissues and formation of localized periodontal lesions.16
Another proposed classification of PGG is into mild, moderate, and complex types according to the depth and complexity of the groove,18,19 which can be clinically more important than the location or number of grooves. Mild grooves are gentle depressions in the coronal enamel that terminate at or immediately after crossing the CEJ.3,4 Moderate grooves extend some distance apically along the root surface in a form of a shallow or fissured defect.3,4,7,8,11,18,20 Complex grooves are deeply invaginated defects which involve the entire length of the root or those with separate accessory root from the main root trunk.3,7,11,20,21 Kogon et al. reported that more than 50% of PGG extended beyond the CEJ onto the root surface of the lateral incisors: 43% of the grooves on the root extended less than 5 mm, 47% between 6 and 10 mm, and 10% more than 10 mm.9 Meanwhile, as the severity and the complexity of PGG increase, the potential for pulp-periodontal ligament space communications also increases.18 In a histological observation of a tooth extracted due to PGG, the dysplastic dentin with islands and clefts seemed to communicate with the periodontal ligament space subjacent to the groove.11,18,20
According to the degree of invagination of the groove toward the pulp cavity, PGG can also be classified as shallow/flat (< 1 mm), deep (> 1 mm), or a closed tube that forms a tunnel-like channel.9,22 Some researchers have used sequential ground sections or histological examinations of deeply invaginated grooves and observed a groove with entrapped enamel (also called a hollow enamel island) that ends in a blind enamel cul-de-sac.8,18 Kogon et al. reported that 54% of all PGG samples were classified as shallow depression, 42% as deep depression, and 4% as closed tube.9
Recently, extracted maxillary lateral incisors with PGG were observed and reconstructed 3 dimensionally using micro-computed tomography. Gu et al. classified 11 extracted teeth into 3 types.23 A type I groove is short (not beyond the coronal third of the root), type II is long (beyond the coronal third of the root) but shallow, corresponding to a normal or simple root canal. Type III is long (beyond the coronal third of the root) and deep, corresponding to a complex root canal.23 Rarely, numerous grooves in a single tooth have been reported; for example, one in the labial surface and another in the lingual surface24 or multiple grooves in the lingual surface of maxillary central incisors.25
Al-Rasheed et al.13 studied the association between PGG and periodontal health by investigating the plaque index (PI), bleeding index (BI), and pocket depth (PD) in 552 maxillary lateral incisors in Saudi adults. They reported that teeth with an apical groove showed PI and BI of 100%; thus, the apical extension of the groove was significantly associated with poor periodontal health. Diseases related to PGG are localized gingivitis and periodontitis with or without pulpal pathology. In several case reports, PGG cases presented as a combined type of primary periodontal lesion with secondary involvement of the pulp.
Some explanations have been presented about possible causes of such combined endodontic-periodontal lesions. Whereas some authors have insisted that the pulpal and periodontal lesions are unrelated with different etiologies that simply coincide in time,24 others have suggested a correlation between pulpal and periodontal lesions.7,26 Those researchers called out the root apex, accessory foramina, lateral canals, and dentinal tubules as possible communication channels between periodontal defects and pulpal tissues, and speculated that bacteria and necrotic debris from a primarily infected root canal could extend into the apical base of the fissure groove with subsequent coronal progression along the groove.7
On the other hand, irritants and microorganisms from the periodontal pocket could progress along the hollow, funnel-shaped PGG surface, advancing to the periodontal breakdown and root surface contamination resulting in retrogenic pulp necrosis even though PGGs do not reach the apex and are not very deep. Accessory foramina or dentinal tubules along the grooves have been regarded as the possible pathway between the pulp canal and the groove.18,26 Peikoff et al. showed the presence of communication like numerous clefts between the pulp canal and the groove in their histological observation.11 In 1989, Gao et al.26 investigated maxillary lateral incisors with PGG by scanning electron microscope. They found some parts as thin as 360 µm between the groove and the pulp cavity, and observed accessory foramina along the grooves. Four of the five specimens had one to three foramina with diameters varying between 15 and 200 µm. They concluded that those accessary foramina along the groove could be primary means of communication between the pulp and periodontium in teeth with PGG. These findings were supported by histological investigations of Goon et al.18
Several PGG cases have been reported recently. Clinically, patients with a pathologic lesion related to PGG often complain of dull intermittent27,28 or acute pain,22,29 mobility of the teeth,30,31,32 pain on percussion,27 pus discharge,29,30,31,33 sinus tract formation (Figure 1a),15,32,33 and gingival swelling.22 They can also present with no symptoms.32 In most cases, the patient had no history of dental caries, trauma, or discoloration of the teeth. Pulp vitality was retained or lost contingent upon pulpal nerve involvement. Cases with advanced lesions along deep grooves frequently show no response to thermal or electric pulp testing.15,22,30,31,32,33,34,35,36 A clinical examination shows funnel-shaped hollow grooves with an accumulation of plaque and calculus, along with loss of epithelial attachment, pocket formation, and bleeding on probing. PGG is often observed bilaterally in the oral cavity. In one study, bilateralism was found in 57.5% of total cases, 63% for coronal and 46.15% for apical grooves among 200 patients.1
Radiographically, teardrop-like radiolucency can be observed in cases with primary pulpal infection,7 and pear-shaped radiolucency (Figure 1c) can be observed in the coronal aspect with apical periodontal widening. In some cases, a radiolucent parapulpal line can be observed as one or more dark lines extending along the length of the root parallel to or superimposed over the root canal.8 In one case report, placement of a gutta-percha cone through the sulcus along the groove could delineate the course and extent of the groove.34 Meanwhile, some authors have indicated difficulties in detecting the groove in conventional radiographs because of overlapping of the structures.37,38 The type of diseases developed from PGG are various: endodontic or periodontal disease or combined endodontic-periodontal disease, resulting in diverse symptoms, including no symptoms even with advanced lesions. It is difficult and simultaneously important to determine the size and location of any bone lesion, the depth and location of the groove onto the root, and the relationship between the lesion and the groove. Three dimensional radiographs using cone beam-computed tomography (CBCT) can provide accurate, sensitive information to assess and plan the treatment of the teeth with PGG (Figure 1d).39 In 2015, Castelo-Baz et al. stated that the information acquired from the CBCT imaging, which showed that the pulp necrosis was not connected to the PGG, is important in making treatment plans, including the choice of an appropriate material to fill the canal.15 However, in consideration of the radiation exposure the patients receive, the use of CBCT should be limited to cases in which conventional imaging fails to provide adequate information.
Dental anomalies that resemble PGG include dens invaginatus, Tomes' root, and extra root variation. Each aberration has been deemed a non-typical form of the others. In the differentiation of dens invaginatus from PPG, PGG presents as a split form rather than the infolding form seen in dens invaginatus. Even in PGG cases that exhibit an infolding form, the groove is shallower than that seen with dens invaginatus. Occasionally, a groove can be detected on the labial surface of the maxillary incisors.40 The prevalence rate of the labial cervical vertical groove has been reported as 3%41 to 6.5%,42 which is lower than that reported for PGG. Accessory root formation occurs most commonly in mandibular canine, premolar, and molar teeth, but it is also rarely observed in maxillary and mandibular incisor teeth.21,43 Yavuz et al. reported a case of accessory root in a maxillary lateral incisor.21
1. Basic principle for treatment
In the past, if a tooth with PGG had both a draining sinus tract and periodontitis, extraction of the tooth was recommended because of poor prognosis.7 However, several reports have shown successful clinical outcomes despite the initial complex condition of a combined endodontic-periodontal lesion related to PGG manifested as normal pocket depth and radiographically normal state of surrounding tissue. Given those favorable clinical outcomes, conservative treatment methods to treat and retain the tooth are now recommended. The change also reflects both patients' increasing desires to retain their teeth as long as possible and difficulties in achieving proper papillary reconstruction or an esthetic outcome with dental implants for maxillary incisors. The treatment approach for teeth with PGG is based on the following three strategies: (i) complete eradication of microbials, (ii) permanent and thorough sealing of the root groove that communicates between the root canal and the periodontium, and (iii) periodontal regeneration and complete healing of the periodontium.
In 2003, Kerezoudis et al.40 summarized the treatment interventions needed in cases of relatively shallow PGG: (i) surgical removal of granulation tissue and irritants,8 (ii) a gingivectomy and apically positioned flap,3 (iii) surgical exposure and flattening of the groove by grinding, with or without application of guided tissue regeneration techniques,44 (iv) positioning a restoration in the groove,3,7 and (v) orthodontic extrusion of the tooth.
Among the various proposed treatment options, clinicians have to choose a method to eliminate the causative pathologic factor and achieve a favorable outcome. First, it is necessary to examine whether the pulp is involved in the pathologic change. If the pulp is in a pathologic state, endodontic treatment is recommended first. It is important to determine the dentin thickness of the palatal surface of the canal near the groove, which can be very thin. Careful instrumentation during canal shaping or post space preparation is needed to prevent perforation of the root canal.23
2. Treatment of mild PGG
In the mild type of PGG with physiologic mobility and shallow grooves, odontoplasty in conjunction with periodontal treatment, including gingivectomy or subgingival root planing, is recommended. The pathology caused by the groove space is expected to be eliminated by grinding the groove or sealing it with one of a variety of filling materials. ‘Saucerization’ is one method for treating the mild form of PGG that involves grinding the groove to the level of the crestal bone with a rotary cutting and polishing instrument.45
3. Treatment of complex PGG
For more complex cases, several methods have been proposed: granulation tissue removal through a flap, elimination of the defect at the level of the crestal bone using rotatory instruments (saucerization) with or without the guided tissue regeneration technique, intentional extraction of a problematic tooth to achieve complete removal of the groove and subsequent reimplantation (intentional replantation), orthodontic extrusion, and extraction. In cases with an extensive groove area, some authors have reported successful treatment outcomes with intentional replantation.29
4. Various regenerative materials currently used to fill the groove
Dragoo et al. indicated that any subgingival restoration material should include the following characteristics: (i) biocompatibility, (ii) a dual-cure set, (iii) adhesiveness, (iv) radiopacity, (v) compactness, (vi) surface hardness, (vii) insolubility in oral fluids, (viii) absence of microleakage, (ix) low coefficient of thermal expansion, and (x) low cure shrinkage.46 In addition, convenience of use and rapid setting that does not interfere with hemostasis are important properties in clinical circumstances. Several materials, including amalgam, glass ionomer cement (GIC), composite resin, and calcium silicate based-cement such as mineral trioxide aggregate (MTA), have been selected for PGG treatment.
1) Amalgam
Amalgam has antibacterial activity related to the presence of mercury, copper, and zinc.47 Along with its biocompatibility, ease of manipulation, and better hardiness to moist conditions than other materials, it has led amalgam to be widely used to fill PGG.7 Tooth discoloration from amalgam is one of its major disadvantages in PGG restoration of anterior teeth. With the development of various filling material, the frequency of use of amalgam has been decreased.
2) Glass ionomer cement (GIC)
GIC has been widely used in the restoration of PGG because of its favorable characteristics for both the tooth surface and periodontal tissues. This material is resistant to water degradation at the tooth-cement interface, shows good sealing ability through chemical bonding, and has an antibacterial effect.48 The fluoride in GIC can interfere with the initial adherence of bacteria and inhibit their metabolism and growth.49 It has also been reported that epithelial and connective tissue attachment occurred on the cement surface.50
3) Composite resin
Controversial data are available for the effects of composite resin on gingival health. Whereas some authors have shown that well-finished composite resin restorations do not damage the health of the gingiva,51 several researchers have reported that patients with composite resin restorations on a subgingival area presented with more gingival bleeding and PD associated with gingival inflammation and higher bacterial counts than patients treated with other restoration materials.52,53 Moreover, in a long-term observation, gingival inflammation adjacent to composite resin restorations increased over time with a significantly higher prevalence rate than when other materials were used.54 Increased wear and marginal leakage over time might adversely affect gingival health.
4) Calcium silicate-based cement
Recently, calcium silicate-based cements showing excellent biocompatible characteristics have been developed for the dental field. In several case reports, MTA has been used to restore the subgingival groove of a tooth. Although MTA has several favorable properties (excellent biocompatibility, sealing ability, and ability to set in moisture), difficulty in material handling and the possibility of wash-off, especially in a transgingival defect with a long setting time, make it difficult to use in PGG cases.35 Because PGG is mostly distributed from the crown area to the root of the tooth, mechanical properties that can endure intraoral conditions and biocompatibility with subgingival conditions are very important considerations. Some authors have reported a successful treatment outcome for PGG with Biodentine, describing advantages such as easy handling, a relatively short setting time of 9 to 12 minutes, improved mechanical properties, good biocompatibility, and regenerative potential (Figure 2b).2,55,56
5. Various regenerative materials currently used to fill intrabony defects
If the groove extends beyond the apical third of the root, surgical interventions are required to access the whole groove area and related lesions. For regeneration of periodontal tissues, diverse barrier or graft materials (bone grafts, platelet-rich plasma, and enamel matrix derivative) have been used, with consideration of the size of the bone defect and the presence of palatal bone loss.
1) Membrane
Attam et al. reported that a combined technique of bone graft and membrane significantly reduced the pocket depth compared with cases treated by open flap debridement (Figures 2c and 2d).35 Anderegg et al. also reported 10 cases of successful treatment after 6 month follow-up using a polytetrafluoroethylene membrane.57
2) Bone graft
To achieve guided tissue regeneration, bone graft materials have been used along with membrane in the bone defect area to enhance the bone fill. McClain et al. reported that the attachment level was more predictable when combined graft/GTR therapy was used.58 Gandhi et al. also reported the use of synthetic bone graft material in an extensive bone defect area related to PGG.31
3) Platelet-rich fibrin
Platelet rich fibrin (PRF) is an immune platelet concentrate collecting all constituents of a blood sample favorable to healing and immunity on a single fibrin membrane.59 Rajendran et al. reported that, in the treatment of PGG, PRF application along with bone graft on the bony defect area resulted in crestal bone formation after 9 month follow-up.32
4) Enamel matrix derivative
The proteins of the enamel matrix secreted by Hertwig's epithelial root sheath in the process of root formation can contribute to apposition of the acellular cementum.60 The justification for the use of enamel matrix derivative (EMD, Emdogain, Straumann, Basel, Switzerland) is to emulate this process and facilitate the role of acellular cementum in the formation and repair mechanisms of the periodontal ligament and alveolar bone.33 Hammarström et al. reported the actual formation of acellular cementum with EMD.60 EMD has also been used to prevent external root resorption and ankylosis onto the bone in cases of intentional replantation.61 Al-Hezaimi et al. reported a favorable 4 year outcome after treatment of PGG with intentional replantation accompanied by EMD.33
The prognosis for a tooth with PGG depends on several factors: the location of the groove, severity of the accompanying periodontal disease, accessibility to the defect area, and type of groove (shallow/deep or long/short).16,62 If the groove is shallow and terminates before the CEJ (confined to the crown of the tooth), the prognosis is good. Even in cases initially thought to have poor prognosis because of the severity and complexity of the disease, several authors have reported successful treatment outcomes with active and multidirectional treatment interventions.
Conclusions
PGG, a rare aberration on the maxillary anterior teeth, occasionally results in combined endodontic-periodontal disease with extensive periodontal destruction of the tooth, which is associated with poor prognosis. In the past, a tooth with PGG showing a complex lesion was regarded as hopeless, and immediate extraction was recommended. However, with the development of new materials, diagnostic tools, and understanding of the characteristics and treatment principles, many recent cases have shown successful treatment outcomes on teeth with PGG. Clinicians should recognize the existence of PGG and manage it properly based on the understanding in order to ensure survival of the tooth.
Acknowledgement
This paper was supported by the Biomedical Research Institute of the Chonbuk National University Hospital in 2017.
- 1. Iqbal N, Tirmazi SM, Majeed HA, Munir MB. Prevalence of palato gingival groove in maxillary lateral incisors. Pak Oral Dent J 2011;31:424-426.
- 2. Sharma S, Deepak P, Vivek S, Ranjan Dutta S. Palatogingival goove: recognizing and managing the hidden tract in a maxillary incisor: a case report. J Int Oral Health 2015;7:110-114.PubMedPMC
- 3. Lee KW, Lee EC, Poon KY. Palato-gingival grooves in maxillary incisors. A possible predisposing factor to localised periodontal disease. Br Dent J 1968;124:14-18.PubMed
- 4. Withers JA, Brunsvold MA, Killoy WJ, Rahe AJ. The relationship of palato-gingival grooves to localized periodontal disease. J Periodontol 1981;52:41-44.ArticlePubMed
- 5. Black GV. Operative dentistry: pathology of the hard tissues of teeth. Chicago: Medico-Dental Publishing; 1908.
- 6. Oehlers FA. The radicular variety of dens invaginatus. Oral Surg Oral Med Oral Pathol 1958;11:1251-1260.ArticlePubMed
- 7. Simon JH, Glick DH, Frank AL. Predictable endodontic and periodontic failures as a result of radicular anomalies. Oral Surg Oral Med Oral Pathol 1971;31:823-826.ArticlePubMed
- 8. Everett FG, Kramer GM. The disto-lingual groove in the maxillary lateral incisor; a periodontal hazard. J Periodontol 1972;43:352-361.ArticlePubMed
- 9. Kogon SL. The prevalence, location and conformation of palato-radicular grooves in maxillary incisors. J Periodontol 1986;57:231-234.ArticlePubMed
- 10. Mittal M, Vashisth P, Arora R, Dwivedi S. Combined endodontic therapy and periapical surgery with MTA and bone graft in treating palatogingival groove. BMJ Case Rep 2013 4 18.Article
- 11. Peikoff MD, Perry JB, Chapnick LA. Endodontic failure attributable to a complex radicular lingual groove. J Endod 1985;11:573-577.ArticlePubMed
- 12. Storrer CM, Sanchez PL, Romito GA, Pustiglioni FE. Morphometric study of length and grooves of maxillary lateral incisor roots. Arch Oral Biol 2006;51:649-654.ArticlePubMed
- 13. Al-Rasheed A. Relationship between palato-radicular groove and periodontal health in maxillary lateral incisors. Pak Oral Dent J 2011;31:154-157.
- 14. Hou GL, Tsai CC. Relationship between palato-radicular grooves and localized periodontitis. J Clin Periodontol 1993;20:678-682.ArticlePubMed
- 15. Castelo-Baz P, Ramos-Barbosa I, Martin-Biedma B, Dablanca-Blanco AB, Varela-Patiño P, Blanco-Carrión J. Combined endodontic-periodontal treatment of a palatogingival groove. J Endod 2015;41:1918-1922.ArticlePubMed
- 16. Bacić M, Karakas Z, Kaić Z, Sutalo J. The association between palatal grooves in upper incisors and periodontal complications. J Periodontol 1990;61:197-199.ArticlePubMed
- 17. Albaricci MF, de Toledo BE, Zuza EP, Gomes DA, Rosetti EP. Prevalence and features of palato-radicular grooves: an in-vitro study. J Int Acad Periodontol 2008;10:2-5.PubMed
- 18. Goon WW, Carpenter WM, Brace NM, Ahlfeld RJ. Complex facial radicular groove in a maxillary lateral incisor. J Endod 1991;17:244-248.ArticlePubMed
- 19. Schäfer E, Cankay R, Ott K. Malformations in maxillary incisors: case report of radicular palatal groove. Endod Dent Traumatol 2000;16:132-137.ArticlePubMedPDF
- 20. Peikoff MD, Trott JR. An endodontic failure caused by an unusual anatomical anomaly. J Endod 1977;3:356-359.ArticlePubMed
- 21. Yavuz MS, Keleş A, Ozgöz M, Ahmetoğlu F. Comprehensive treatment of the infected maxillary lateral incisor with an accessory root. J Endod 2008;34:1134-1137.ArticlePubMed
- 22. Sharma S, Deepak P, Vivek S, Ranjan Dutta S. Palatogingival groove: recognizing and managing the hidden tract in a maxillary incisor: a case report. J Int Oral Health 2015;7:110-114.PubMedPMC
- 23. Gu YC. A micro-computed tomographic analysis of maxillary lateral incisors with radicular grooves. J Endod 2011;37:789-792.ArticlePubMed
- 24. Smith BE, Carroll B. Maxillary lateral incisor with two developmental grooves. Oral Surg Oral Med Oral Pathol 1990;70:523-525.Article
- 25. Nanba K, Ito K. Palatal radicular multigrooves associated with severe periodontal defects in maxillary central incisors. J Clin Periodontol 2001;28:372-375.ArticlePubMedPDF
- 26. Gao ZR, Shi JN, Wang Y, Gu FY. Scanning electron microscopic investigation of maxillary lateral incisors with a radicular lingual groove. Oral Surg Oral Med Oral Pathol 1989;68:462-466.ArticlePubMed
- 27. Vipin A, Srinivas SR, Pooja A, Jithendra KD, Shashit S. Surgical management of a palatal radicular groove and an associated periodontal lesion in a maxillary lateral incisor. J Pierre Fauchard Acad (India Section) 2009;23:104-106.Article
- 28. Suchetha A, Heralgi R, Bharwani AG, Mundinamane D. Treatment of an intrabony osseous lesion associated with a palatoradicular groove. Contemp Clin Dent 2012;3:S260-S263.PubMedPMC
- 29. Forero-López J, Gamboa-Martinez L, Pico-Porras L, Niño-Barrera JL. Surgical management with intentional replantation on a tooth with palato-radicular groove. Restor Dent Endod 2015;40:166-171.ArticlePubMed
- 30. Ballal NV, Jothi V, Bhat KS, Bhat KM. Salvaging a tooth with a deep palatogingival groove: an endo-perio treatment-a case report. Int Endod J 2007;40:808-817.ArticlePubMed
- 31. Gandhi A, Yadav P, Gandhi T. Endodontic-periodontal management of a maxillary lateral incisor with an associated radicular lingual groove and severe periapical osseous destruction-a case report. J Ir Dent Assoc 2012;58:95-100.PubMed
- 32. Rajendran M, Sivasankar K. Palato-radicular groove-hidden route to destruction-case report. Int J Curr Res Rev 2013;5:85-92.
- 33. Al-Hezaimi K, Naghshbandi J, Simon JH, Rotstein I. Successful treatment of a radicular groove by intentional replantation and Emdogain therapy: four years follow-up. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:e82-e85.ArticlePubMed
- 34. Schwartz SA, Koch MA, Deas DE, Powell CA. Combined endodontic-periodontic treatment of a palatal groove: a case report. J Endod 2006;32:573-578.ArticlePubMed
- 35. Attam K, Tiwary R, Talwar S, Lamba AK. Palatogingival groove: endodontic-periodontal management-case report. J Endod 2010;36:1717-1720.ArticlePubMed
- 36. Kishan KV, Hegde V, Ponnappa KC, Girish TN, Ponappa MC. Management of palato radicular groove in a maxillary lateral incisor. J Nat Sci Biol Med 2014;5:178-181.ArticlePubMedPMC
- 37. Kozlovsky A, Tal H, Yechezkiely N, Mozes O. Facial radicular groove in a maxillary central incisor. A case report. J Periodontol 1988;59:615-617.PubMed
- 38. Estrela C, Pereira HL, Pécora JD. Radicular grooves in maxillary lateral incisor: case report. Braz Dent J 1995;6:143-146.PubMed
- 39. Durack C, Patel S. The use of cone beam computed tomography in the management of dens invaginatus affecting a strategic tooth in a patient affected by hypodontia: a case report. Int Endod J 2011;44:474-483.ArticlePubMed
- 40. Kerezoudis NP, Siskos GJ, Tsatsas V. Bilateral buccal radicular groove in maxillary incisors: case report. Int Endod J 2003;36:898-906.ArticlePubMedPDF
- 41. Kovacs I. A systematic description of dental roots. In: Dahlberg AA, editor. Dental morphology and evolution. Chicago and London: The University of Chicago Press; 1971. p. 211-256.
- 42. Brin I, Ben-Bassat Y. Appearance of a labial notch in maxillary incisors: a population survey. Am J Phys Anthropol 1989;80:25-29.ArticlePubMed
- 43. Wei PC, Geivelis M, Chan CP, Ju YR. Successful treatment of pulpal-periodontal combined lesion in a birooted maxillary lateral incisor with concomitant palato-radicular groove. A case report. J Periodontol 1999;70:1540-1546.ArticlePubMed
- 44. Rankow HJ, Krasner PR. Endodontic applications of guided tissue regeneration in endodontic surgery. J Endod 1996;22:34-43.ArticlePubMed
- 45. Jeng JH, Lu HK, Hou LT. Treatment of an osseous lesion associated with a severe palato-radicular groove: a case report. J Periodontol 1992;63:708-712.ArticlePubMed
- 46. Dragoo MR. Resin-ionomer and hybrid-ionomer cements: part I. comparison of three materials for the treatment of subgingival root lesions. Int J Periodontics Restorative Dent 1996;16:594-601.PubMed
- 47. Morrier JJ, Suchett-Kaye G, Nguyen D, Rocca JP, Blanc-Benon J, Barsotti O. Antimicrobial activity of amalgams, alloys and their elements and phases. Dent Mater 1998;14:150-157.ArticlePubMed
- 48. Vermeersch G, Leloup G, Delmée M, Vreven J. Antibacterial activity of glass-ionomer cements, compomers and resin composites: relationship between acidity and material setting phase. J Oral Rehabil 2005;32:368-374.ArticlePubMed
- 49. Paolantonio M, D'ercole S, Perinetti G, Tripodi D, Catamo G, Serra E, Bruè C, Piccolomini R. Clinical and microbiological effects of different restorative materials on the periodontal tissues adjacent to subgingival class V restorations. J Clin Periodontol 2004;31:200-207.ArticlePubMed
- 50. Dragoo MR. Resin-ionomer and hybrid-ionomer cements: part II. human clinical and histologic wound healing responses in specific periodontal lesions. Int J Periodontics Restorative Dent 1997;17:75-87.PubMed
- 51. Blank LW, Caffesse RG, Charbeneau GT. The gingival response to well-finished composite resin restorations. J Prosthet Dent 1979;42:626-632.ArticlePubMed
- 52. Larato DC. Influence of a composite resin restoration on the gingiva. J Prosthet Dent 1972;28:402-404.ArticlePubMed
- 53. Willershausen B, Köttgen C, Ernst CP. The influence of restorative materials on marginal gingiva. Eur J Med Res 2001;6:433-439.PubMed
- 54. van Dijken JW, Sjöström S, Wing K. The effect of different types of composite resin fillings on marginal gingiva. J Clin Periodontol 1987;14:185-189.ArticlePubMed
- 55. Liji MP, Rameshkumar M. Integration of PRF and biodentine in palatogingival groove case. IOSR J Dent Med Sci 2013;6:26-30.Article
- 56. Johns DA, Shivashankar VY, Shobha K, Johns M. An innovative approach in the management of palatogingival groove using Biodentine and platelet-rich fibrin membrane. J Conserv Dent 2014;17:75-79.ArticlePubMedPMC
- 57. Anderegg CR, Metzler DG. Treatment of the palatogingival groove with guided tissue regeneration. Report of 10 cases. J Periodontol 1993;64:72-74.ArticlePubMed
- 58. McClain PK, Schallhorn RG. Long-term assessment of combined osseous composite grafting, root conditioning, and guided tissue regeneration. Int J Periodontics Restorative Dent 1993;13:9-27.PubMed
- 59. Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:e56-e60.ArticlePubMed
- 60. Hammarström L. Enamel matrix, cementum development and regeneration. J Clin periodontol 1997;24:658-668.ArticlePubMed
- 61. Drain DE, Petrone JA. Intentional replantation: a case report and review of the literature. J N J Dent Assoc 1995;66:63-65.
- 62. Lara VS, Consolaro A, Bruce RS. Macroscopic and microscopic analysis of the palato-gingival groove. J Endod 2000;26:345-350.ArticlePubMed
REFERENCES
Figure 1
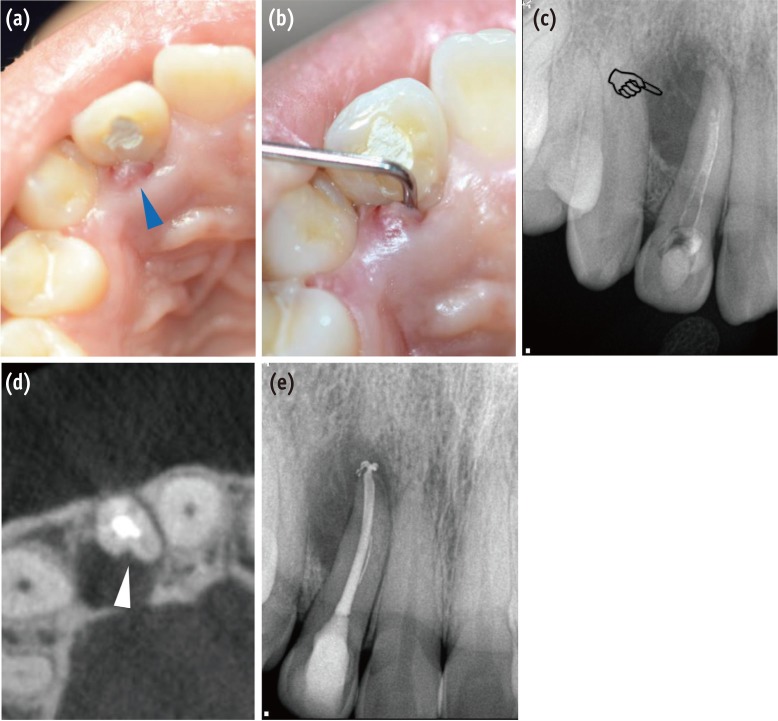
Clinical and radiographic findings for diagnosis of the periradicular pathosis associated with PGG. (a) A sinus tract was observed on the palatal side of the right maxillary lateral incisor (blue triangle); (b) Deep and narrow pocket depth (> 10 mm) was detected on the palatal side along the root in the vertical direction; (c) A diagnostic periapical radiograph shows pear-shaped or tear-drop-like radiolucency in the coronal aspect of the root apex (pointing finger); (d) A CBCT scan shows a groove with adjacent radiolucency on the palatal aspect of the tooth (white triangle). It is strongly suspected that the pathogen was associated primarily with the PGG; (e) Endodontic treatment was performed to eliminate the possible irritant in the root canal space. PGG, palatogingival groove; CBCT, cone beam-computed tomography.

Figure 2
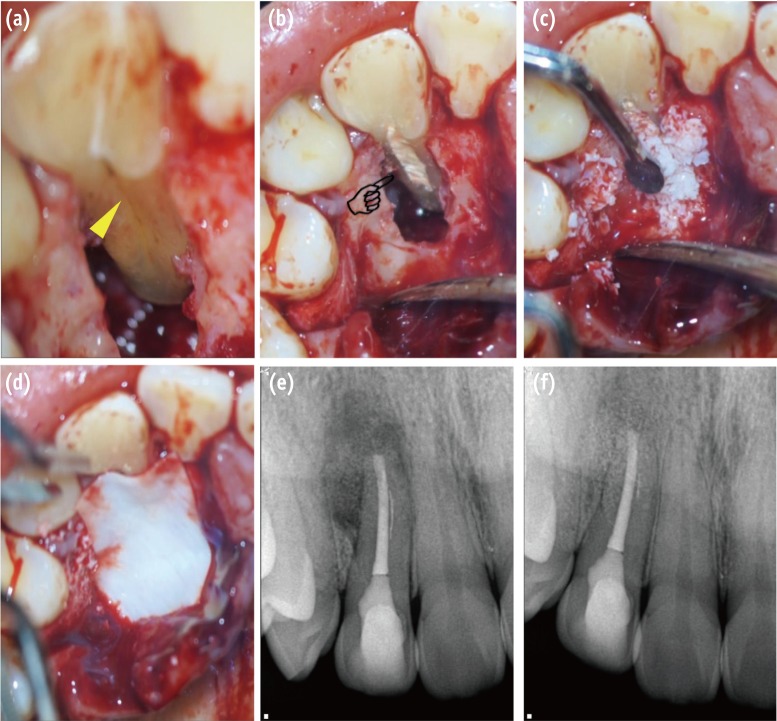
Surgical procedure for management of PGG-related periradicular pathosis. (a) PGG is verified along the root surface to the region of the apical third (yellow triangle); (b) The PGG was filled with Biodentine after saucerization (pointing finger); (c) The bony defect was filled with artificial bone graft material; (d) The bone-filled lesion was covered by an absorbable membrane; (e) Post-operative radiograph; (f) 8 month follow-up radiograph reveals healing of the periradicular lesion. PGG, palatogingival groove.

Table 1
Classifications of palatogingival groove
| Classification | Feature |
|---|---|
| Location of groove9 | 1) Distal |
| 2) Mesial | |
| 3) Central (or midpalatal) | |
| Extent and complexity of groove18 | 1) Mild: the grooves are gentle depressions of the coronal enamel that terminate at or immediately after crossing the CEJ |
| 2) Moderate: the grooves extend some distance apically along the root surface in the form of a shallow or fissured defect | |
| 3) Complex: the grooves are deeply invaginated defects that involve the entire length of the root or that separate an accessory root from the main root trunk | |
| Degree of invagination of the groove towards the pulp cavity9 | 1) Shallow/flat (< 1 mm) |
| 2) Deep (> 1 mm) | |
| 3) Closed tube | |
| Degree of severity based on microcomputed tomography studies23 | 1) Type I: the groove is short (not beyond the coronal third of the root) |
| 2) Type II: the groove is long (beyond the coronal third of the root) but shallow, corresponding to a normal or simple root canal | |
| 3) Type III: the groove is long (beyond the coronal third of the root) and deep, corresponding to a complex root canal system |
Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Prevalence of Palatal Grooves on Maxillary Anterior Teeth Using Cone-beam Computed Tomography: A Systematic Review and Meta-Analysis
Oscar Lozano González, Marco Felipe Salas Orozco, Nuria Patiño Marín, Paul V. Abbott, Marc Garcia-Font, Francesc Abella Sans
Journal of Endodontics.2026; 52(1): 14. CrossRef - Endodontic bioceramics: current and futurity aspects
Roma M, Karthik Shetty, Laxmish Mallya, Krishna Prasad Shetty
Frontiers in Oral Health.2026;[Epub] CrossRef - A Unified Deep Learning Framework for Visual Diagnosis of Palatal Radicular Grooves in CBCT Scans: A Multicenter Validation Study
Qikui Zhu, Weitao Fu, Yeyu Lin, Jiaxing Li, Wenhui Tang, Ying Zhang, Rui Zhang, Guanfan Lu, Yao Lin, Jing Shen, Zhuan Bian, Liuyan Meng
Journal of Endodontics.2026;[Epub] CrossRef - Endodontic and Periodontal Treatment of a Two‐Rooted Maxillary Lateral Incisor With a Type III Palatoradicular Groove: A Case Report With 2‐Year Follow‐Up
Katsuhiro Takeda, Tomoya Naruse, Yohei Takahashi, Reina Kawai, Kimiaki Yuhi, Hideki Shiba, Barbara Lapinska
Case Reports in Dentistry.2026;[Epub] CrossRef - Three-year follow-up case report: root canal treatment combined with intentional replantation for treating type III palatogingival groove in a maxillary lateral incisor
Jixu Jia, Miao Cheng, Sumeng Shi, Yanchun Qiao
Frontiers in Oral Health.2025;[Epub] CrossRef - Prevalence of palatogingival groove and its association with periapical lesions and periodontal bone loss: a cone beam computed tomography study
Dilan Pelin Yildirim, Selin Goker Kamali
BMC Oral Health.2025;[Epub] CrossRef - Evaluation of Morphology and Prevalence of Palatoradicular Grooves on Affected Maxillary Anterior Teeth Using Cone-Beam Computed Tomography: An Institutional Retrospective Study
Dilara Baştuğ, Leyla Benan Ayrancı
Applied Sciences.2025; 15(14): 8031. CrossRef - Sulco palato-gengival e suas consequências: Revisão de literatura
Marielli de Paula Gonçalves, Maria Júlia Ribeiro Chalita Vieira, Mikaelly Kawany Martins da Silva, Fabiana Tavares Lunardi Palhari, Maria Isabel Gonçalves Fialho
Research, Society and Development.2025; 14(8): e5014849388. CrossRef - Credibility of Intentional Reimplantation Techniques for Periodontally Compromised Teeth: A Report of Two Cases
Satarupa Suklabaidya, Ilakiya Mathi, Kennedy Babu, Gandhimadhi D, Manoj Margabandhu
Cureus.2025;[Epub] CrossRef - Prevalence of Palatal Radicular Groove in upper Lateral Incisors: A CBCT study at Isfahan Azad dental school
Amirreza Zefreh, Azadeh Torkzadeh, Hajar Shekarchizadeh, Maryam Zare Jahromi, Rojin Ardalani
Contemporary Orofacial Science.2025;[Epub] CrossRef - A classification of radicular grooves from the perspective of periodontology
Huxiao Li, Zhaowei Tai, Jiachen Dong, Zhongchen Song
BMC Oral Health.2025;[Epub] CrossRef - Advancements in Root Canal Therapy: Translational Innovations and the Role of Nanoparticles in Endodontic Treatment
Noha M. Badawi, Mohamed M. Kataia, Hadeel A. Mousa, Mozhgan Afshari
Journal of Nanotechnology.2025;[Epub] CrossRef - Cone-beam computed tomographic evaluation to estimate the prevalence of palatogingival groove in the maxillary anterior teeth and its radiographic characteristics: An institutional retrospective study
Mousumi Biswas, Dibyendu Mazumdar, Binayak Saha, Siddhi Agarwala, Kallol Kumar Saha, Kuntal Chowdhury
Journal of Conservative Dentistry and Endodontics.2024; 27(3): 233. CrossRef - A Three-Dimensional Assessment of a Type I Shallow Palatogingival Groove by Cone Beam Computed Tomography: A Case Report
Ramachandra Reddy Gowda Venkatesha, Karthik Rajaram Mohan, Saramma Mathew Fenn, Sabitha Gokulraj, Kumar Appusamy
Cureus.2024;[Epub] CrossRef - Diagnostic Approaches of Palatogingival Groove: A Systematic Review
Greta Venskutė
Journal of Dental Health and Oral Research.2024; : 1. CrossRef - Palatal groove associated with periodontal lesions: a systematic review illustrated by a decisional tree for management
Yvan Gaudex, Vianney Gandillot, Isabelle Fontanille, Philippe Bouchard, Stephane Kerner, Maria Clotilde Carra
BMC Oral Health.2024;[Epub] CrossRef - Palatogingival Groove: The Known–unknown Devourer
Sandeep Tandon, Rinku Mathur, Ambika S Rathore, Tripti S Rai, Kanchan Kumari Dhaker, Sumedha Gupta
International Journal of Clinical Pediatric Dentistry.2024; 17(S1): S95. CrossRef - Nomogram to predict radicular grooves in maxillary lateral incisors in preoperative orthodontic population
Xiuneng Zhou, Jie Deng, Nianke Liu, Chunhui Yang, Shiyu Li, Yaling Song
Clinical Oral Investigations.2024;[Epub] CrossRef - Management of Palatogingival Groove in Maxillary Lateral Incisor: A Report of a Rare Case With a Brief Review of Literature
Irfan Ansari, Sanjay Miglani, Vijay Yadav, Shamimul Hasan
Cureus.2023;[Epub] CrossRef - Prevalence of palatogingival groove affecting maxillary anterior teeth in Saudi subpopulation: A cone-beam computed tomographic study with literature review
Ali Ibrahim Aljuailan, Roqayah Aljuailan, Rahul N. Gaikwad, Shaul Hameed Kolarkodi, Nasser Rufaydan Alamri
The Saudi Dental Journal.2023; 35(8): 1039. CrossRef - Bioceramics in Endodontics: Updates and Future Perspectives
Xu Dong, Xin Xu
Bioengineering.2023; 10(3): 354. CrossRef - Interdisciplinary approach for diagnosis and management of the tooth with type III palatogingival groove
Harakh Chand Baranwal, Jyoti Yadav
Saudi Endodontic Journal.2023; 13(2): 211. CrossRef - Progress in Diagnosis and Treatment of Palatogingival Groove
倩 郑
Advances in Clinical Medicine.2022; 12(04): 2723. CrossRef - Palatogingival grooves associated with periodontal bone Loss of maxillary incisors in a Chinese population
Rui Zhang, Jie Xiong, Markus Haapasalo, Ya Shen, Liuyan Meng
Australian Endodontic Journal.2022; 48(2): 313. CrossRef - Surgical management of lateral lesions with intentional replantation in single-rooted mandibular first premolars with radicular groove
Ya-Hsin Yu, Minje Kim, Samuel Kratchman, Bekir Karabucak
The Journal of the American Dental Association.2022; 153(4): 371. CrossRef - Management of the palato-radicular groove with a periodontal regenerative procedure and prosthodontic treatment: A case report
Dan-Hua Ling, Wei-Ping Shi, Yan-Hong Wang, Dan-Ping Lai, Yan-Zhen Zhang
World Journal of Clinical Cases.2022; 10(17): 5732. CrossRef - Combined Periodontal and Endodontic Management of Palatal Radicular Groove with Platelet‐Rich Fibrin and Biodentine®
Arjun Hari Rijal, Bhageshwar Dhami, Pratistha Ghimire, Konstantinos Michalakis
Case Reports in Dentistry.2022;[Epub] CrossRef - Intentional replantation combined root resection therapy for the treatment of type III radicular groove with two roots: A case report
Dan Tan, Shi-Ting Li, Hao Feng, Zhong-Chao Wang, Cai Wen, Min-Hai Nie
World Journal of Clinical Cases.2022; 10(20): 6991. CrossRef - DENTAL DEFECTS WITH SUBGINGIVAL EXTENSION: A RESTORATIVE CONUNDRUM
Seema Yadav
INTERNATIONAL JOURNAL OF SCIENTIFIC RESEARCH.2021; : 20. CrossRef - Misdiagnosis or Missed Diagnosis? Cone-Beam Computed Tomography-Aided Multidisciplinary Management of Maxillary Central Incisor with Palatogingival Groove
R. Kurinji Amalavathy, K.M. Vidya, Sonali Nabil Sarooshi, Hrudi Sundar Sahoo
Indian Journal of Dental Sciences.2021; 13(1): 46. CrossRef - Root and Root Canal Morphology: Study Methods and Classifications
Duaa M Shihab , Anas F Mahdee
Journal of Baghdad College of Dentistry.2021; 33(4): 11. CrossRef - Prevalence and radiological characteristics of palatogingival groove: A retrospective cone-beam computed tomography study in an Indian cohort
MS Lekshmi, Sheetal Sharma, ShaliniR Gupta, Sidhartha Sharma, Vijay Kumar, Amrita Chawla, Ajay Logani
Journal of Conservative Dentistry.2021; 24(4): 359. CrossRef - Successful Multidisciplinary Management of an Endodontic‐Periodontal Lesion Associated With a Palato‐Radicular Groove: A Case Report
Diksha Katwal, Jennifer K. Fiorica, Jane Bleuel, Stephen J. Clark
Clinical Advances in Periodontics.2020; 10(2): 88. CrossRef - Anatomical, microbiological, and genetic considerations in treatment of Chinese periodontal patients
Edwin X. J. Goh, Marianne M. A. Ong
Journal of Investigative and Clinical Dentistry.2019;[Epub] CrossRef - A new system for classifying tooth, root and canal anomalies
H. M. A. Ahmed, P. M. H. Dummer
International Endodontic Journal.2018; 51(4): 389. CrossRef
Recognition and management of palatogingival groove for tooth survival: a literature review
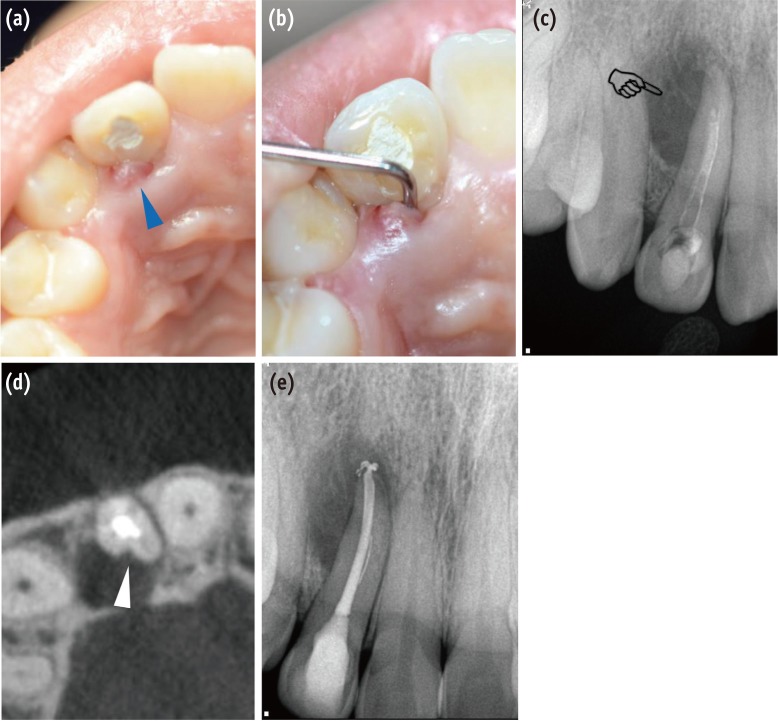
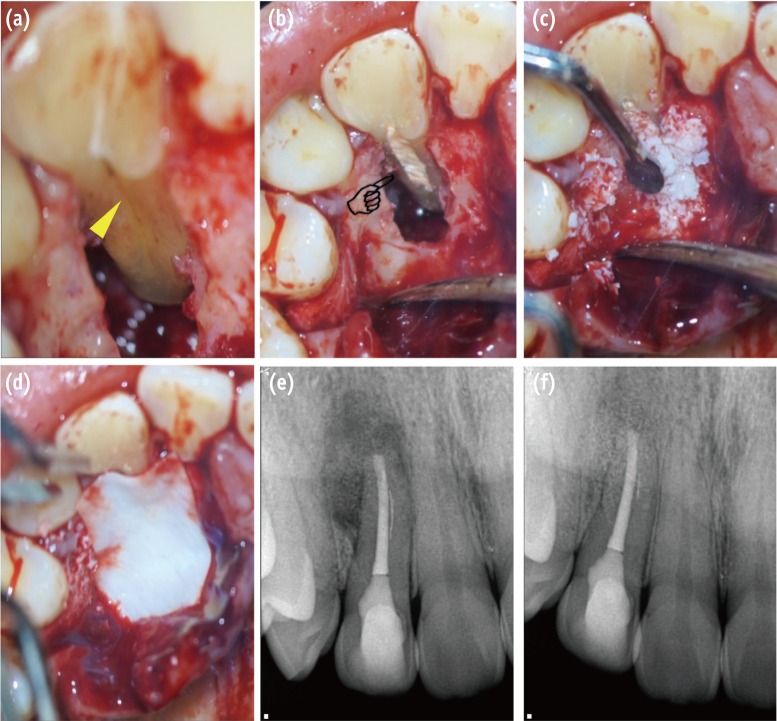
Figure 1 Clinical and radiographic findings for diagnosis of the periradicular pathosis associated with PGG. (a) A sinus tract was observed on the palatal side of the right maxillary lateral incisor (blue triangle); (b) Deep and narrow pocket depth (> 10 mm) was detected on the palatal side along the root in the vertical direction; (c) A diagnostic periapical radiograph shows pear-shaped or tear-drop-like radiolucency in the coronal aspect of the root apex (pointing finger); (d) A CBCT scan shows a groove with adjacent radiolucency on the palatal aspect of the tooth (white triangle). It is strongly suspected that the pathogen was associated primarily with the PGG; (e) Endodontic treatment was performed to eliminate the possible irritant in the root canal space. PGG, palatogingival groove; CBCT, cone beam-computed tomography.
Figure 2 Surgical procedure for management of PGG-related periradicular pathosis. (a) PGG is verified along the root surface to the region of the apical third (yellow triangle); (b) The PGG was filled with Biodentine after saucerization (pointing finger); (c) The bony defect was filled with artificial bone graft material; (d) The bone-filled lesion was covered by an absorbable membrane; (e) Post-operative radiograph; (f) 8 month follow-up radiograph reveals healing of the periradicular lesion. PGG, palatogingival groove.
Figure 1
Figure 2
Recognition and management of palatogingival groove for tooth survival: a literature review
Classifications of palatogingival groove
| Classification | Feature |
|---|---|
| Location of groove | 1) Distal |
| 2) Mesial | |
| 3) Central (or midpalatal) | |
| Extent and complexity of groove | 1) Mild: the grooves are gentle depressions of the coronal enamel that terminate at or immediately after crossing the CEJ |
| 2) Moderate: the grooves extend some distance apically along the root surface in the form of a shallow or fissured defect | |
| 3) Complex: the grooves are deeply invaginated defects that involve the entire length of the root or that separate an accessory root from the main root trunk | |
| Degree of invagination of the groove towards the pulp cavity | 1) Shallow/flat (< 1 mm) |
| 2) Deep (> 1 mm) | |
| 3) Closed tube | |
| Degree of severity based on microcomputed tomography studies | 1) Type I: the groove is short (not beyond the coronal third of the root) |
| 2) Type II: the groove is long (beyond the coronal third of the root) but shallow, corresponding to a normal or simple root canal | |
| 3) Type III: the groove is long (beyond the coronal third of the root) and deep, corresponding to a complex root canal system |
Table 1 Classifications of palatogingival groove

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

