Articles
- Page Path
- HOME > Restor Dent Endod > Volume 42(4); 2017 > Article
- Research Article Evaluation of antimicrobial activity and efficacy of herbal oils and extracts in disinfection of gutta percha cones before obturation
-
Chetana S. Makade
 , Pratima R. Shenoi
, Pratima R. Shenoi , Elakshi Morey
, Elakshi Morey , Ameya V. Paralikar
, Ameya V. Paralikar
-
2017;42(4):-272.
DOI: https://doi.org/10.5395/rde.2017.42.4.264
Published online: October 30, 2017
Department of Conservative Dentistry and Endodontics, VSPM Dental College & Research Centre, Nagpur, MH, India.
- Correspondence to Chetana S. Makade, MDS. Associate Professor, Department of Conservative Dentistry and Endodontics, VSPM Dental College & Research Centre, Digdoh Hills, Hingna Road, Nagpur, MH 4400019, India. Tel: +91-937-269-0962, Fax: +91-7104-232904/5, makade.chetana@gmail.com
Copyright © 2017. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,751 Views
- 13 Download
- 9 Crossref
Abstract
-
Objectives Literature has shown that micro-organisms contaminate gutta percha (GP) during storage and manipulation. Till date herbal extracts are not explored as an alternative medicament for pre-operative chairside disinfection of GP cones. The purpose of our study was to evaluate the antimicrobial activity and efficacy of lemon grass oil (LG), basil oil (BO), and obicure tea extract (OT) in disinfecting GP cones before obturation.
-
Materials and Methods Agar diffusion method was used to evaluate the antimicrobial efficacy of LG, BO, OT, and sodium hypochlorite (control) against common contaminants, namely, Enterococcus faecalis, Staphylococcus aureus, and Candida albicans. One hundred and twenty GP cones were contaminated and cut into 2. First half was placed in the broth and incubated; whereas the second was treated with herbal extracts for 1 minute and then incubated for 24 hours in the broth. Any inhibition in bacterial growth was noted with presence/absence of turbidity. Two-way analysis of variance and χ2 test were used to assess the effectiveness of herbal extracts to decontaminate GP.
-
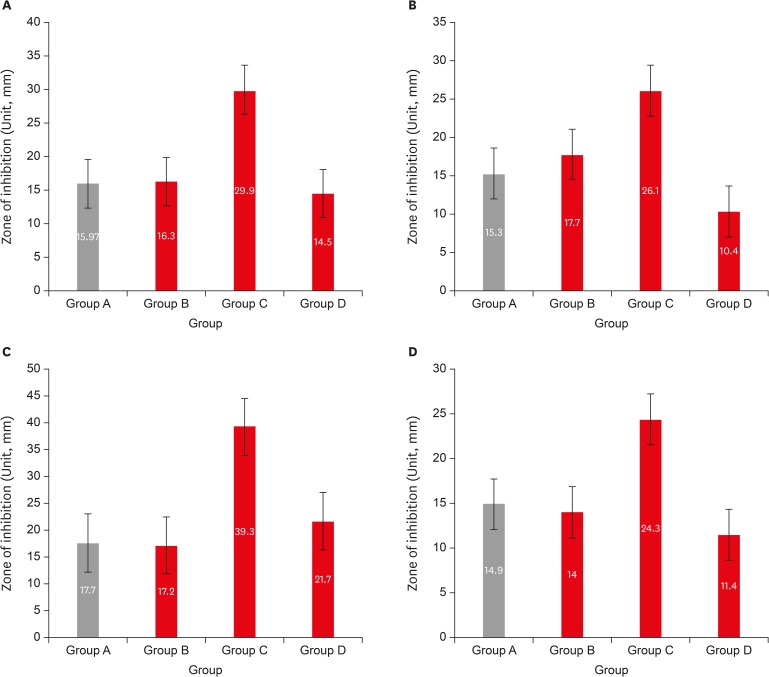
Results LG showed the highest inhibition zones (29.9 ± 6.9 mm) for all tested organisms, followed by OT extract (16.3 ± 1.8 mm), sodium hypochlorite (16.0 ± 1.6 mm), and BO (14.5 ± 5.3 mm). Statistically significant difference was observed between LG and other herbal extracts (p < 0.05).
-
Conclusions All extracts proved to be potential rapid chairside disinfectants of GP cones with LG showing the highest antimicrobial activity.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
Descriptive statistics for tested organisms by 4 comparison groups (n = 10)
Two-way analysis of variance (ANOVA) for tested organisms and medicaments
Efficacy of herbal extracts in decontamination of gutta percha (GP) cones (n = 30)
| Turbidity absent | Group A (control) | Group B (OT) | Group C (LG) | Group D (BO) | χ2 test | p value |
|---|---|---|---|---|---|---|
| No. (%) | 26 (86.7) | 25 (83.3) | 30 (100.0) | 28 (93.3) | 5.90 | 0.110 (NS) |
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENT
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Makade CS, Shenoi PR.
Data curation: Makade CS, Morey E.
Formal analysis: Makade CS, Paralikar AV.
Funding acquisition: Makade CS, Shenoi PR.
Investigation: Makade CS.
Methodology: Makade CS, Shenoi PR, Morey E.
Project administration: Makade CS.
Resources: Makade CS.
Software: Makade CS, Paralikar AV.
Supervision: Shenoi PR.
Validation: Makade CS, Shenoi PR.
Visualization: Makade CS, Shenoi PR.
Writing - original draft: Makade CS.
Writing - review & editing: Makade CS, Shenoi PR, Morey E, Paralikar AV.
- 1. Siqueira JF Jr, da Silva CH. Cerqueira M das D, Lopes HP, de Uzeda M. Effectiveness of four chemical solutions in eliminating Bacillus subtilis spores on gutta-percha cones. Endod Dent Traumatol 1998;14:124-126.PubMed
- 2. Moorer WR, Genet JM. Evidence for antibacterial activity of endodontic gutta-percha cones. Oral Surg Oral Med Oral Pathol 1982;53:503-507.ArticlePubMed
- 3. Higgins JR, Newton CW, Palenik CJ. The use of paraformaldehyde powder for the sterile storage of gutta-percha cones. J Endod 1986;12:242-248.ArticlePubMed
- 4. da Motta PG, de Figueiredo CB, Maltos SM, Nicoli JR, Ribeiro Sobrinho AP, Maltos KL, Carvalhais HP. Efficacy of chemical sterilization and storage conditions of gutta-percha cones. Int Endod J 2001;34:435-439.ArticlePubMedPDF
- 5. Panuganti V, Vivek VJ, Jayashankara CM, Anilkumar S, Girish SA, Nanjundasett JK. Gutta-percha disinfection: a knowledge, attitude, and practice study among endodontic postgraduate students in India. Saudi Endod J 2016;6:127-130.Article
- 6. Subha N, Prabhakar V, Koshy M, Abinaya K, Prabu M, Thangavelu L. Efficacy of peracetic acid in rapid disinfection of Resilon and gutta-percha cones compared with sodium hypochlorite, chlorhexidine, and povidone-iodine. J Endod 2013;39:1261-1264.ArticlePubMed
- 7. Chandrappa MM, Mundathodu N, Srinivasan R, Nasreen F, Kavitha P, Shetty A. Disinfection of gutta-percha cones using three reagents and their residual effects. J Conserv Dent 2014;17:571-574.ArticlePubMedPMC
- 8. Hamza MO, Gufran K, Baroudi K. Assessment of the potential of CFC (Calcium hydroxide Flagyl Ciprofloxacin) for the rapid disinfection of Resilon and gutta-percha. J Clin Diagn Res 2015;9:ZC40-ZC43.Article
- 9. Senia ES, Marraro RV, Mitchell JL, Lewis AG, Thomas L. Rapid sterilization of gutta-percha cones with 5.25% sodium hypochlorite. J Endod 1975;1:136-140.ArticlePubMed
- 10. Short RD, Dorn SO, Kuttler S. The crystallization of sodium hypochlorite on gutta-percha cones after the rapid-sterilization technique: an SEM study. J Endod 2003;29:670-673.ArticlePubMed
- 11. Pallotta RC, Ribeiro MS, de Lima Machado ME. Determination of the minimum inhibitory concentration of four medicaments used as intracanal medication. Aust Endod J 2007;33:107-111.ArticlePubMed
- 12. Nabeshima CK, Machado ME, Britto ML, Pallotta RC. Effectiveness of different chemical agents for disinfection of gutta-percha cones. Aust Endod J 2011;37:118-121.ArticlePubMed
- 13. Gomes BP, Vianna ME, Matsumoto CU, Rossi Vde P, Zaia AA, Ferraz CC, Souza Filho FJ. Disinfection of gutta-percha cones with chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:512-517.ArticlePubMed
- 14. Pang NS, Jung IY, Bae KS, Baek SH, Lee WC, Kum KY. Effects of short-term chemical disinfection of gutta-percha cones: identification of affected microbes and alterations in surface texture and physical properties. J Endod 2007;33:594-598.ArticlePubMed
- 15. Kumar G, Jalaluddin M, Rout P, Mohanty R, Dileep CL. Emerging trends of herbal care in dentistry. J Clin Diagn Res 2013;7:1827-1829.PubMedPMC
- 16. Rajesvari R, Lakshmi T. Lemon grass oil for improvement of oral health. Dent Hypotheses 2013;4:115-117.Article
- 17. Shenoi PR, Morey ES, Makade C, Gunwal MK, Wanmali SS. To evaluate the antimicrobial activity of herbal extracts and their efficacy in disinfecting gutta percha cones before obturation-an in vitro study. J Med Sci Clin Res 2014;2:2676-2684.
- 18. Athiban PP, Borthakur BJ, Ganesan S, Swathika B. Evaluation of antimicrobial efficacy of Aloe vera and its effectiveness in decontaminating gutta percha cones. J Conserv Dent 2012;15:246-248.ArticlePubMedPMC
- 19. Falcão MA, Fianco AL, Lucas AM, Pereira MA, Torres FC, Vargas RM, Cassel E. Determination of antibacterial activity of vacuum distillation fractions of lemongrass essential oil. Phytochem Rev 2012;11:405-412.ArticlePDF
- 20. Garcia LS, Isenberg HD. Clinical microbiology procedures handbook ??volume 1. 3rd ed. Washington, D.C.: ASM Press; 2010.
- 21. Gomes BP, Pedroso JA, Jacinto RC, Vianna ME, Ferraz CC, Zaia AA, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of five root canal sealers. Braz Dent J 2004;15:30-35.ArticlePubMed
- 22. Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod 2006;32:93-98.ArticlePubMed
- 23. Love RM. Enterococcus faecalis--a mechanism for its role in endodontic failure. Int Endod J 2001;34:399-405.ArticlePubMedPDF
- 24. Waltimo TM, Haapasalo M, Zehnder M, Meyer J. Clinical aspects related to endodontic yeast infections. Endod Topics 2004;9:66-78.Article
- 25. Baumgartner JC, Siqueira JF Jr, Sedgley CM, Kishen A. Microbiology of endodontic disease. In: Ingle JI, Bakland LK, Baumgartner JC, editors. Ingle's endodontics. 6th ed. Hamilton: BC Decker; 2008. p. 221-308.
- 26. Street RA, Prinsloo G. Commercially important medicinal plants of South Africa: a review. J Chem 2013;2013:205048.ArticlePDF
- 27. Jaikaria A, Thakur S, Jayam C. Natural products used in dentistry - a review. Int J Oral Health Dent 2016;2:209-212.
- 28. Saddiq AA, Khayyat SA. Chemical and antimicrobial studies of monoterpene: citral. Pestic Biochem Physiol 2010;98:89-93.Article
- 29. Silva CB, Guterres SS, Weisheimer V, Schapoval EE. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz J Infect Dis 2008;12:63-66.ArticlePubMed
- 30. Tyagi AK, Malik A. Liquid and vapour-phase antifungal activities of selected essential oils against Candida albicans: microscopic observations and chemical characterization of Cymbopogon citratus . BMC Complement Altern Med 2010;10:65.ArticlePubMedPMCPDF
- 31. Naik MI, Fomda BA, Jaykumar E, Bhat JA. On antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacteria. Asian Pac J Trop Med 2010;3:535-538.
- 32. Suppakul P, Miltz J, Sonneveld K, Bigger SW. Antimicrobial properties of basil and its possible application in food packaging. J Agric Food Chem 2003;51:3197-3207.ArticlePubMed
- 33. Hossain MA, Kabir MJ, Salehuddin SM, Rahman SM, Das AK, Singha SK, Alam MK, Rahman A. Antibacterial properties of essential oils and methanol extracts of sweet basil Ocimum basilicum occurring in Bangladesh. Pharm Biol 2010;48:504-511.ArticlePubMed
- 34. Elgayyar M, Draughon FA, Golden DA, Mount JR. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J Food Prot 2001;64:1019-1024.ArticlePubMedPDF
- 35. Hovijitra RS, Choonharuangdej S, Srithavaj T. Effect of essential oils prepared from Thai culinary herbs on sessile Candida albicans cultures. J Oral Sci 2016;58:365-371.PubMed
- 36. Burt S. Essential oils: their antibacterial properties and potential applications in foods--a review. Int J Food Microbiol 2004;94:223-253.ArticlePubMed
- 37. Prabhakar J, Senthilkumar M, Priya MS, Mahalakshmi K, Sehgal PK, Sukumaran VG. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: an in vitro study. J Endod 2010;36:83-86.ArticlePubMed
- 38. Shiva Rani SK, Saxena N. Udaysree. Antimicrobial activity of black pepper (Piper nigrum L). Glob J Pharmacol 2013;7:87-90.
- 39. Roder E. The synergistic and individual anti-microbial impacts of green tea and ginger on common gastro-intestinal bacteria. Curr Med Chem J 2004;11:34-38.
REFERENCES
Tables & Figures
REFERENCES
Citations

- Effect of various disinfectant solutions on the tensile strength of gutta-percha using the rapid sterilization technique
Sandeep Rudranaik, Yoganatha Hanasoge Nagashetty, Sahadev Chikmagarvalli Krishna Gowda, Bharath Makonahalli Jaganath, K. B. Nirmala, M. C. Bharath Gowda
Journal of Conservative Dentistry and Endodontics.2024; 27(2): 154. CrossRef - Evaluation of antimicrobial efficacy of herbal extracts and their effect on the surface characteristics of gutta-percha cones: An in vitro study
Anshuman Shetty, Shivprasad Rai, Shetty Suhani Sudhakar
Endodontology.2023; 35(2): 142. CrossRef - Comparative evaluation of various herbal agents for the disinfection of guttapercha cones – An in vitro study
Gunnam Anjany Chowdary
IP Indian Journal of Conservative and Endodontics.2023; 8(2): 86. CrossRef - Dynamics of herbal medicine processing and production in Benue State Nigeria
P. Adigwe Obi, F. Builders Philip, Alfa John, Oladosu Peter
African Journal of Pharmacy and Pharmacology.2022; 16(7): 110. CrossRef - Antimicrobial Efficacy of Acacia Nilotica (Babul) Extract and its Effectiveness in Disinfecting Gutta Percha Cones - An In Vitro Study
Dolly R. Jagyasi, Neelam D. Chandwani, Mohit K. Gunwal, Aastha S. Ranka
Indian Journal of Dental Research.2021; 32(2): 221. CrossRef - The Anti-Inflammatory and Antimicrobial Potential of Selected Ethnomedicinal Plants from Sri Lanka
Mayuri Napagoda, Jana Gerstmeier, Hannah Butschek, Sudhara De Soyza, Simona Pace, Sybille Lorenz, Mallique Qader, Sanjeeva Witharana, Ajith Nagahawatte, Gaya Wijayaratne, Aleš Svatoš, Lalith Jayasinghe, Andreas Koeberle, Oliver Werz
Molecules.2020; 25(8): 1894. CrossRef - Comparación de desinfección de diferentes marcas de punta de gutapercha con hipoclorito de sodio
Jorge Morales García, Mónica Badillo Barba, María Guadalupe Chávez García, Vanessa García Ruíz, Adolfo Gutiérrez García
Revista de la Asociación Dental Mexicana.2020; 77(4): 185. CrossRef - Current herbal medicine as an alternative treatment in dentistry: In vitro, in vivo and clinical studies
Ehsan Tafazoli Moghadam, Mohsen Yazdanian, Elahe Tahmasebi, Hamid Tebyanian, Reza Ranjbar, Alireza Yazdanian, Alexander Seifalian, Ali Tafazoli
European Journal of Pharmacology.2020; 889: 173665. CrossRef - Gutta-percha in endodontics - A comprehensive review of material science
Vijetha Vishwanath, HMurali Rao
Journal of Conservative Dentistry.2019; 22(3): 216. CrossRef
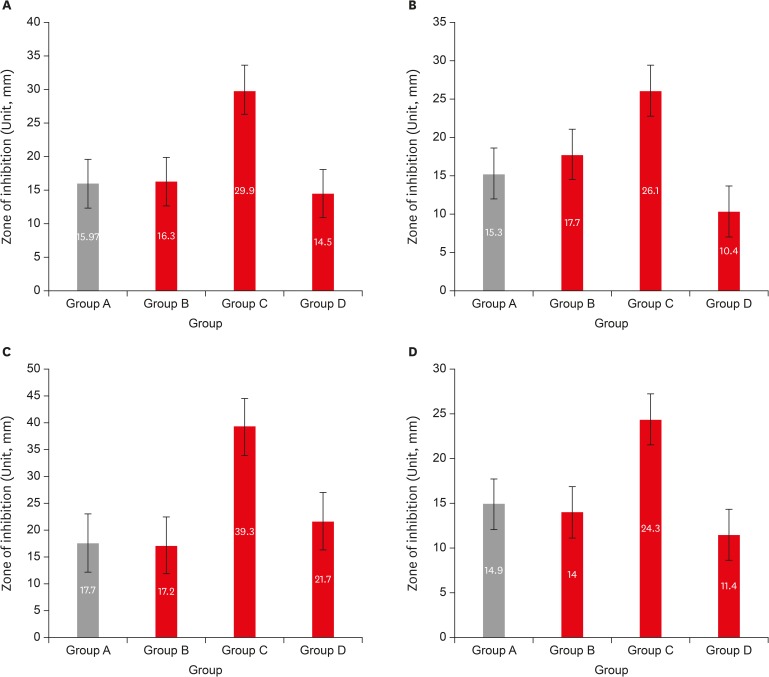
Figure 1
Descriptive statistics for tested organisms by 4 comparison groups (n = 10)
| Organism | Group A (control) | Group B (OT) | Group C (LG) | Group D (BO) |
|---|---|---|---|---|
| E. faecalis | 15.30 ± 0.78 | 17.70 ± 0.90 | 26.10 ± 0.94 | 10.40 ± 1.02 |
| S. aureus | 17.70 ± 1.10 | 17.20 ± 0.75 | 39.30 ± 1.19 | 21.70 ± 0.90 |
| C. albicans | 14.90 ± 0.94 | 14.00 ± 0.63 | 24.30 ± 0.90 | 11.40 ± 0.80 |
All the data are presented as means and standard deviations; Unit, mm.
Group A (control), 5.25% sodium hypochlorite; group B (OT), obicure tea extract; group C (LG), lemon grass oil; group D (BO), basil oil.
Two-way analysis of variance (ANOVA) for tested organisms and medicaments
| Source | Partial SS | df | MS | F | p value |
|---|---|---|---|---|---|
| Model | 6,913.87 | 11 | 628.53 | 673.43 | 0.0001 |
| Group | 4,663.2 | 3 | 1,554.4 | 1,665.43 | 0.0001 |
| Org. | 1,417.22 | 2 | 708.61 | 759.22 | 0.0001 |
| Interaction (group×org.) | 833.45 | 6 | 138.91 | 148.83 | 0.0001 |
| Residual | 100.8 | 108 | 0.93 | - | - |
| Total | 7,014.67 | 119 | 58.95 | - | - |
SS, sum of squares; df, degrees of freedom; MS, mean sum of squares; org., organism.
Efficacy of herbal extracts in decontamination of gutta percha (GP) cones (n = 30)
| Turbidity absent | Group A (control) | Group B (OT) | Group C (LG) | Group D (BO) | χ2 test | p value |
|---|---|---|---|---|---|---|
| No. (%) | 26 (86.7) | 25 (83.3) | 30 (100.0) | 28 (93.3) | 5.90 | 0.110 (NS) |
Group A (control), sodium hypochlorite; group B (OT), obicure tea extract; group C (LG), lemon grass oil; Group D (BO), basil oil; NS, not significant.
All the data are presented as means and standard deviations; Unit, mm.
Group A (control), 5.25% sodium hypochlorite; group B (OT), obicure tea extract; group C (LG), lemon grass oil; group D (BO), basil oil.
SS, sum of squares; df, degrees of freedom; MS, mean sum of squares; org., organism.
Group A (control), sodium hypochlorite; group B (OT), obicure tea extract; group C (LG), lemon grass oil; Group D (BO), basil oil; NS, not significant.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

