Articles
- Page Path
- HOME > Restor Dent Endod > Volume 41(1); 2016 > Article
- Research Article Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry
-
Jia Kim1, Young-Sang Song1, Kyung-San Min2, Sun-Hun Kim3, Jeong-Tae Koh3, Bin-Na Lee1
 , Hoon-Sang Chang1
, Hoon-Sang Chang1 , In-Nam Hwang1
, In-Nam Hwang1 , Won-Mann Oh1
, Won-Mann Oh1 , Yun-Chan Hwang1,3
, Yun-Chan Hwang1,3 -
2016;41(1):-36.
DOI: https://doi.org/10.5395/rde.2016.41.1.29
Published online: January 4, 2016
1Department of Conservative Dentistry, School of Dentistry, Dental Science Research Institute, Chonnam National University, Gwangju, Korea.
2Department of Conservative Dentistry, School of Dentistry, Chonbuk National University, Jeonju, Korea.
3Research Center for Biomineralization Disorders, Chonnam National University, Gwangju, Korea.
- Correspondence to Yun-Chan Hwang, DDS, PhD. Professor, Department of Conservative Dentistry, School of Dentistry, Chonnam National University, 77 Yongbong-ro, Buk-gu, Gwangju, Korea 61186. TEL, +82-62-530-5831; FAX, +82-62-530-5629; ychwang@chonnam.ac.kr
• Received: August 13, 2015 • Accepted: November 9, 2015
©Copyrights 2016. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 3,289 Views
- 37 Download
- 57 Crossref
Abstract
-
Objectives The purpose of this study was to assess the ability of two new calcium silicate-based pulp-capping materials (Biodentine and BioAggregate) to induce healing in a rat pulp injury model and to compare them with mineral trioxide aggregate (MTA).
-
Materials and Methods Eighteen rats were anesthetized, cavities were prepared and the pulp was capped with either of ProRoot MTA, Biodentine, or BioAggregate. The specimens were scanned using a high-resolution micro-computed tomography (micro-CT) system and were prepared and evaluated histologically and immunohistochemically using dentin sialoprotein (DSP).
-
Results On micro-CT analysis, the ProRoot MTA and Biodentine groups showed significantly thicker hard tissue formation (p < 0.05). On H&E staining, ProRoot MTA showed complete dentin bridge formation with normal pulpal histology. In the Biodentine and BioAggregate groups, a thick, homogeneous hard tissue barrier was observed. The ProRoot MTA specimens showed strong immunopositive reaction for DSP.
-
Conclusions Our results suggest that calcium silicate-based pulp-capping materials induce favorable effects on reparative processes during vital pulp therapy and that both Biodentine and BioAggregate could be considered as alternatives to ProRoot MTA.
Introduction
To preserve vitality of the pulp tissue and to prevent its pathological changes, cariously or mechanically exposed vital pulp must be sealed with a biocompatible material to protect the pulp from additional injury and to promote healing and repair. The healing process of dental pulp is characterized by the formation of a hard tissue and maintenance of vitality without inflammation.1,2 Calcium hydroxide has played an important role in vital pulp therapy by inducing formation of a hard tissue barrier. However, despite the long history, its use in vital pulp therapy remains controversial because of its destructive cytotoxicity and tunneling of the dentin bridge.3
Mineral trioxide aggregate (MTA) has received attention as an alternative to calcium hydroxide. MTA contains tricalcium silicate, tricalcium aluminate, tricalcium oxide, and silicate oxide. Its ability to stimulate formation of a dentin bridge, consequently leading to pulp healing has been well demonstrated in previous studies.4,5,6,7 MTA has excellent biocompatibility without mutagenic potential, sealing capacity and the deposition of cementum, which may promote the regeneration of the periodontal tissue and the formation of mineralized tissue.8,9,10,11,12,13,14 However, it has a long setting time and a tendency to become discolored.15
Recently, a new calcium silicate-based cement, Biodentine (Septodont, Saint-Maur-des-Fosses, France), has been introduced. The Biodentine powder contains mainly tricalcium silicate, dicalcium silicate, and calcium oxide, and the liquid consists of calcium chloride and a carboxylate-based hydrosoluble polymer (water-reducing agent). Its prominent characteristics are shorter setting time (12 minutes) and better compressive strength and sealing ability than MTA.16 Biodentine can be applied as a dentin substitute. Previous studies on its interactions with pulp cells demonstrated its biocompatibility and its ability to induce odontogenic differentiation and mineralization in cultured pulp cells.16,17,18,19,20,21,22 However, its ability to stimulate reparative dentin in direct pulp-capping remains to be studied further.
BioAggregate (Innovative BioCeramix Inc., Vancouver, BC, Canada) is a new bioceramic material for perforation repair and retrograde filling. It mainly consists of tricalcium silicate, dicalcium silicate, calcium phosphate monobasic, amorphous silicone dioxide, and tantalum oxide as a radiopacifier. It is claimed to stimulate cementogenesis, to form a hermetic seal, and to have effects on osteoblast differentiation and odontoblastic differentiation.16,21,22,23,24,25,26
Although many studies can be found regarding MTA, Biodentine, and BioAggregate, the reactions of pulp tissue to these three materials have not been simultaneously compared histologically. The objective of this study was to evaluate and characterize the reparative dentin formation of MTA, Biodentine, and BioAggregate using micro-computed tomography (micro-CT) and histology, and to provide guidelines for selecting the most appropriate biomaterials for pulp-capping procedures.
Materials and Methods
Eighteen Sprague-Dawley rats, 9 weeks old, were evenly divided into three treatment groups (n = 6). The rats were anesthetized with an intraperitoneal injection of 50 mg/kg of Zoletil 50 (Virbac, Carros, France) and 15 mg/kg of Rompun (Bayer, Leuverkeusen, Germany). Class I cavities were prepared to induce pulp exposure on the occlusal surfaces of the left and right maxillary first molars using a 1/4 round bur (n = 36). Bleeding was controlled by applying light pressure with wet cotton pellets and by irrigation with sodium hypochlorite (NaOCl) and sterile saline.
The cavities were then filled with either of MTA (ProRoot MTA, Dentsply Tulsa Dental, Tulsa, OK, USA), Biodentine (Septodont), or BioAggregate (Innovative BioCeramix Inc.), each of which was mixed according to the manufacturers' instructions. The exposed site was acid-etched (ETCH-37, Bisco Inc., Schaumburg, IL, USA), dentin adhesive (Xeno V, Dentsply DeTrey GmbH, Constanz, Germany) was applied, and the area was restored with flowable resin (G-aenial Universal Flo, GC Corp., Tokyo, Japan). After 4 weeks, the animals were anesthetized and sacrificed by means of intracardiac perfusion with 4% paraformaldehyde buffered with sodium cacodylate 0.1 M at pH 7.2 - 7.4. All procedures were performed in accordance with the animal experimental guidelines of the Institutional Animal Care and Use Committee of Chonnam National University Dental Hospital in Korea.
Block sections including molar specimens were dissected from the maxillas of all the rats and were placed in 10% formalin for storage before micro-CT scanning. Fixed block sections were scanned using a high-resolution micro-CT system (SkyScan 1172, SkyScan, Aartselaar, Belgium). Each section was mounted in a plastic container on the scanning platform with the root oriented vertically. The x-ray transmission was set at 180 degrees of rotation, with the x-ray source set at 70 kV/141 µm. A 0.5 mm aluminum filter was used to cut off the softest x-ray. The raw data were reconstructed into images using SkyScan's cluster reconstruction software (NRecon/NRecon Server).24,25 After reconstruction, gray images were displaced by color images to visualize mineral density. The Image J program (version 1.47, National Institutes of Health, Bethesda, MD, USA) was used for quantitative analysis. Areas of newly formed reparative dentin and pulp cavity were measured in five randomly selected transverse sections, and the relative ratio of reparative dentin to pulp cavity was calculated.
Specimens were immersed in a 4% paraformaldehyde solution for 24 hours at 4℃. Samples for demineralization were placed in a decalcifying agent (Calci-Clear Rapid, National Diagnostics, Atlanta, GA, USA) at 4℃ for a month. The tissues were treated with ethanol dehydration, embedded in paraffin, and cut into 5 µm thick sections. Tissue samples were stained with hematoxylin and eosin for evaluation of dentine bridge formation and pulpal inflammation. Slides were scanned with a Panoramic MIDI scanner (3DHISTECH, Budapest, Hungary), and digital image analysis was performed with Panoramic Viewer software (3DHISTECH).
Immunohistochemical reactions were performed with a streptavidin-biotin system (LSAB System-HRP, Dako, Glostrup, Denmark) according to the manufacturer's protocol. Briefly, specimens were de-waxed in xylene, rehydrated in a graded alcohol series, placed in an endogenous peroxide blocker for 10 minutes, and washed with Tris-buffered saline (TBS). The primary anti-dentin sialoprotein (DSP) antibody (dilution 1:100) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was incubated at 4℃ for 1 hour, followed by the biotin-streptavidin peroxidase complex. The tissues were counterstained with hematoxylin. A positive control was prepared from samples with no pulp treatment. Negative controls consisted of 1% bovine serum albumin substituted for DSP antibody. The immunostained samples were scanned and reviewed, as described under Histological examination.
The results were analyzed by one-way analysis of variation (ANOVA), and the Duncan test was used for post hoc analysis. A p value of less than 0.05 was considered statistically significant.
Results
In normal dental tissue, green color represents dentin and bone, blue color represents enamel, and purple color represents pulp and soft tissue (Figures 1a and 1b). In the MTA sample, green-colored thick homogeneous hard tissue was observed beneath the pulp exposure site. A complete hard tissue bridge with a high-intensity green hue was uniformly seen (Figures 1c and 1d). The mineral density of this newly formed green tissue was similar to that of dentin. The Biodentine specimens showed calcified materials beneath the pulpotomy site, with the calcification tissue exhibiting different degrees of saturation (light green, Figures 1e and 1f). In the BioAggregate group, newly formed thick hard tissue was also observed at the pulpal floor and lateral wall of the pulp chamber. This newly formed tissue, which had a light-green hue, was unevenly distributed (Figures 1g and 1h).
The relative ratios of newly formed reparative dentin to pulp cavity were 0.50 ± 0.02, 0.47 ± 0.03, and 0.44 ± 0.02 for MTA, Biodentine, and BioAggregate, respectively. The difference between MTA and BioAggregate in forming hard tissue was statistically significant (p < 0.05, Figure 2).
The results of histological analysis showed that the dentin bridge was created in all three experimental groups. Specimens in the MTA group showed complete calcified bridges. Remaining pulp tissue was vital with an intact odontoblastic layer. Few inflammatory cells were observed beneath the site of pulp exposure in any specimens (Figures 3a and 3b). In all the Biodentine specimens, the pulp wound healed with thick, hard tissue formation. The access cavity was completely closed by the dentin bridge. The hard tissue had an irregular pattern beneath the site of pulp exposure. Necrotic changes in the pulp were not observed (Figures 3c and 3d). The BioAggregate specimens showed a dense calcified area at the pulp exposure interface. Vital pulp was observed without acute inflammation or necrosis (Figures 3e and 3f).
Newly formed hard tissues from all experimental groups demonstrated positive immunoreactivity for DSP. In the MTA group, newly formed, strong immunopositive layer was observed within and around the pulp tissue that formed at the exposure site. In the Biodentine group, immunolabeled mineralized tissue was also seen within the pulp exposure site. In the BioAggregate group, there were positive expressions of DSP within the newly formed tissue at the site of the defect (Figure 4).
Discussion
In this study, micro-CT was used to evaluate newly formed reparative dentin. Micro-CT is a noninvasive technique that presents morphological features in a nondestructive manner. It allows imaging of the interior microstructure of the subject with high spatial resolution, and its data offer detailed internal morphology. This imaging technique provides insights into the calcification patterns of dentin.27,28 On micro-CT, the hard tissue barrier observed in the MTA group was thicker than that seen in the BioAggregate group. When compared with the MTA group, the Biodentine group showed an irregular, heterogeneous distribution of mineralization nodules within a uniform thickness of hard tissue barrier. In the BioAggregate group, considerable reparative dentin with relatively lower mineral density than dentin was observed. All these findings suggest that MTA, Biodentine, and BioAggregate have a dentinogenic capacity, but MTA and Biodentine have superior dentinogenic effects, when compared with BioAggregate.
Current results showed that MTA and the two calcium silicate-based materials showed favorable outcomes as a direct pulp capping material in rodent model. Some studies have reported that the pulpal response to capping materials after direct capping is affected by bacterial microleakage, which has an inhibitory effect.29,30,31 In the present study, acute inflammatory responses and necrosis were not observed in the experimental groups. This can be explained by the fact that MTA, Biodentine, and BioAggregate all have excellent sealing ability, which prevents microleakage and pulpal inflammation and thus provides a predictable barrier.
Newly formed dentin in the MTA group had the characteristics of homogeneous reparative dentin. Specimens of Biodentine had engulfed cellular inclusions within newly formed hard tissue, and this could have been the result of the rapid initial disorganized formation of the reparative dentin. The exact mechanism by which MTA formed the dentin bridge is not completely understood. Pulp capping with MTA induced cytological and functional changes in the pulp cells, leading to the formation of fibrodentin and reparative dentin.30 MTA contains calcium oxide, which forms calcium hydroxide when mixed with water. The reaction of calcium hydroxide and the carbon dioxide from pulp tissue produced calcite crystals.32 Seux et al. demonstrated the role of calcite crystals and fibronectin as an initiating step in the formation of a hard tissue barrier.33 Laurent et al. reported that Biodentine induced an early form of reparative dentin synthesis owing to a modulation of transforming growth factor beta-1 secretion by pulp cells.34 These authors showed that Biodentine might promote the mineralization process, as shown with MTA-based cements. Tricalcium silicate, which is one of the main components of Biodentine and BioAggregate, might be associated with the stimulation of cell proliferation and differentiation.17,34,35,36
Previous studies have shown that BioAggregate is nontoxic to osteoblasts and human periodontal ligament fibroblasts and is able to induce mineralization and odontoblastic differentiation-associated gene expression in human dental pulp cells.22,37,38,39 Because of the absence of aluminum in its chemical composition, BioAggregate has fewer negative effects on the inflammatory cell response.40 In this study, we did not observe any acute inflammation or necrosis in the BioAggregate specimens. However, when compared with MTA, BioAggregate differed significantly with respect to forming a dentin bridge. Because BioAggregate differs from MTA in containing tantalum oxide instead of bismuth oxide as a radiopacifier, it may be important to further evaluate the differences between bismuth oxide and tantalum oxide in forming dentin bridges.
Vital pulp therapy requires three types of healing processes: (1) rapid formation of a hard tissue bridge to protect the pulp from other stimuli, (2) formation of a barrier that prevents secondary pulp infections, and (3) induction of hard tissue formation at the interface to avoid obliteration of the pulp.41 Above all, compact hard tissue formation without bacterial invasion is the crucial key to the success of the vital pulp therapy. We observed that MTA, Biodentine, and BioAggregate induced adequate hard tissue formation that would preserve the integrity of the pulp. All three materials formed a tight barrier and might be associated with stimulating dentinogenesis.
DSP protein was specifically found in odontoblasts and the dentinal matrix, and its expression was evaluated for dentinogenesis. DSP is synthesized as a precursor dentin sialophosphoprotein and is synthesized by terminally differentiated odontoblasts. A previous study showed positive immunostaining for DSP in the reparative dentin induced by MTA and Biodentine.36 In another study, Min et al. demonstrated that MTA-induced reparative dentin showed greater DSP immunostaining than did the calcium hydroxide groups.42 We observed higher levels of DSP expression in the MTA-treated pulp tissue than in the tissue treated with Biodentine or BioAggregate. Although, treatments with Biodentine or BioAggregate formed comparable reparative dentin, they showed weaker DSP immunostaining than did the MTA group. Some embedded immunolabeled cells were observed within the newly formed tissue in the Biodentine and BioAggregate groups. It appeared that the characteristics of newly formed reparative dentin with Biodentine and BioAggregate treatment were closer to osteodentin. These findings indicate that MTA, Biodentine, and BioAggregate induced new reparative dentin and that MTA induced dentin with better characteristics. However, further studies will be needed to demonstrate the exact mechanism and characteristics of reparative dentin induced by these three materials.
Conclusions
The results of this study suggested that Biodentine and BioAggregate might provide an optimal environment for pulp healing and repair and were comparable to MTA. Although there were some differences in the thickness and morphology of the new hard tissue, all three materials showed acceptable biocompatibility. Based on these results, calcium silicate-based materials induced favorable effects on the reparative process during vital pulp therapy and could be considered as alternatives to MTA. Further long-term studies are required for thorough evaluation of the pulpal response to these materials.
Acknowledgement
This study was supported by a Chonnam National University (2013) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2011-0030121).
- 1. Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod 2003;29:324-333.ArticlePubMed
- 2. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod 1999;25:197-205.ArticlePubMed
- 3. de Souza V, Holland R. Treatment of the inflamed dental pulp. Aust Dent J 1974;19:191-196.ArticlePubMed
- 4. Eskandarizadeh A, Shahpasandzadeh MH, Shahpasandzadeh M, Torabi M, Parirokh M. A comparative study on dental pulp response to calcium hydroxide, white and grey mineral trioxide aggregate as pulp capping agents. J Conserv Dent 2011;14:351-355.ArticlePubMedPMC
- 5. Faraco IM Jr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol 2001;17:163-166.ArticlePubMedPDF
- 6. Accorinte ML, Loguercio AD, Reis A, Carneiro E, Grande RH, Murata SS, Holland R. Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper Dent 2008;33:488-495.ArticlePubMedPDF
- 7. Zarrabi MH, Javidi M, Jafarian AH, Joushan B. Histologic assessment of human pulp response to capping with mineral trioxide aggregate and a novel endodontic cement. J Endod 2010;36:1778-1781.ArticlePubMed
- 8. Torabinejad M, Hong CU, Pitt Ford TR, Kaiyawasam SP. Tissue reaction to implanted super-EBA and mineral trioxide aggregate in the mandible of guinea pigs: a preliminary report. J Endod 1995;21:569-571.ArticlePubMed
- 9. Moretton TR, Brown CE Jr, Legan JJ, Kafrawy AH. Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. J Biomed Mater Res 2000;52:528-533.ArticlePubMed
- 10. Kettering JD, Torabinejad M. Investigation of mutagenicity of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod 1995;21:537-542.ArticlePubMed
- 11. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod 1993;19:591-595.ArticlePubMed
- 12. Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod 1993;19:541-544.ArticlePubMed
- 13. Schwartz RS, Mauger M, Clement DJ, Walker WA 3rd. Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc 1999;130:967-975.ArticlePubMed
- 14. Holland R, de Souza V, Nery MJ, Faraco Júnior IM, Bernabé PF, Otoboni Filho JA, Dezan Júnior E. Reaction of rat connective tissue to implanted dentin tube filled with mineral trioxide aggregate, Portland cement or calcium hydroxide. Braz Dent J 2001;12:3-8.PubMed
- 15. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part III: clinical applications, drawbacks, and mechanism of action. J Endod 2010;36:400-413.ArticlePubMed
- 16. Jang YE, Lee BN, Koh JT, Park YJ, Joo NE, Chang HS, Hwang IN, Oh WM, Hwang YC. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor Dent Endod 2014;39:89-94.ArticlePubMedPMC
- 17. Zanini M, Sautier JM, Berdal A, Simon S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod 2012;38:1220-1226.ArticlePubMed
- 18. Koubi S, Elmerini H, Koubi G, Tassery H, Camps J. Quantitative evaluation by glucose diffusion of microleakage in aged calcium silicate-based open-sandwich restorations. Int J Dent 2012;2012:105863.ArticlePubMedPDF
- 19. Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, Kaczmarek W, Buczkowska-Radlińska J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod 2013;39:743-747.ArticlePubMed
- 20. Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater 2013;29:580-593.ArticlePubMed
- 21. Lee BN, Lee KN, Koh JT, Min KS, Chang HS, Hwang IN, Hwang YC, Oh WM. Effects of 3 endodontic bioactive cements on osteogenic differentiation in mesenchymal stem cells. J Endod 2014;40:1217-1222.ArticlePubMed
- 22. Jung JY, Woo SM, Lee BN, Kon JT, Nör JE, Hwang YC. Effect of Biodentine and Bioaggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int Endod J 2015;48:177-184.ArticlePubMed
- 23. Zhang H, Pappen FG, Haapasalo M. Dentin enhances the antibacterial effect of mineral trioxide aggregate and bioaggregate. J Endod 2009;35:221-224.ArticlePubMed
- 24. Hashem AA, Wanees Amin SA. The effect of acidity on dislodgment resistance of mineral trioxide aggregate and bioaggregate in furcation perforations: an in vitro comparative study. J Endod 2012;38:245-249.ArticlePubMed
- 25. Grech L, Mallia B, Camilleri J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent Mater 2013;29:e20-e28.ArticlePubMed
- 26. Batur YB, Acar G, Yalcin Y, Dindar S, Sancakli H, Erdemir U. The cytotoxic evaluation of mineral trioxide aggregate and bioaggregate in the subcutaneous connective tissue of rats. Med Oral Patol Oral Cir Bucal 2013;18:e745-e751.ArticlePubMedPMC
- 27. Nielsen RB, Alyassin AM, Peters DD, Carnes DL, Lancaster J. Microcomputed tomography: an advanced system for detailed endodontic research. J Endod 1995;21:561-568.ArticlePubMed
- 28. Verma P, Love RM. A Micro CT study of the mesiobuccal root canal morphology of the maxillary first molar tooth. Int Endod J 2011;44:210-217.ArticlePubMed
- 29. Iwamoto CE, Adachi E, Pameijer CH, Barnes D, Romberg EE, Jefferies S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am J Dent 2006;19:85-90.PubMed
- 30. Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J 2003;36:225-231.ArticlePubMedPDF
- 31. Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J 2008;41:128-150.ArticlePubMed
- 32. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. J Endod 1998;24:543-547.ArticlePubMed
- 33. Seux D, Couble ML, Hartmann DJ, Gauthier JP, Magloire H. Odontoblast-like cytodifferentiation of human dental pulp cells in vitro in the presence of a calcium hydroxide-containing cement. Arch Oral Biol 1991;36:117-128.ArticlePubMed
- 34. Laurent P, Camps J, About I. Biodentine(TM) induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J 2012;45:439-448.ArticlePubMed
- 35. Laurent P, Camps J, De Méo M, Déjou J, About I. Induction of specific cell responses to a Ca3SiO5-based posterior restorative material. Dent Mater 2008;24:1486-1494.ArticlePubMed
- 36. Tran XV, Gorin C, Willig C, Baroukh B, Pellat B, Decup F, Opsahl Vital S, Chaussain C, Boukpessi T. Effect of a calcium-silicate-based restorative cement on pulp repair. J Dent Res 2012;91:1166-1171.ArticlePubMedPDF
- 37. Yan P, Yuan Z, Jiang H, Peng B, Bian Z. Effect of bioaggregate on differentiation of human periodontal ligament fibroblasts. Int Endod J 2010;43:1116-1121.ArticlePubMed
- 38. Yuan Z, Peng B, Jiang H, Bian Z, Yan P. Effect of bioaggregate on mineral-associated gene expression in osteoblast cells. J Endod 2010;36:1145-1148.ArticlePubMed
- 39. Zhang S, Yang X, Fan M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int Endod J 2013;46:923-929.PubMed
- 40. Saghiri MA, Tanideh N, Garcia-Godoy F, Lotfi M, Karamifar K, Amanat D. Subcutaneous connective tissue reactions to various endodontic biomaterials: an animal study. J Dent Res Dent Clin Dent Prospects 2013;7:15-21.PubMedPMC
- 41. Tziafas D, Belibasakis G, Veis A, Papadimitriou S. Dentin regeneration in vital pulp therapy: design principles. Adv Dent Res 2001;15:96-100.ArticlePubMedPDF
- 42. Min KS, Park HJ, Lee SK, Park SH, Hong CU, Kim HW, Lee HH, Kim EC. Effect of mineral trioxide aggregate on dentin bridge formation and expression of dentin sialoprotein and heme oxygenase-1 in human dental pulp. J Endod 2008;34:666-670.ArticlePubMed
REFERENCES
Figure 1
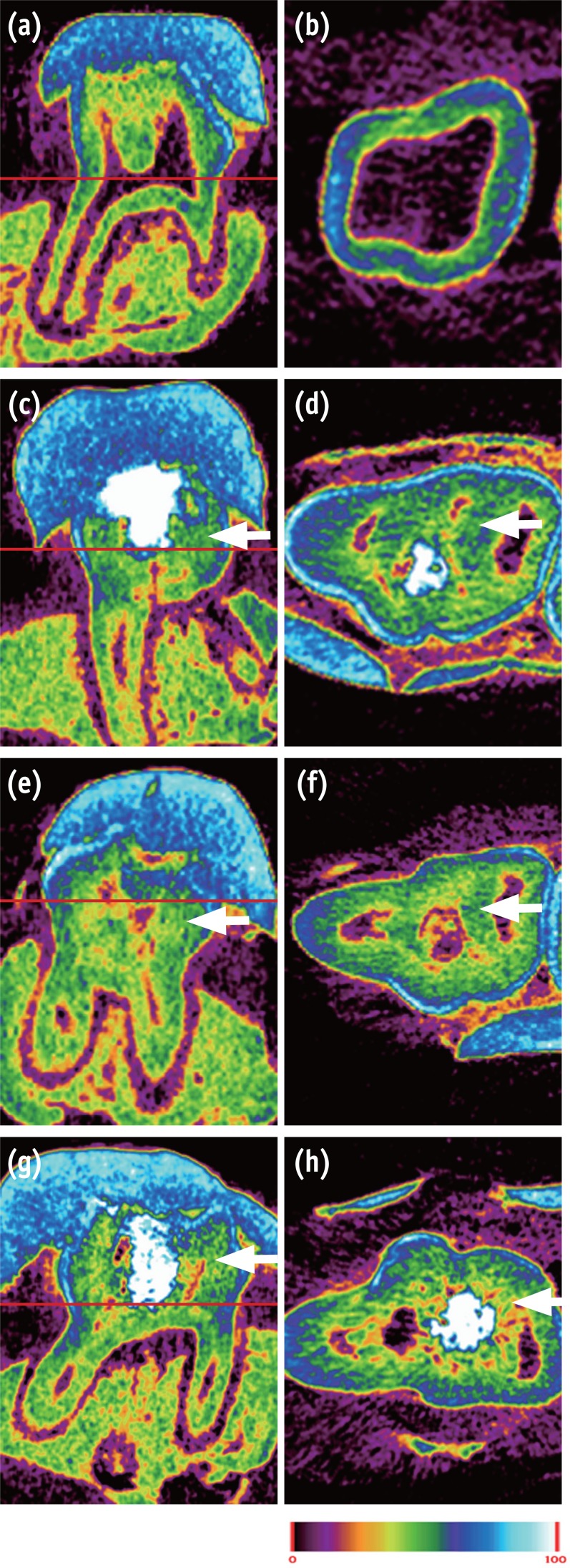
Micro-CT image of pulp capped rat molar teeth after 4 weeks. MTA and Biodentine showed thicker hard tissue formation than did BioAggregate. (a, b) Normal pulp; (c, d) MTA; (e, f) Biodentine; (g, h) BioAggregate group. Color scale bar indicates mineral density from 0 (corresponding to black in radiograph) to 100 (corresponding to white in radiograph). White arrow indicates reparative dentin.

Figure 2
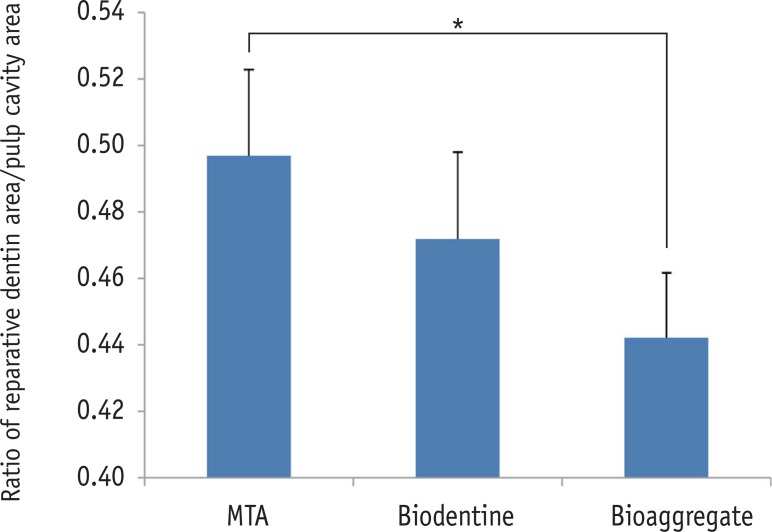
The relative ratio of newly formed reparative dentin to pulp cavity. Area of mineralized tissue and pulp cavity was measured by Image J (version 1.47, National Institutes of Health, Bethesda, MD, USA). MTA and BioAggregate differed significantly in forming a hard tissue.
*p < 0.05.
MTA, Mineral trioxide aggregate.

Figure 3
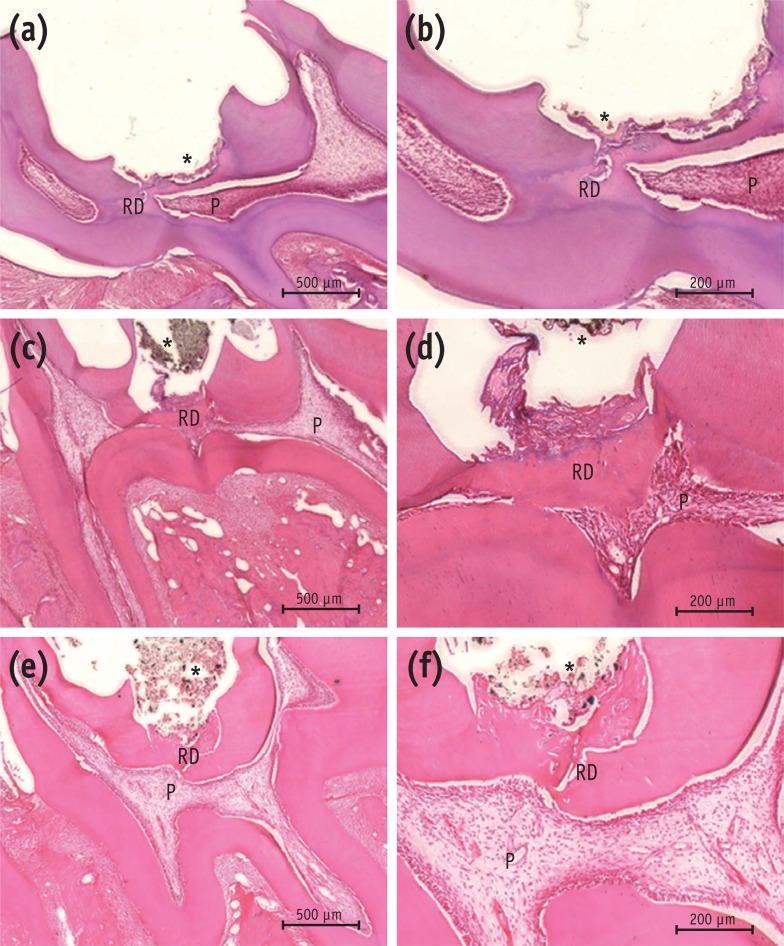
Histological analysis of rat molar teeth. (a, b) MTA; (c, d) Biodentine; (e, f) BioAggregate. At 4 weeks, hematoxylin and eosin stained sections showed reparative dentin bridge formation in all samples. A thick, homogeneous reparative dentin bridge and reactionary dentin could be seen in the MTA group (a, b). Reparative tissue was continuous and thick in the Biodentine group (c, d). Bridges in the BioAggregate group had a dense mineralized structure (e, f).
*Biomaterial.
P, pulp; RD, reparative dentin.

Figure 4
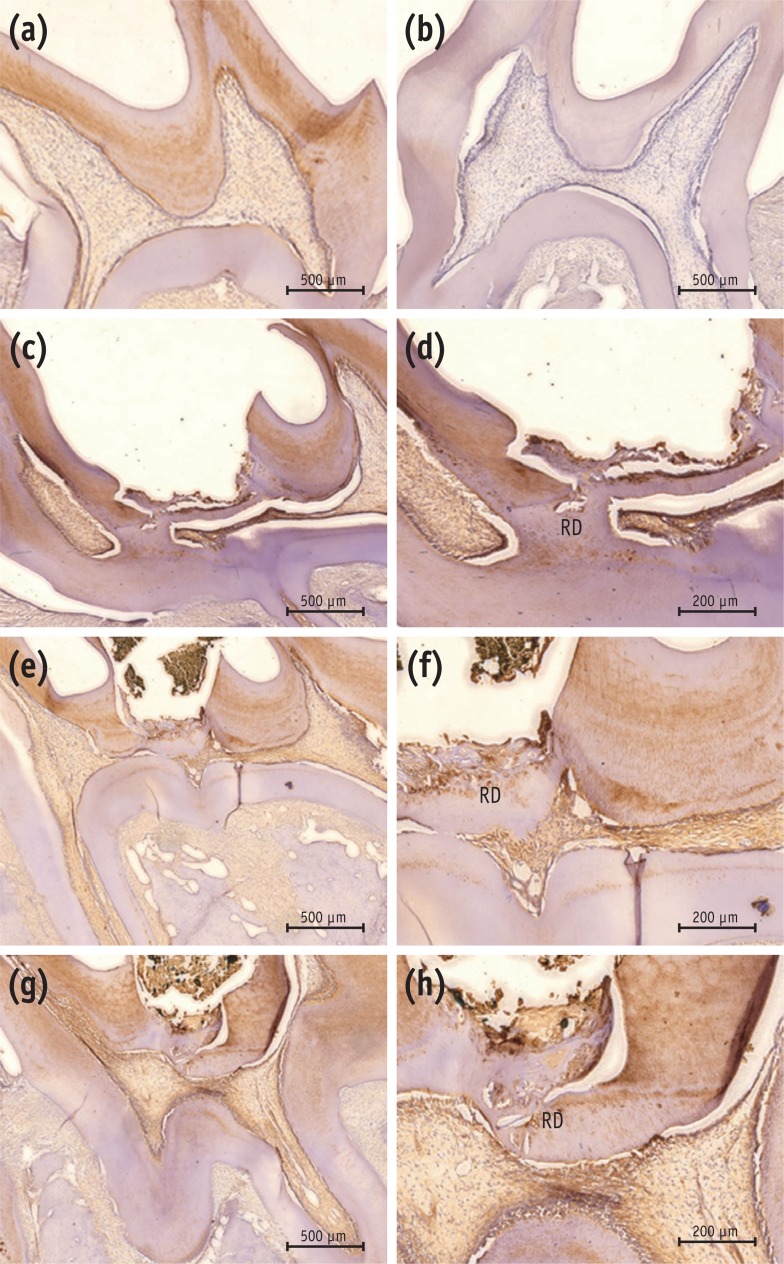
Immunohistochemical results with DSP antibody. (a) Positive control; (b) negative control; (c, d) MTA; (e, f) Biodentine; (g, h) BioAggregate. The positive control showed positive immunoreactivity to DSP in dentin and pulp (a), while the negative control was immunonegative to the DSP antibody (b). Reparative dentin from the MTA group showed strong immunoreactivity to DSP (c, d). Newly formed tissue from the Biodentine group stained lightly positive to DSP (e, f). Weakly-stained DSP immunolabeled tissue was seen in the BioAggregate group (g, h). Some immunolabeled cells were embedded within the newly formed tissue.
DSP, dentin sialoprotein; RD, reparative dentin.

Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Evaluation of microhardness, monomer conversion, and antibacterial properties of an experimental pulp-capping material containing collagen–hydroxyapatite nanocomposite and/or chlorhexidine
Hacer Balkaya, Sezer Demirbuğa, Fatih Duman, Ahmet Ceylan, Ömer Aydın
Odontology.2026; 114(1): 204. CrossRef - Clinical applications and classification of calcium silicate-based cements based on their history and evolution: a narrative review
Kenta Tsuchiya, Salvatore Sauro, Hidehiko Sano, Jukka P. Matinlinna, Monica Yamauti, Shuhei Hoshika, Yu Toida, Rafiqul Islam, Atsushi Tomokiyo
Clinical Oral Investigations.2025;[Epub] CrossRef - Impact of diode laser irradiation along with Biodentine on dental pulp stem cell proliferation and pluripotent gene expression
Ladan Alborzy, Sedighe Sadat Hashemikamangar, Mahshid Hodjat, Nasim Chiniforush, Behnaz Behniafar
Scientific Reports.2025;[Epub] CrossRef - The effect of different treatment methods on apical closure and treatment success in immature permanent first molars with reversible pulpitis
Muhammed ALAGOZ, Sera SIMSEK DERELIOĞLU
BMC Oral Health.2025;[Epub] CrossRef - Novelties in pulp capping materials
Vani Grover, Namith Rai, Nidambur Ballal
Acta stomatologica Naissi.2025; 41(91): 3086. CrossRef - Histological evaluation of pulp response to alendronate and Biodentine as pulp capping agents: an animal study
Thangavel Boopathi, Sekar Manimaran, Joseline Charles Kerena, Mathew Sebeena, Kumaravadivel Karthick, Natesan Thangaraj Deepa
Restorative Dentistry & Endodontics.2024;[Epub] CrossRef - Comparative Clinical and Radiographic Success Rate of Bioceramic Premix vs Biosilicate-based Medicament as Indirect Pulp Treatment Materials in Primary Molars: A Double-blind Randomized Trial with a Follow-up of 12 Months
Aditi Mathur, Meenakshi Nankar, Sunnypriyatham Tirupathi, Payal Kothari, Rashmi Chauhan, Ashrita Suvarna
International Journal of Clinical Pediatric Dentistry.2024; 17(7): 748. CrossRef - Effects of mineral trioxide aggregate and methyl sulfonyl methane on pulp exposure via RUNX2 and RANKL pathways
Altar Ateş, Ayca Kurt, Tolga Mercantepe
Odontology.2024; 112(3): 895. CrossRef - Evaluation of biocompatibility and bioactive potential of Well-Root PT by comparison with ProRoot MTA and Biodentine
Yong Kwon Chae, Ju Ri Ye, Ok Hyung Nam
Journal of Dental Sciences.2024; 19(4): 2218. CrossRef - Dentine Remineralisation Induced by “Bioactive” Materials through Mineral Deposition: An In Vitro Study
Marta Kunert, Ireneusz Piwonski, Louis Hardan, Rim Bourgi, Salvatore Sauro, Francesco Inchingolo, Monika Lukomska-Szymanska
Nanomaterials.2024; 14(3): 274. CrossRef - Different pulp capping agents and their effect on pulp inflammatory response: A narrative review
Mustafa Tariq Mutar, Anas F Mahdee
The Saudi Dental Journal.2024; 36(10): 1295. CrossRef - Clinical application of calcium silicate-based bioceramics in endodontics
Xinyuan Wang, Yizhi Xiao, Wencheng Song, Lanxiang Ye, Chen Yang, Yuzhen Xing, Zhenglin Yuan
Journal of Translational Medicine.2023;[Epub] CrossRef - Evaluation of the pulp response following direct pulp capping with exogenous nitric oxide and Mineral Trioxide Aggregate (MTA) a histologic study
Amirah Alnour, Ghassan Almohammad, Anas Abdo, Kinda Layous
Heliyon.2023; 9(7): e17458. CrossRef - Histological evaluation of dental pulp response to Biodentine, enamel matrix derivative (Emdogain), and mineral trioxide aggregate as direct pulp-capping agents – A randomized clinical trial
Takhellambam Premlata Devi, Amandeep Kaur, Shamurailatpam Priyadarshini, B. S. Deepak, Sumita Banerjee, Ngairangbam Sanjeeta
Journal of Medical Society.2023; 37(3): 107. CrossRef - Effect of Intracoronal Sealing Biomaterials on the Histological Outcome of Endodontic Revitalisation in Immature Sheep Teeth—A Pilot Study
Elanagai Rathinam, Sivaprakash Rajasekharan, Heidi Declercq, Christian Vanhove, Peter De Coster, Luc Martens
Journal of Functional Biomaterials.2023; 14(4): 214. CrossRef - Restorative management of the posterior tooth that has undergone a pulpotomy
Nicholas N Longridge, James S Hyde, Fadi Jarad, Sondos Albadri
Dental Update.2023; 50(11): 932. CrossRef - Direct pulp capping procedures – Evidence and practice
Rafiqul Islam, Md Refat Readul Islam, Toru Tanaka, Mohammad Khursheed Alam, Hany Mohamed Aly Ahmed, Hidehiko Sano
Japanese Dental Science Review.2023; 59: 48. CrossRef - A novel analysis of the formation and resorption changes in dental hard tissue using longitudinal in vivo micro computed tomography
Yeon-Jee YOO, Joonil HWANG, So-Hyun PARK, Jaehong HWANG, Seungryong CHO, Sun-Young KIM
Dental Materials Journal.2023; 42(5): 708. CrossRef - Evaluation of pH and Calcium Ion Diffusion from Intracanal MTA and Bioaggregate to Simulated External Resorption Cavities Through Dentinal Tubules
Umut AKSOY, Kaan POLATOĞLU, Feridun ŞAKLAR
European Annals of Dental Sciences.2022; 49(3): 108. CrossRef - Pulpa Kuafajı ve Kuafaj Materyallerine Güncel Bir Bakış: Derleme
Dilek AKIN, Çiğdem ATALAYIN ÖZKAYA
Selcuk Dental Journal.2022; 9(2): 617. CrossRef - The Influence of New Bioactive Materials on Pulp–Dentin Complex Regeneration in the Assessment of Cone Bone Computed Tomography (CBCT) and Computed Micro-Tomography (Micro-CT) from a Present and Future Perspective—A Systematic Review
Mirona Paula Palczewska-Komsa, Bartosz Gapiński, Alicja Nowicka
Journal of Clinical Medicine.2022; 11(11): 3091. CrossRef - Evaluation of shear bond strength of e-mineral trioxide aggregate and biodentine with glass ionomer cement
Hemalatha Hiremath, Aishwarya Singh Solanki, Shivangi Trivedi, Devansh Verma
Endodontology.2022; 34(2): 127. CrossRef - Multiple growth factors accommodated degradable submicron calcium sulfate hemihydrate/porous hydroxyapatite for dentin-pulp regeneration
Chih-Wen Chi, Bharathi Priya Lohanathan, Ching-Ching Wong, Che-Lun Chen, Hsun-Chang Lin, Yu-Chih Chiang
Biomaterials Advances.2022; 140: 213045. CrossRef - THE EFFECT OF BLOOD CONTAMINATION ON SHEAR BOND STRENGTH OF CALCIUM SILICATE-BASED PULP CAPPING MATERIALS
Hasan Fatih YAVUZ, Güneş BULUT EYÜBOĞLU
Cumhuriyet Dental Journal.2022; 24(4): 371. CrossRef - Comparison of Four Dental Pulp-Capping Agents by Cone-Beam Computed Tomography and Histological Techniques—A Split-Mouth Design Ex Vivo Study
Jayanandan Muruganandhan, Govindarajan Sujatha, Saravanan Poorni, Manali Ramakrishnan Srinivasan, Nezar Boreak, Ahmed Al-Kahtani, Mohammed Mashyakhy, Hitesh Chohan, Shilpa Bhandi, A. Thirumal Raj, Alessio Zanza, Luca Testarelli, Shankargouda Patil
Applied Sciences.2021; 11(7): 3045. CrossRef - Effect of Naturally Occurring Biogenic Materials on Human Dental Pulp Stem Cells (hDPSC): an In Vitro Study.
Prasanna T. Dahake, Vinod V. Panchal, Yogesh J. Kale, Mahesh V. Dadpe, Shrikant B. Kendre, Vijay M. Kumbar
Regenerative Engineering and Translational Medicine.2021; 7(4): 506. CrossRef - Influence of Ultrasonic Activation on the Physicochemical Properties of Calcium Silicate-Based Cements
Fredson Márcio Acris De Carvalho, Yara Teresinha Corrêa Silva-Sousa, Carlos Eduardo Saraiva Miranda, Paulo Henrique Miller Calderon, Ana Flávia Simões Barbosa, Luciana Martins Domingues De Macedo, Fuad Jacob Abi Rached-Junior, Boonlert Kukiattrakoon
International Journal of Dentistry.2021; 2021: 1. CrossRef - Tailored 70S30C Bioactive glass induces severe inflammation as pulpotomy agent in primary teeth: an interim analysis of a randomised controlled trial
Yasmine Elhamouly, Rania M. El Backly, Dalia M. Talaat, Samia S. Omar, Maha El Tantawi, Karin M. L. Dowidar
Clinical Oral Investigations.2021; 25(6): 3775. CrossRef - Response of dental pulp capped with calcium-silicate based material, calcium hydroxide and adhesive resin in rabbit teeth
Cynthia Kassis, Pierre Khoury, Karim Corbani, Charbel Mansour, Louis Hardan, Ghassan Yared, Carole Chakar
Brazilian Journal of Oral Sciences.2021;[Epub] CrossRef - Effectiveness of Direct Pulp Capping Bioactive Materials in Dentin Regeneration: A Systematic Review
Ermin Nie, Jiali Yu, Rui Jiang, Xiangzhen Liu, Xiang Li, Rafiqul Islam, Mohammad Khursheed Alam
Materials.2021; 14(22): 6811. CrossRef - Immunohistochemical expression of non-collagenous extracellular matrix molecules involved in tertiary dentinogenesis following direct pulp capping: a systematic review
C. Călin, M. Sajin, V.T. Moldovan, C. Coman, S.I. Stratul, A.C. Didilescu
Annals of Anatomy - Anatomischer Anzeiger.2021; 235: 151674. CrossRef - Recent Advances in Indirect Pulp Treatment Materials for Primary Teeth: A Literature Review
Omar AES El Meligy, Afnan M Saber, Sumer M Alaki
International Journal of Clinical Pediatric Dentistry.2021; 14(6): 795. CrossRef - Chitosan-Based Accelerated Portland Cement Promotes Dentinogenic/Osteogenic Differentiation and Mineralization Activity of SHED
Hasan Subhi, Adam Husein, Dasmawati Mohamad, Nik Rozainah Nik Abdul Ghani, Asma-Abdullah Nurul
Polymers.2021; 13(19): 3358. CrossRef - Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping
Ahmed A. Holiel, Elsayed M. Mahmoud, Wegdan M. Abdel-Fattah, Khadiga Y. Kawana
Clinical Oral Investigations.2021; 25(4): 2101. CrossRef - Minimal Intervention in Dentistry: A Literature Review on Biodentine as a Bioactive Pulp Capping Material
Naji Ziad Arandi, Mohammad Thabet, Mona Abbassy
BioMed Research International.2021;[Epub] CrossRef - Potential of tailored amorphous multiporous calcium silicate glass for pulp capping regenerative endodontics—A preliminary assessment
Jie Liu, Chao-An Chen, Xiaofei Zhu, Brian R. Morrow, Ukrit Thamma, Tia J. Kowal, Hassan M. Moawad, Matthias M. Falk, Himanshu Jain, George T.-J. Huang
Journal of Dentistry.2021; 109: 103655. CrossRef - Tomographic evaluation of direct pulp capping using a novel injectable treated dentin matrix hydrogel: a 2-year randomized controlled clinical trial
Ahmed A. Holiel, Elsayed M. Mahmoud, Wegdan M. Abdel-Fattah
Clinical Oral Investigations.2021; 25(7): 4621. CrossRef - Hard tissue formation after direct pulp capping with osteostatin and MTA in vivo
Ji-Hye Yoon, Sung-Hyeon Choi, Jeong-Tae Koh, Bin-Na Lee, Hoon-Sang Chang, In-Nam Hwang, Won-Mann Oh, Yun-Chan Hwang
Restorative Dentistry & Endodontics.2021;[Epub] CrossRef - Hydraulic cements for various intra-coronal applications: Part 1
Stephen J Bonsor, Josette Camilleri
Dental Update.2021; 48(8): 653. CrossRef - In vivo Biocompatibility and Bioactivity of Calcium Silicate-Based Bioceramics in Endodontics
Wencheng Song, Wei Sun, Lili Chen, Zhenglin Yuan
Frontiers in Bioengineering and Biotechnology.2020;[Epub] CrossRef - Evaluation of dentinogenesis inducer biomaterials: an in vivo study
Anabela B. Paula, Mafalda Laranjo, Carlos-Miguel Marto, Siri Paulo, Ana M. Abrantes, Bruno Fernandes, João Casalta-Lopes, Manuel Marques-Ferreira, Maria Filomena Botelho, Eunice Carrilho
Journal of Applied Oral Science.2020;[Epub] CrossRef - Micro-computed tomographic evaluation of a new system for root canal filling using calcium silicate-based root canal sealers
Mario Tanomaru-Filho, Fernanda Ferrari Esteves Torres, Jader Camilo Pinto, Airton Oliveira Santos-Junior, Karina Ines Medina Carita Tavares, Juliane Maria Guerreiro-Tanomaru
Restorative Dentistry & Endodontics.2020;[Epub] CrossRef - Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article
Marta Kunert, Monika Lukomska-Szymanska
Materials.2020; 13(5): 1204. CrossRef - Micro-computed tomographic evaluation of the flow and filling ability of endodontic materials using different test models
Fernanda Ferrari Esteves Torres, Juliane Maria Guerreiro-Tanomaru, Gisselle Moraima Chavez-Andrade, Jader Camilo Pinto, Fábio Luiz Camargo Villela Berbert, Mario Tanomaru-Filho
Restorative Dentistry & Endodontics.2020;[Epub] CrossRef - Release of Transforming Growth Factor Beta 1 from Human Tooth Dentin after Application of Either ProRoot MTA or Biodentine as a Coronal Barrier
Kunlada Wattanapakkavong, Tanida Srisuwan
Journal of Endodontics.2019; 45(6): 701. CrossRef - Effect of Leptin on Odontoblastic Differentiation and Angiogenesis: An In Vivo Study
Sung-Hyeon Choi, Ji-Hyun Jang, Jeong-Tae Koh, Hoon-Sang Chang, Yun-Chan Hwang, In-Nam Hwang, Bin-Na Lee, Won-Mann Oh
Journal of Endodontics.2019; 45(11): 1332. CrossRef - Análise da composição química dos cimentos MTA Angelus® branco, cinza e HP Repair® através de Microscopia Eletrônica de Varredura (MEV) acoplada a Espectrômetro de Energia Dispersiva (EDS)
Gabriela Duarte Rocha SARZEDA, Marcelo Santos BAHIA, Paulo Victor Teixeira DORIGUÊTTO, Karina Lopes DEVITO, Anamaria Pessoa Pereira LEITE
Revista de Odontologia da UNESP.2019;[Epub] CrossRef - Direct Pulp Capping: Which is the Most Effective Biomaterial? A Retrospective Clinical Study
Anabela Paula, Eunice Carrilho, Mafalda Laranjo, Ana M. Abrantes, João Casalta-Lopes, Maria Filomena Botelho, Carlos Miguel Marto, Manuel M. Ferreira
Materials.2019; 12(20): 3382. CrossRef - Characterization of Odontoblast-like Cell Phenotype and Reparative Dentin Formation In Vivo: A Comprehensive Literature Review
Dimitrios Tziafas
Journal of Endodontics.2019; 45(3): 241. CrossRef - Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview – part I: vital pulp therapy
M. Parirokh, M. Torabinejad, P. M. H. Dummer
International Endodontic Journal.2018; 51(2): 177. CrossRef - Effects of calcium silicate cements on dental pulp cells: A systematic review
Ramy Emara, Karim Elhennawy, Falk Schwendicke
Journal of Dentistry.2018; 77: 18. CrossRef - Biodentine™ material characteristics and clinical applications: a 3 year literature review and update
S. Rajasekharan, L. C. Martens, R. G. E. C. Cauwels, R. P. Anthonappa
European Archives of Paediatric Dentistry.2018; 19(1): 1. CrossRef - The Relationship of Surface Characteristics and Antimicrobial Performance of Pulp Capping Materials
Cher Farrugia, Christie Y.K. Lung, Pierre Schembri Wismayer, Maria Teresa Arias-Moliz, Josette Camilleri
Journal of Endodontics.2018; 44(7): 1115. CrossRef - Effect of iRoot Fast Set root repair material on the proliferation, migration and differentiation of human dental pulp stem cells in vitro
Yan Sun, Tao Luo, Ya Shen, Markus Haapasalo, Ling Zou, Jun Liu, Gianpaolo Papaccio
PLOS ONE.2017; 12(10): e0186848. CrossRef - Bioactive-glass in Endodontic Therapy and Associated Microsurgery
Andrea Corrado Profeta, Gian Marco Prucher
The Open Dentistry Journal.2017; 11(1): 164. CrossRef - Influence of Biodentine® - A Dentine Substitute - On Collagen Type I Synthesis in Pulp Fibroblasts In Vitro
Frangis Nikfarjam, Kim Beyer, Anke König, Matthias Hofmann, Manuel Butting, Eva Valesky, Stefan Kippenberger, Roland Kaufmann, Detlef Heidemann, August Bernd, Nadja Nicole Zöller, Dimitrios Karamichos
PLOS ONE.2016; 11(12): e0167633. CrossRef - Effect of an Experimental Direct Pulp-capping Material on the Properties and Osteogenic Differentiation of Human Dental Pulp Stem Cells
Fan Yu, Yan Dong, Yan-wei Yang, Ping-ting Lin, Hao-han Yu, Xiang Sun, Xue-fei Sun, Huan Zhou, Li Huang, Ji-hua Chen
Scientific Reports.2016;[Epub] CrossRef
Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry
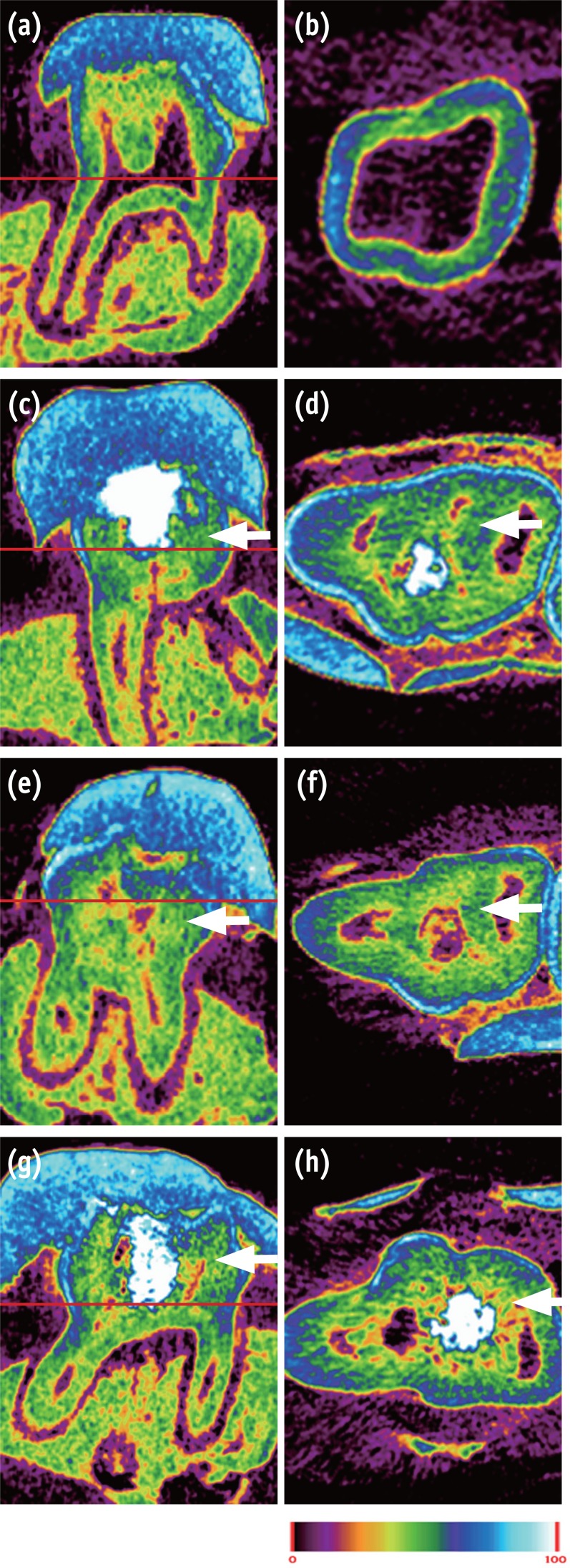
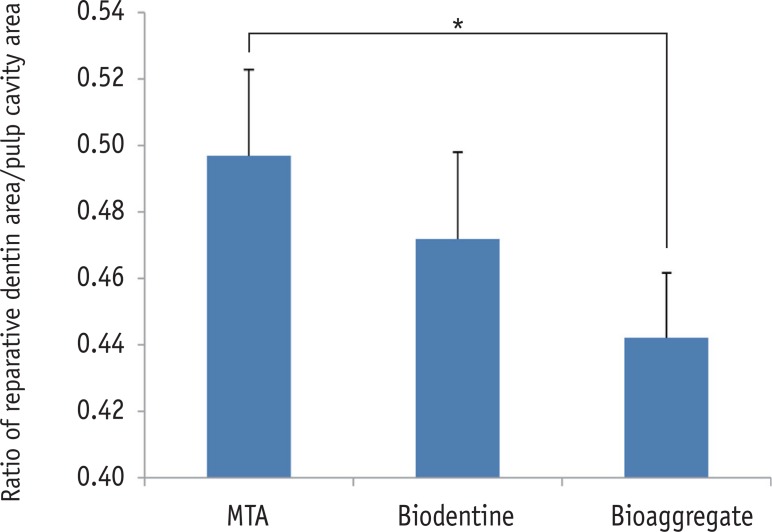
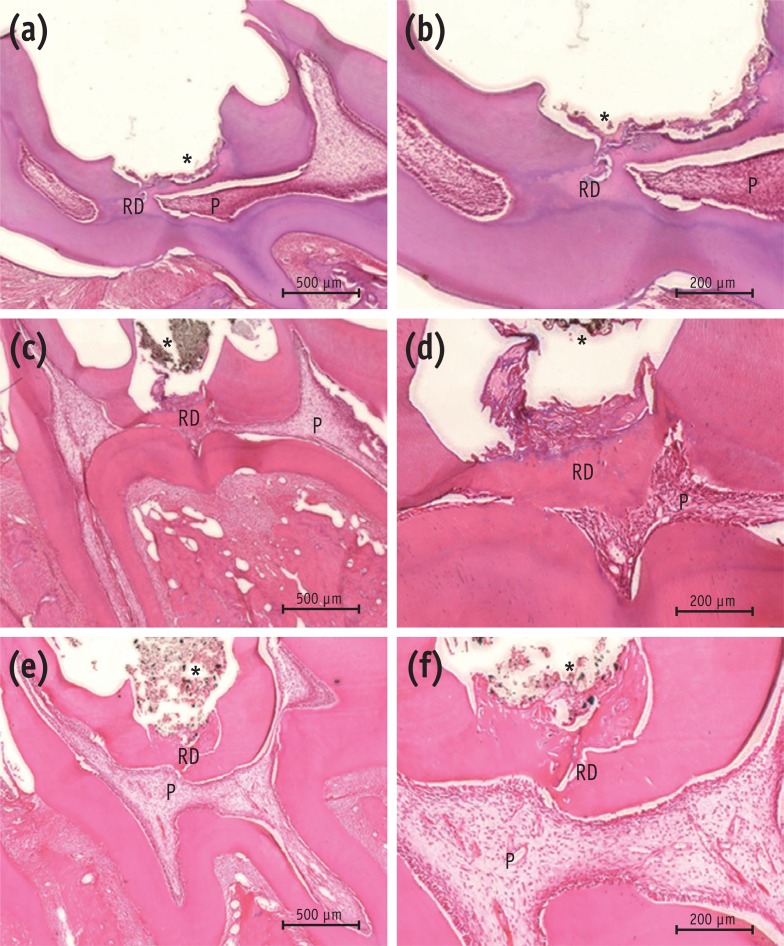
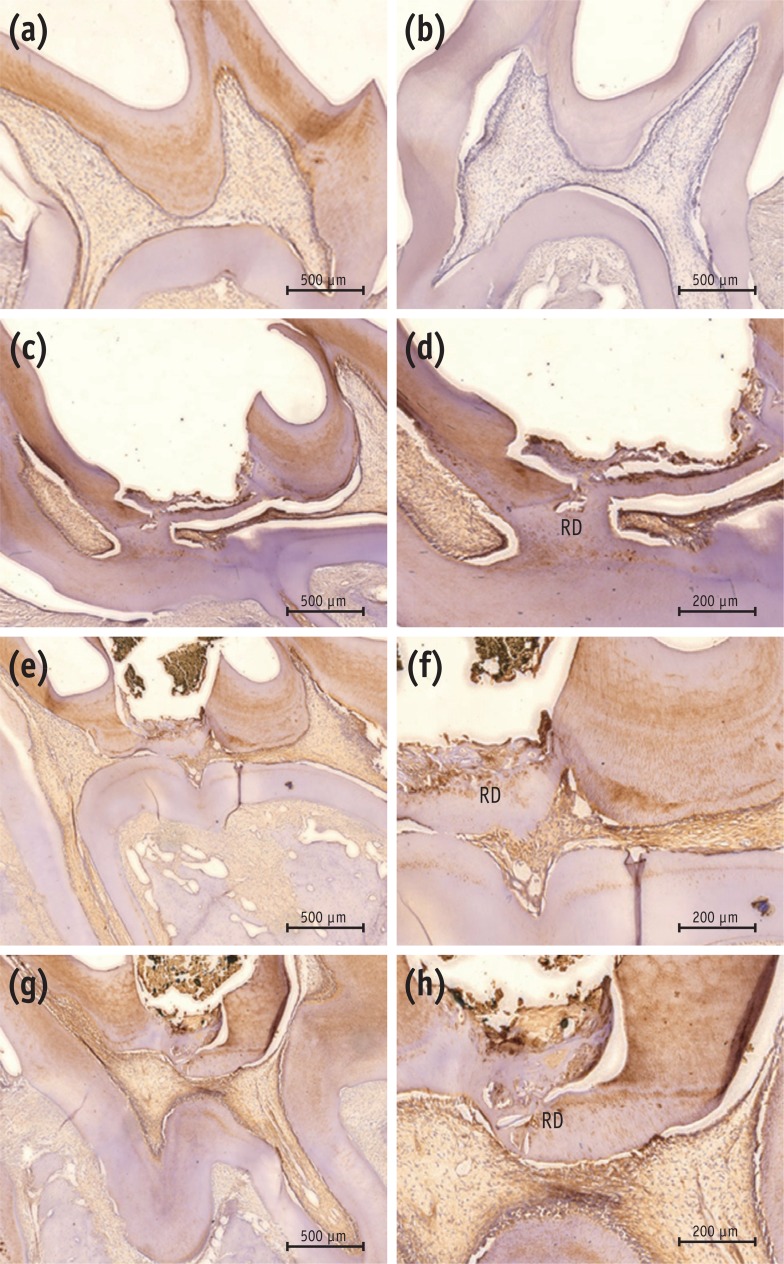
Figure 1 Micro-CT image of pulp capped rat molar teeth after 4 weeks. MTA and Biodentine showed thicker hard tissue formation than did BioAggregate. (a, b) Normal pulp; (c, d) MTA; (e, f) Biodentine; (g, h) BioAggregate group. Color scale bar indicates mineral density from 0 (corresponding to black in radiograph) to 100 (corresponding to white in radiograph). White arrow indicates reparative dentin.
Figure 2 The relative ratio of newly formed reparative dentin to pulp cavity. Area of mineralized tissue and pulp cavity was measured by Image J (version 1.47, National Institutes of Health, Bethesda, MD, USA). MTA and BioAggregate differed significantly in forming a hard tissue.*p < 0.05.MTA, Mineral trioxide aggregate.
Figure 3 Histological analysis of rat molar teeth. (a, b) MTA; (c, d) Biodentine; (e, f) BioAggregate. At 4 weeks, hematoxylin and eosin stained sections showed reparative dentin bridge formation in all samples. A thick, homogeneous reparative dentin bridge and reactionary dentin could be seen in the MTA group (a, b). Reparative tissue was continuous and thick in the Biodentine group (c, d). Bridges in the BioAggregate group had a dense mineralized structure (e, f).*Biomaterial.P, pulp; RD, reparative dentin.
Figure 4 Immunohistochemical results with DSP antibody. (a) Positive control; (b) negative control; (c, d) MTA; (e, f) Biodentine; (g, h) BioAggregate. The positive control showed positive immunoreactivity to DSP in dentin and pulp (a), while the negative control was immunonegative to the DSP antibody (b). Reparative dentin from the MTA group showed strong immunoreactivity to DSP (c, d). Newly formed tissue from the Biodentine group stained lightly positive to DSP (e, f). Weakly-stained DSP immunolabeled tissue was seen in the BioAggregate group (g, h). Some immunolabeled cells were embedded within the newly formed tissue.DSP, dentin sialoprotein; RD, reparative dentin.
Figure 1
Figure 2
Figure 3
Figure 4
Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

