Articles
- Page Path
- HOME > Restor Dent Endod > Volume 40(3); 2015 > Article
- Research Article Chelating and antibacterial properties of chitosan nanoparticles on dentin
- Aldo del Carpio-Perochena1, Clovis Monteiro Bramante1, Marco Antonio Hungaro Duarte1, Marcia Regina de Moura2,3, Fauze Ahmad Aouada2, Anil Kishen4
-
2015;40(3):-201.
DOI: https://doi.org/10.5395/rde.2015.40.3.195
Published online: March 30, 2015
1Department of Dentistry, Endodontics and Dental Materials, Bauru Dental School, University of São Paulo, Bauru-São Paulo, Brazil.
2Department of Physics and Chemistry, FEIS, São Paulo State University, Ilha Solteira-São Paulo, Brazil.
3National Nanotechnology Laboratory for Agriculture, Embrapa, São Carlos-São Paulo, Brazil.
4Discipline of Endodontics, Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada.
- Correspondence to Aldo del Carpio Perochena, DDS, PhD. Research associate, Department of Dentistry, Endodontics and Dental Materials, Bauru Dental School, University of São Paulo, Faculdade de Odontologia de Bauru-USP. Al. Octávio Pinheiro Brisolla, 9-75, Bauru-São Paulo, Brazil 17012-901. TEL, +55-14-3235-8344; FAX, +55-14-3223-4679; aldodelcp@usp.br
©Copyrights 2015. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,692 Views
- 42 Download
- 95 Crossref
Abstract
-
Objectives The use of chitosan nanoparticles (CNPs) in endodontics is of interest due to their antibiofilm properties. This study was to investigate the ability of bioactive CNPs to remove the smear layer and inhibit bacterial recolonization on dentin.
-
Materials and Methods One hundred bovine dentin sections were divided into five groups (n = 20 per group) according to the treatment. The irrigating solutions used were 2.5% sodium hypochlorite (NaOCl) for 20 min, 17% ethylenediaminetetraacetic acid (EDTA) for 3 min and 1.29 mg/mL CNPs for 3 min. The samples were irrigated with either distilled water (control), NaOCl, NaOCl-EDTA, NaOCl-EDTA-CNPs or NaOCl-CNPs. After the treatment, half of the samples (n = 50) were used to assess the chelating effect of the solutions using portable scanning electronic microscopy, while the other half (n = 50) were infected intra-orally to examine the post-treatment bacterial biofilm forming capacity. The biovolume and cellular viability of the biofilms were analysed under confocal laser scanning microscopy. The Kappa test was performed for examiner calibration, and the non-parametric Kruskal-Wallis and Dunn tests (p < 0.05) were used for comparisons among the groups.
-
Results The smear layer was significantly reduced in all of the groups except the control and NaOCl groups (p < 0.05). The CNPs-treated samples were able to resist biofilm formation significantly better than other treatment groups (p < 0.05).
-
Conclusions CNPs could be used as a final irrigant during root canal treatment with the dual benefit of removing the smear layer and inhibiting bacterial recolonization on root dentin.
Introduction
Materials and Methods
Results
Discussion
Conclusions
Acknowledgement
- 1. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318-1322.ArticlePubMed
- 2. Siqueira JF Jr, Paiva SS, Rôças IN. Reduction in the cultivable bacterial populations in infected root canals by a chlorhexidine-based antimicrobial protocol. J Endod 2007;33:541-547.ArticlePubMed
- 3. Pascon FM, Kantovitz KR, Sacramento PA, Nobredos-Santos M, Puppin-Rontani RM. Effect of sodium hypochlorite on dentin mechanical properties. A review. J Dent 2009;37:903-908.PubMed
- 4. Del Carpio-Perochena AE, Bramante CM, Duarte MA, Cavenago BC, Villas-Boas MH, Graeff MS, Bernardineli N, de Andrade FB, Ordinola-Zapata R. Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J Endod 2011;37:1134-1138.ArticlePubMed
- 5. Zhang K, Kim YK, Cadenaro M, Bryan TE, Sidow SJ, Loushine RJ, Ling JQ, Pashley DH, Tay FR. Effects of different exposure times and concentrations of sodium hypochlorite/ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. J Endod 2010;36:105-109.ArticlePubMed
- 6. Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod 2002;28:17-19.ArticlePubMed
- 7. Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:658-666.ArticlePubMed
- 8. Violich DR, Chandler NP. The smear layer in endodontics - a review. Int Endod J 2010;43:2-15.ArticlePubMed
- 9. Garberoglio R, Becce C. Smear layer removal by root canal irrigants. A comparative scanning electron microscopic study. Oral Surg Oral Med Oral Pathol 1994;78:359-367.PubMed
- 10. Kishen A, Sum CP, Mathew S, Lim CT. Influence of irrigation regimens on the adherence of Enterococcus faecalis to root canal dentin. J Endod 2008;34:850-854.ArticlePubMed
- 11. Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, Dhawan S. Chitosan microspheres as a potential carrier for drugs. Int J Pharm 2004;274:1-33.PubMed
- 12. Xu Z, Neoh KG, Lin CC, Kishen A. Biomimetic deposition of calcium phosphate minerals on the surface of partially demineralized dentin modified with phosphorylated chitosan. J Biomed Mater Res B Appl Biomater 2011;98:150-159.PubMed
- 13. Shrestha A, Friedman S, Kishen A. Photodynamically crosslinked and chitosan-incorporated dentin collagen. J Dent Res 2011;90:1346-1351.ArticlePubMedPDF
- 14. No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 2002;74:65-72.ArticlePubMed
- 15. Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod 2008;34:1515-1520.ArticlePubMed
- 16. Silva PV, Guedes DF, Nakadi FV, Pécora JD, Cruz-Filho AM. Chitosan: a new solution for removal of smear layer after root canal instrumentation. Int Endod J 2013;46:332-338.ArticlePubMed
- 17. Calamari SE, Bojanich MA, Barembaum SR, Berdicevski N, Azcurra AI. Antifungal and post-antifungal effects of chlorhexidine, fluconazole, chitosan and its combinations on Candida albicans. Med Oral Patol Oral Cir Bucal 2011;16:e23-e28.ArticlePubMed
- 18. Magura ME, Kafrawy AH, Brown CE Jr, Newton CW. Human saliva coronal microleakage in obturated root canals: an in vitro study. J Endod 1991;17:324-331.ArticlePubMed
- 19. Silva PV, Guedes DF, Pécora JD, da Cruz-Filho AM. Time-dependent effects of chitosan on dentin structures. Braz Dent J 2012;23:357-361.ArticlePubMed
- 20. De Moura MR, Aouada FA, Avena-Bustillos RJ, McHugh TH, Krochta JM, Mattoso LHC. Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. J Food Eng 2009;92:448-453.Article
- 21. Zandim DL, Corrêa FO, Rossa Júnior C, Sampaio JE. In vitro evaluation of the effect of natural orange juices on dentin morphology. Braz Oral Res 2008;22:176-183.ArticlePubMed
- 22. Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000;146:2395-2407.ArticlePubMed
- 23. Chávez de Paz LE. Image analysis software based on color segmentation for characterization of viability and physiological activity of biofilms. Appl Environ Microbiol 2009;75:1734-1739.ArticlePubMedPMCPDF
- 24. Wegehaupt F, Gries D, Wiegand A, Attin T. Is bovine dentin an appropriate substitute for human dentin in erosion/abrasion tests? J Oral Rehabil 2008;35:390-394.PubMed
- 25. Whitehead KA, Rogers D, Colligon J, Wright C, Verran J. Use of the atomic force microscope to determine the effect of substratum surface topography on the ease of bacterial removal. Colloids Surf B Biointerfaces 2006;51:44-53.ArticlePubMed
- 26. Lopes MB, Sinhoreti MA, Gonini Júnior A, Consani S, McCabe JF. Comparative study of tubular diameter and quantity for human and bovine dentin at different depths. Braz Dent J 2009;20:279-283.ArticlePubMed
- 27. Del Carpio-Perochena A, Bramante CM, Hungaro Duarte MA, de Andrade FB, Cavenago BC, Villas-Bôas MH, Ordinola-Zapata R, Amoroso-Silva P. Application of laser scanning microscopy for the analysis of oral biofilm dissolution by different endodontic irrigants. Dent Res J (Isfahan) 2014;11:442-447.PubMedPMC
- 28. Ordinola-Zapata R, Bramante CM, Cavenago B, Graeff MS, Gomes de Moraes I, Marciano M, Duarte MA. Antimicrobial effect of endodontic solutions used as final irrigants on a dentin biofilm model. Int Endod J 2012;45:162-168.PubMed
- 29. Inoue K, Yoshizuka K, Ohto K. Adsorptive separation of some metal ions by complexing agent types of chemically modified chitosan. Anal Chim Acta 1999;388:209-218.Article
- 30. Bassi R, Prasher SO, Simpson BK. Effects of organic acids on the adsorption of heavy metal ions by chitosan flakes. J Environ Sci Health 1999;34:289-294.Article
- 31. Blair HS, Ho TC. Studies in the adsorption and diffusion of ions in chitosan. J Chem Technol Biotechnol 1981;31:6-10.Article
- 32. Vold IMN, Vårum KM, Guibal E, Smidsrød O. Binding of ions to chitosan-selectivity studies. Carbohydr Polym 2003;54:471-477.Article
- 33. Pimenta JA, Zaparolli D, Pécora JD, Cruz-Filho AM. Chitosan: effect of a new chelating agent on the microhardness of root dentin. Braz Dent J 2012;23:212-217.ArticlePubMed
- 34. Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Annu Rev Microbiol 1987;41:435-464.ArticlePubMed
- 35. Wang QQ, Zhang CF, Chu CH, Zhu XF. Prevalence of Enterococcus faecalis in saliva and filled root canals of teeth associated with apical periodontitis. Int J Oral Sci 2012;4:19-23.ArticlePubMedPMCPDF
- 36. Saunders WP, Saunders EM. Coronal leakage as a cause of failure in root-canal therapy: a review. Endod Dent Traumatol 1994;10:105-108.ArticlePubMed
- 37. Hasan NA, Young BA, Minard-Smith AT, Saeed K, Li H, Heizer EM, McMillan NJ, Isom R, Abdullah AS, Bornman DM, Faith SA, Choi SY, Dickens ML, Cebula TA, Colwell RR. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS One 2014;9:e97699.ArticlePubMedPMC
- 38. Madison S, Swanson K, Chiles SA. An evaluation of coronal microleakage in endodontically treated teeth. Part II. Sealer types. J Endod 1987;13:109-112.ArticlePubMed
- 39. Madison S, Wilcox LR. An evaluation of coronal microleakage in endodontically treated teeth. Part III. In vivo study. J Endod 1988;14:455-458.ArticlePubMed
- 40. Gray GW, Wilkinson SG. The effect of ethylenediaminetetra-acetic acid on cell walls of some gram-negative bacteria. J Gen Microbiol 1965;39:385-399.PubMed
- 41. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003;4:1457-1465.ArticlePubMed
- 42. Young DH, Köhle H, Kauss H. Effect of chitosan on membrane-permeability of suspension-cultured glycine-max and phaseolus-vulgaris cells. Plant Physiol 1982;70:1449-1454.ArticlePubMedPMC
- 43. Persadmehr A, Torneck CD, Cvitkovitch DG, Pinto V, Talior I, Kazembe M, Shrestha S, McCulloch CA, Kishen A. Bioactive chitosan nanoparticles and photodynamic therapy inhibit collagen degradation in vitro. J Endod 2014;40:703-709.ArticlePubMed
- 44. Grande NM, Plotino G, Falanga A, Pomponi M, Somma F. Interaction between EDTA and sodium hypochlorite: a nuclear magnetic resonance analysis. J Endod 2006;32:460-464.ArticlePubMed
- 45. Yoo SH, Lee JS, Park SY, Kim YS, Chang PS, Lee HG. Effects of selective oxidation of chitosan on physical and biological properties. Int J Biol Macromol 2005;35:27-31.ArticlePubMed
REFERENCES
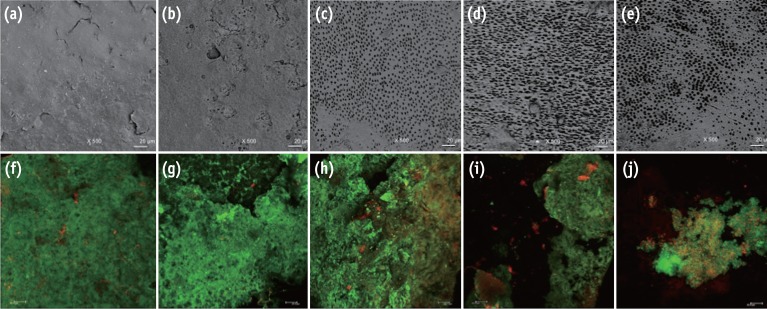
Representative images from the portable scanning electron microscope (×500) and confocal laser scanning microscope (×40). The irrigated pre-infection samples can be seen in images (a) - (e), and the infected samples after experimental irrigation protocols can be seen in images (f) - (j). A substantial amount of smear layer was observed when the samples were irrigated with sterile distilled water (a) and NaOCl (b). Visible dentinal tubules were seen in the samples treated with NaOCl-EDTA (c), NaOCl-EDTA-CNPs (d) and NaOCl-CNPs (e). A positive cellular viability and evident biomass were observed in the control (f), NaOCl (g) and NaOCl-EDTA (h) groups. The NaOCl-EDTA-CNPs and NaOCl-CNPs groups had decreased biomass (i) and interfered with bacterial growth (j). All bars represent 20 µm.
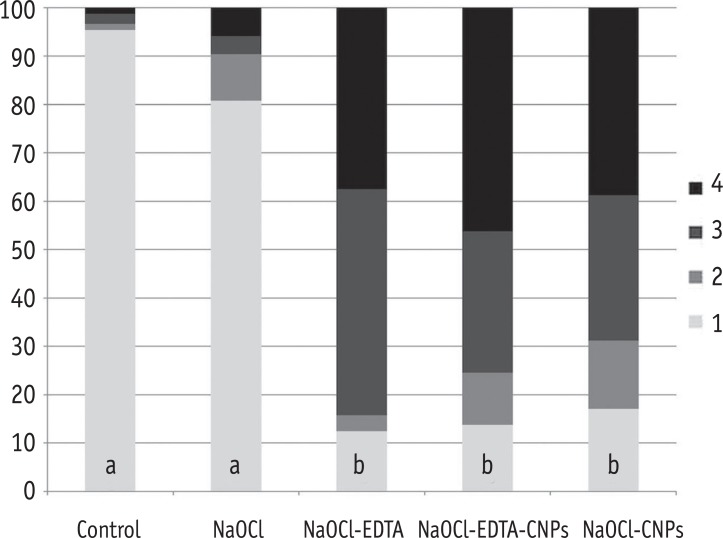
The percentage of areas with open dentinal tubules for each score (1 to 4). Having less than 10% of the area containing open dentinal tubules was scored as one, having 10 - 50% of the area containing open dentinal tubules was scored as two, having 50 - 70% of the area containing open dentinal tubules was scored as three and having more than 70% of the area containing open dentinal tubules was scored as four.
Medians (25 - 75 percentiles) of the total biovolume and the percentage of live cells of the comparisons among the groups
*Different superscript letters in each column represent significant differences (p < 0.05).
NaOCl, Sodium hypochlorite; NaOCl-EDTA, Sodium hypochlorite-ethylenediaminetetraacetic acid; NaOCl-EDTA-CNPs, Sodium hypochlorite-ethylenediaminetetraacetic acid-chitosan nanoparticles; NaOCl-CNPs, Sodium hypochlorite-chitosan nanoparticles.
Tables & Figures
REFERENCES
Citations

- Effect of experimental dentifrices containing epigallocatechin-3-gallate–loaded chitosan nanoparticles on permeability, tubule occlusion, microhardness, and wear in eroded dentin
Karen Pintado-Palomino, Letícia de Sousa Franco, Renata Siqueira Scatolin, Luiza Araújo Gusmão, Antonio Claudio Tedesco, Mario Sadaiti Ogasawara, Raissa Manoel Garcia, Tais Scaramucci, Silmara Aparecida Corona
JADA Foundational Science.2026; 5: 100057. CrossRef - Advanced nanoparticle-based antibacterial delivery for endodontic disinfection: A systematic review and meta-analysis
Kanwalpreet Kaur, Seerat Kaura, Ravinder S Saini, Maurya Manjunath, Shashit Shetty Bavabeedu, Mario Alberto Alarcón-Sánchez, Javier Flores-Fraile, Artak Heboyan
Journal of Dentistry.2026; 166: 106347. CrossRef - Comparison of Various Irrigation Techniques for the Removal of Silicone Oil-Based Calcium Hydroxide Intracanal Medicament from the Apical Third: An SEM Study
Shalin Ann Saji, Chitharanjan Shetty, Gurmeen Kaur, Sunheri Bajpe, Chandraprabha Chandraprabha, Rashi Shroff, Shazeena Qaiser, Surabhi Gupta
Journal of Health and Allied Sciences NU.2025; 15(01): 103. CrossRef - Comparative evaluation of smear layer removal and dentin wettability using 1% phytic acid with and without 0.2% chitosan nanoparticles: An in vitro study
Rahul Halkai, Kiran R. Halkai, Syeda Uzma Mahveen
Saudi Endodontic Journal.2025; 15(1): 38. CrossRef - Chitosan’s Ability to Remove the Smear Layer—A Systematic Review of Ex Vivo Studies
Ana Ferreira-Reguera, Inês Ferreira, Irene Pina-Vaz, Benjamín Martín-Biedma, José Martín-Cruces
Medicina.2025; 61(1): 114. CrossRef - Nanoparticles modified bioceramic sealers on solubility, antimicrobial efficacy, pushout bond strength and marginal adaptation at apical-third of canal dentin
Basil Almutairi, Fahad Alkhudhairy
PeerJ.2025; 13: e18840. CrossRef - Optimization of chitosan nanoparticle dentin pretreatment with different concentrations and application times to improve bonding at resin-dentin interface
Rinki Meher, Rashmi Rekha Mallick, Priyanka Sarangi, Amit Jena, Shradha Suman, Gaurav Sharma
Journal of Conservative Dentistry and Endodontics.2025; 28(3): 248. CrossRef - Innovative strategy for chitosan nanoparticles biosynthesis using Gelidium amansii, statistical optimization, characterization, cytotoxicity and molecular docking against hepatocellular carcinoma
Noura El-Ahmady El-Naggar, Naglaa Elshafey, Hagar I. Alafifi, Manar A. Eltahy, Reem I. Haikl, Hagar A. ElShazly, Yasmin W. Ahmed, Hossam I. Hassan, Mohamed M. Safo, S.A. Haroun, Asmaa A. El-Sawah
International Journal of Biological Macromolecules.2025; 311: 143687. CrossRef - Enhancing root canal sealing: Exploring the sealing potential of epoxy and calcium silicate-based sealers with chitosan nanoparticle enhancement
S. Harishma, Srilekha Jayakumar, K Shibani Shetty, Barkavi Panchatcharam, Jwaalaa Rajkumar, S. Harshini
Endodontology.2025; 37(3): 306. CrossRef - An in vitro comparative evaluation of silver and chitosan nanoparticles on shear bond strength of nanohybrid composite using different adhesion protocols
Roopadevi Garlapati, Nagesh Bolla, Mayana Aameena Banu, Anila Bandlapally Sreenivasa Guptha, Niharika Halder, Ram Chowdary Basam
Journal of Conservative Dentistry and Endodontics.2025; 28(6): 522. CrossRef - Comparative evaluation of the effect of chitosan and titanium dioxide nanoparticles on the pushout bond strength of mineral trioxide aggregate: An in vitro comparative study
Garima Poddar, Suparna Ganguly Saha, Rolly S. Agarwal, Geetika Pable, Affrin Shaikh, Shakti Singh
Endodontology.2025; 37(3): 289. CrossRef - Antibacterial efficacy of chitosan nanoparticles against Enterococcus faecalis in planktonic and biofilm forms
Raras Ajeng Enggardipta, Minato Akizuki, Kazumitsu Sekine, Kenichi Hamada, Tomoko Sumitomo, Hiromichi Yumoto
Journal of Applied Microbiology.2025;[Epub] CrossRef - Corrosion Inhibition Properties of Chitosan Doped With Fe, Cu, Zn, and Co on the Fe(110) Surface: A Combined DFT and Monte Carlo Simulation Study
D. M. Mamand, Peshawa O. Hama, Rebaz Anwar Omer, Rebaz Obaid Kareem, Dana S. Muhammad, Sarkawt A. Hussen, Yousif Hussein Azeez
Surface and Interface Analysis.2025; 57(12): 936. CrossRef - Comparison of penetration depth of chitosan, zinc oxide, and silica-doped titanium novel nanoparticle irrigant solutions – A confocal laser scanning microscopic in vitro study
Sree Laksmi Bademela, T. B. V. G. Raju, Krishna Prasad Parvathaneni, Abitha Seshadri, Nadimpalli Mahendra Varma, Gowtam Dev Dondapati
Endodontology.2024; 36(3): 280. CrossRef - Combined use of XP-Endo Finisher and different chelating agents on the smear layer
Meenu Elizabeth Saju, Ramya Raghu, Ashish Shetty, Lekha Santhosh, Subhashini Rajasekhara, Priya C. Yadav
Endodontology.2024;[Epub] CrossRef - Therapeutic efficacy of chitosan-based hybrid nanomaterials to treat microbial biofilms and their infections – A review
Anisha Salim, Palanivel Sathishkumar
International Journal of Biological Macromolecules.2024; 283: 137850. CrossRef - Local and systemic adverse effects of nanoparticles incorporated in dental materials- a critical review
Harini Karunakaran, Jogikalmat Krithikadatta, Mukesh Doble
The Saudi Dental Journal.2024; 36(1): 158. CrossRef - Effect of final irrigation protocols with chitosan nanoparticle and genipin on dentine against collagenase degradation: An ex‐vivo study
S. N. Şengül, S. Ozturk, K. Ulubayram, N. Pekel Bayramgil, S. Kucukkaya Eren
International Endodontic Journal.2024; 57(4): 477. CrossRef - Application of Nanomaterials in Endodontics
Farzaneh Afkhami, Yuan Chen, Laurence J. Walsh, Ove A. Peters, Chun Xu
BME Frontiers.2024;[Epub] CrossRef - Evaluation of the Effect of Chitosan-Based Irrigation Solutions on the Bond Strength of Mineral Trioxide Aggregate to Bulk-Fill Composite
Arzu Şahin Mantı, Bağdagül Helvacıoğlu Kıvanç
Journal of Functional Biomaterials.2024; 15(12): 370. CrossRef - In vitro analysis of compressive strength of root dentin on application of intracanal medicaments for different time periods
Kushal Kumar Ghosh, Sayantan Mukherjee, Paromita Mazumdar, Sahil Ali, Lovely Das
Journal of Conservative Dentistry and Endodontics.2024; 27(12): 1289. CrossRef - The comparative of chitosan and chitosan nanoparticle versus ethylenediaminetetraacetic acid on the smear layer removal: A systematic review and meta‐analysis of in vitro study
Hasan İlhan, Elif Bahar Cakici, Fatih Cakici
Microscopy Research and Technique.2024; 87(2): 181. CrossRef - Final Irrigant Temoporfin, Femtosecond Laser, and Chitosan Nanoparticles on Extrusion Bond Strength of Glass Fiber Post, Microhardness, and Modulus of Elasticity of Canal Dentin
Lujain Ibrahim N. Aldosari
Journal of Biomaterials and Tissue Engineering.2024; 14(2): 78. CrossRef - Comparative analysis of an epoxy resin-based and a premixed calcium silicate-based sealer’s push-out bond strength with and without incorporation of chitosan nanoparticles: An in vitro investigation
S. Harishma, K. B. Jeyalakshmi, K. Shibani Shetty, S. Harshini
Journal of Conservative Dentistry and Endodontics.2024; 27(9): 970. CrossRef - Chitosan: A Versatile Biomaterial Revolutionizing Endodontic Therapy
Akash Thakare, Shweta Sedani, Simran Kriplani , Aditya Patel, Utkarsh Umre
Cureus.2024;[Epub] CrossRef - Evaluation of the Effect of Farnesol and/or Chitosan as a Final Irrigation on Enterococcus faecalis Biofilm; An In-vitro Study
Ardavan Moinafshar, Hanieh Paik, Rashid Ramazanzadeh, Amjad Ahmadi, Mohammad Rastegar Khosravi
Scientific Journal of Kurdistan University of Medical Sciences.2024; 29(1): 85. CrossRef - Bionanomaterials an emerging field of nanotechnology
A.R. Shelin, S. Meenakshi
Archives of Materials Science and Engineering.2023; 121(1): 33. CrossRef - Bonding of chitosan and nanochitosan modified universal adhesive to dentin
Yasmin Ezz El-Din, Ahmed El-Banna, Tarek Salah Hussein
International Journal of Adhesion and Adhesives.2023; 125: 103432. CrossRef - Nanoparticles and Their Antibacterial Application in Endodontics
Nicoletta Capuano, Alessandra Amato, Federica Dell’Annunziata, Francesco Giordano, Veronica Folliero, Federica Di Spirito, Pragati Rajendra More, Anna De Filippis, Stefano Martina, Massimo Amato, Massimiliano Galdiero, Alfredo Iandolo, Gianluigi Franci
Antibiotics.2023; 12(12): 1690. CrossRef - In vitro techniques for evaluating smear layer removal by root canal irrigants: a literature review
Luis Hernán Carrillo Varguez, Aracely Serrano-Medina, Eduardo Alberto López Maldonado, Eustolia Rodríguez Velázquez, José Manuel Cornejo-Bravo
Horizon Interdisciplinary Journal.2023; 1(2): 58. CrossRef - Applicability of a Natural Nano-derivative as a Mouth Rinse on Salivary pH and S. mutans Count: An Ex Vivo Study
Raja S Prathigudupu, Deepthi N Gavarraju, Sai S Kallam, Sai Sankar J Avula, Chaitanya M Sattenapalli, Amrutha Valli Audipudi
World Journal of Dentistry.2023; 14(3): 207. CrossRef - Nanopartículas antimicrobianas en endodoncia: Revisión narrativa
Gustavo Adolfo Tovar Rangel , Fanny Mildred González Sáenz , Ingrid Ximena Zamora Córdoba , Lina María García Zapata
Revista Estomatología.2023;[Epub] CrossRef - Quantification of Calcium Ions From the Irrigants Activated With Erbium-Doped Yttrium Aluminum Garnet (Er:YAG) Laser in the Root Dentin: An In Vitro Atomic Absorption Spectrophotometer Study
Dhanalakshmi P, Kiran Kumar N, K Rashmi, Biji Brigit, Shwetha R S, Sourabh T J
Cureus.2023;[Epub] CrossRef - Evaluation of chelating effect of chitosan as intracanal lubricant and an irrigant on smear layer removal – An in-vitro scanning electron microscope study
Thati Jyotsnanjali, M. A. Ranjini, G. R. Krishna Kumar, D. V. Swapna, S. N. Joshi, Roopa R. Nadig
Endodontology.2023; 35(3): 254. CrossRef - Assessment of the Effectiveness of Two Different Dentin Biomodifiers on Shear Bond Strength of Dentin and Resin Interface: A Comparative Study
Narendra V Penumatsa, AlWaleed Abushanan, Uthman S Uthman, Abdulhamid Al Ghwainem, Adel S Alqarni, Abdulfatah Alazmah
World Journal of Dentistry.2023; 14(1): 16. CrossRef - Scanning electron microscopy evaluation of smear layer removal using ethylenediaminetetraacetic acid, etidronic acid, and chitosan nanoparticle solution as root canal irrigants
Sunheri Bajpe, Chitharanjan Shetty, Aditya Shetty, Gurmeen Kaur, Shalin Ann Saji, Chandra Prabha
Endodontology.2023; 35(1): 48. CrossRef - Green fabrication of chitosan nanoparticles using Lavendula angustifolia, optimization, characterization and in‑vitro antibiofilm activity
Noura El-Ahmady El-Naggar, Marwa Eltarahony, Elsayed E. Hafez, Shimaa I. Bashir
Scientific Reports.2023;[Epub] CrossRef - Nanobiotechnology: Synthesis components and a few approaches for controlling plant diseases
Malavika Ram A K, Ramji Singh, Meenakshi Rana, S.A. Dwivedi, Kshitij Parmar, Abha Sharma, Chitranjan Kumar, Vineeta Pandey, Vikash Kumar, Shashank Mishra, Ajay Tomar
Plant Nano Biology.2023; 4: 100038. CrossRef - Physicochemical and biological properties of a biostimulating membrane (BBio) for pulp capping
Natalino Lourenço Neto, Luciana Lourenço Ribeiro Vitor, Silgia Aparecida da Costa, Sirlene Maria da Costa, Thiago Cruvinel, Thais Marchini Oliveira, Rodrigo Cardoso Oliveira, Maria Aparecida Andrade Moreira Machado
Materials Letters.2022; 308: 131186. CrossRef - In Vitro Study of Irrigation solution of Chitosan Nanoparticles to Inhibit the Adhesion and Biofilm Formation of Enterococcus faecalis in the Root Canal
Imelda Darmawi, Trimurni Abidin, Harry Agusnar, Basri A. Gani
Research Journal of Pharmacy and Technology.2022; : 2691. CrossRef - Nanoparticles in Endodontics Disinfection: State of the Art
Xavier Roig-Soriano, Eliana B. Souto, Firas Elmsmari, Maria Luisa Garcia, Marta Espina, Fernando Duran-Sindreu, Elena Sánchez-López, Jose Antonio González Sánchez
Pharmaceutics.2022; 14(7): 1519. CrossRef - An In Vitro Study Comparing the Antimicrobial Efficacy of 0.2% Chitosan, 3% Sodium Hypochlorite, 2% Chlorhexidine against Enterococcus faecalis, Alone and in Conjunction with Diode Laser
Sameer Makkar, Tamanpreet Kaur, Pallavi Goel, Virat Galhotra, Jatinder Mohan, Neetu Bala
International Journal of Clinical Pediatric Dentistry.2022; 15(1): 109. CrossRef - Chitosan-Based Carbon Dots with Applied Aspects: New Frontiers of International Interest in a Material of Marine Origin
Angel M. Villalba-Rodríguez, Reyna Berenice González-González, Manuel Martínez-Ruiz, Elda A. Flores-Contreras, María Fernanda Cárdenas-Alcaide, Hafiz M. N. Iqbal, Roberto Parra-Saldívar
Marine Drugs.2022; 20(12): 782. CrossRef - The Effect of Final Irrigation Protocols on the Apical Sealing Ability of Epoxy Resin-based and Bioceramic-based Root Canal Sealers
Anan Medhat, Angie Ghoneim, Nehal Nabil Roshdy
Open Access Macedonian Journal of Medical Sciences.2022; 10(D): 458. CrossRef - Molecular docking reveals Chitosan nanoparticle protection mechanism for dentin against Collagen-binding bacteria
Ziliang Zhou, Yanyan Yang, Lu He, Junmei Wang, Jie Xiong
Journal of Materials Science: Materials in Medicine.2022;[Epub] CrossRef - Evaluation of Free Available Chlorine of Sodium Hypochlorite When Admixed with 0.2% Chitosan: A Preliminary Study
Rupali Karale, Nithin K Shetty, Prashanth Bytarahosalli Rajachar, Mythreyee S Vidhya, Vinay Kumar Govindaraju
The Journal of Contemporary Dental Practice.2022; 22(10): 1171. CrossRef - Effect of chitosan irrigant solutions on the release of bioactive proteins from root dentin
Sara Quijano-Guauque, Lilia J. Bernal-Cepeda, Félix G. Delgado, Jaime E. Castellanos, Claudia García-Guerrero
Clinical Oral Investigations.2022; 27(2): 691. CrossRef - Chemical and morphological characterization of self-etch primers incorporated with nanochitosan
Pâmella Coelho Dias, Isabela Barbosa Quero, Juliana Jendiroba Faraoni, Regina Guenka Palma-Dibb
International Journal of Adhesion and Adhesives.2022; 118: 103215. CrossRef - The effects of different root canal irrigation protocols and artificial aging procedures on the bond strength between dentin and hybrid ceramic posts
Celalettin Topbaş, Şevki Çınar, Bike Altan, Dursun Ali Şirin, Mehmet Ali Fildişi
BMC Oral Health.2022;[Epub] CrossRef - Effect of two different concentrations of chitosan irrigation on smear layer removal during root canal treatment
Doaa M. Abd El-latif, Abeer M. Darrag, Dalia A. Sherif
Tanta Dental Journal.2022; 19(4): 204. CrossRef - Impact of Dentin Conditioning and Sealer Modification With Chitosan-Hydroxyapatite Nanocomplexes on the Antibacterial and Mechanical Characteristics of Root Dentin
Aldo del Carpio-Perochena, Eric Nicholson, Chandra Veer Singh, Josette Camilleri, Anil Kishen
Journal of Endodontics.2022; 48(10): 1319. CrossRef - Assessment of Antimicrobial Efficacy of Nano Chitosan, Chlorhexidine, Chlorhexidine/Nano Chitosan Combination versus Sodium Hypochlorite Irrigation in Patients with Necrotic Mandibular Premolars: A Randomized Clinical Trial
Maha Nasr, Alaa Diab, Nehal Roshdy, Amira Farouk
Open Access Macedonian Journal of Medical Sciences.2021; 9(D): 235. CrossRef - Enhanced visualization of the root canal morphology using a chitosan-based endo-radiopaque solution
Shashirekha Govind, Amit Jena, Satabdi Pattanaik, Mahaprasad Anarasi, Satyajit Mohapatra, Vinay Shivagange
Restorative Dentistry & Endodontics.2021;[Epub] CrossRef - Chitosan-Based Biomaterial, Calcium Hydroxide and Chlorhexidine for Potential Use as Intracanal Medication
Bruna de Siqueira Nunes, Rosana Araújo Rosendo, Abrahão Alves de Oliveira Filho, Marcus Vinícius Lia Fook, Wladymyr Jefferson Bacalhau de Sousa, Rossemberg Cardoso Barbosa, Hermano de Vasconcelos Pina, João Emídio da Silva Neto, Solomon Kweku Sagoe Amoah,
Materials.2021; 14(3): 488. CrossRef - Nanostructures as Targeted Therapeutics for Combating Oral Bacterial Diseases
Shima Afrasiabi, Nasim Chiniforush, Hamid Reza Barikani, Alireza Partoazar, Ramin Goudarzi
Biomedicines.2021; 9(10): 1435. CrossRef - Microbiological Aspects of Root Canal Infections and Disinfection Strategies: An Update Review on the Current Knowledge and Challenges
Jasmine Wong, Daniel Manoil, Peggy Näsman, Georgios N. Belibasakis, Prasanna Neelakantan
Frontiers in Oral Health.2021;[Epub] CrossRef - Nanomaterials Application in Endodontics
Wojciech Zakrzewski, Maciej Dobrzyński, Anna Zawadzka-Knefel, Adam Lubojański, Wojciech Dobrzyński, Mateusz Janecki, Karolina Kurek, Maria Szymonowicz, Rafał Jakub Wiglusz, Zbigniew Rybak
Materials.2021; 14(18): 5296. CrossRef - Preparation and application of chitosan biomaterials in dentistry
Chenxi Zhang, Didi Hui, Colin Du, Huan Sun, Wei Peng, Xiaobing Pu, Zhengyong Li, Jianxun Sun, Changchun Zhou
International Journal of Biological Macromolecules.2021; 167: 1198. CrossRef - The Potential Translational Applications of Nanoparticles in Endodontics
Jasmine Wong, Ting Zou, Angeline Hui Cheng Lee, Chengfei Zhang
International Journal of Nanomedicine.2021; Volume 16: 2087. CrossRef - Chitosan Enhances the Anti-Biofilm Activity of Biodentine against an Interkingdom Biofilm Model
Sumaya Abusrewil, Jason L. Brown, Christopher Delaney, Mark C. Butcher, Mohammed Tiba, J. Alun Scott, Gordon Ramage, William McLean
Antibiotics.2021; 10(11): 1317. CrossRef - Evaluation of Anti-Biofilm Activity of Mouthrinses Containing Tannic Acid or Chitosan on Dentin In Situ
Anton Schestakow, Moritz S. Guth, Tobias A. Eisenmenger, Matthias Hannig
Molecules.2021; 26(5): 1351. CrossRef - An All-inclusive Estimation of Antibacterial and Antifungal Efficiencies of Propolis and Cetrimide Root Canal Irrigants against Enterococcus faecalis and Candida albicans: An In vitro (Original Research) Study
Sumita Giri Nishad
Journal of Research and Advancement in Dentistry.2021; 12(5): 185. CrossRef - Carbohydrate-containing nanoparticles as vaccine adjuvants
Xinyuan Zhang, Zhigang Zhang, Ningshao Xia, Qinjian Zhao
Expert Review of Vaccines.2021; 20(7): 797. CrossRef - RANDOMIZED CLINICAL TRIAL OF ANTIMICROBIAL EFFICACY OF TWO HERBAL PRODUCTS AS ROOT CANAL IRRIGANTS IN PRIMARY ENDODONTIC INFECTIONS.
Sonam Dhall, Rakesh Mittal, Monika Tandan
Journal of Indian Dental Association.2021;[Epub] CrossRef - Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging
Murat Yanat, Karin Schroën
Reactive and Functional Polymers.2021; 161: 104849. CrossRef -
Effect of the Incorporation of Chitosan and TiO
2
Nanoparticles on the Shear Bond Strength of an Orthodontic Adhesive: An In Vitro Study
Fahimeh Farzanegan, Hooman Shafaee, Majid Darroudi, Abdolrasoul Rangrazi
Journal of Advanced Oral Research.2021; 12(2): 261. CrossRef - Antibacterial effect of hyaluronan/chitosan nanofilm in the initial adhesion of Pseudomonas aeruginosa wild type, and IV pili and LPS mutant strains
Jacobo Hernandez-Montelongo, Gianlucca G. Nicastro, Thays de O. Pereira, Mariana Zavarize, Marisa M. Beppu, Waldemar A.A. Macedo, Regina L. Baldini, Monica A. Cotta
Surfaces and Interfaces.2021; 26: 101415. CrossRef - Randomized Clinical Trial of Antimicrobial Effi cacy of two Herbal Products as Root Canal Irrigants in Primary Endodontic Infections
Sonam Dhall, Rakesh Mittal, Monika Tandan
Journal of Indian Dental Association.2021;[Epub] CrossRef - Comparative Evaluation Of Fracture Resistance Of Root Dentin To Different Intracanal Medicaments: In-Vitro Study
Anita Sanap-Tandale, Nikhil Borse, Kunal Kunjir, Karan Bhargava
Annals of Dental Specialty.2021; 9(2): 86. CrossRef - Engineering Polymeric Nanosystems against Oral Diseases
Valeria Mercadante, Edoardo Scarpa, Valeria De Matteis, Loris Rizzello, Alessandro Poma
Molecules.2021; 26(8): 2229. CrossRef - Chelation capability of chitosan and chitosan derivatives: Recent developments in sustainable corrosion inhibition and metal decontamination applications
Chandrabhan Verma, M.A. Quraishi
Current Research in Green and Sustainable Chemistry.2021; 4: 100184. CrossRef - Comparative effects of final canal irrigation with chitosan and EDTA
Polliana Vilaça Silva Antunes, Luis Eduardo Souza Flamini, Jardel Francisco Mazzi Chaves, Ricardo Gariba Silva, Antonio Miranda da Cruz Filho
Journal of Applied Oral Science.2020;[Epub] CrossRef - Antibacterial property of chitosan against E. faecalis standard strain and clinical isolates
Apimon SUPOTNGARMKUL, Anchana PANICHUTTRA, Chootima RATISOONTORN, Mettachit NAWACHINDA, Oranart MATANGKASOMBUT
Dental Materials Journal.2020; 39(3): 456. CrossRef - Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry
Pooyan Makvandi, Jun Ting Gu, Ehsan Nazarzadeh Zare, Behnaz Ashtari, Arash Moeini, Franklin R. Tay, Li-na Niu
Acta Biomaterialia.2020; 101: 69. CrossRef - A chitosan-based irrigant improves the dislocation resistance of a mineral trioxide aggregate-resin hybrid root canal sealer
Esin Ozlek, Priti Pragati Rath, Anil Kishen, Prasanna Neelakantan
Clinical Oral Investigations.2020; 24(1): 151. CrossRef - Detection, treatment and prevention of endodontic biofilm infections: what’s new in 2020?
Sumaya Abusrewil, Om Alkhir Alshanta, Khawlah Albashaireh, Saeed Alqahtani, Christopher J. Nile, James Alun Scott, William McLean
Critical Reviews in Microbiology.2020; 46(2): 194. CrossRef - Cytotoxicity of Chelating Agents Used In Endodontics and Their Influence on MMPs of Cell Membranes
Kellin Pivatto, Fabio Luis Miranda Pedro, Orlando Aguirre Guedes, Adriana Fernandes da Silva, Evandro Piva, Thiago Machado Pereira, Welligton Luiz de Oliveira da Rosa, Alvaro Henrique Borges
Brazilian Dental Journal.2020; 31(1): 32. CrossRef - The Effect of Chitosan Nanoparticle as A Final Irrigation Solution on The Smear Layer Removal, Micro-hardness and Surface Roughness of Root Canal Dentin
Diatri Nari Ratih, Raras Ajeng Enggardipta, Aqilla Tiara Kartikaningtyas
The Open Dentistry Journal.2020; 14(1): 19. CrossRef - Time-Dependent Effect of Chitosan Nanoparticles as Final Irrigation on the Apical Sealing Ability and Push-Out Bond Strength of Root Canal Obturation
Diatri Nari Ratih, Nikita Ika Sari, Pribadi Santosa, Nofa Mardia Ningsih Kaswati
International Journal of Dentistry.2020; 2020: 1. CrossRef - Targeting tuberculosis infection in macrophages using chitosan oligosaccharide nanoplexes
Uday Koli, Kayzad Nilgiriwala, Kalpana Sriraman, Ratnesh Jain, Prajakta Dandekar
Journal of Nanoparticle Research.2019;[Epub] CrossRef - Application of Antimicrobial Nanoparticles in Dentistry
Wenjing Song, Shaohua Ge
Molecules.2019; 24(6): 1033. CrossRef - Assessment of antibacterial activity of 2.5% NaOCl, chitosan nano-particles against Enterococcus faecalis contaminating root canals with and without diode laser irradiation: an in vitro study
Nehal Nabil Roshdy, Engy M. Kataia, Neveen A. Helmy
Acta Odontologica Scandinavica.2019; 77(1): 39. CrossRef - In Vitro Antimicrobial Effect of Bioadhesive Oral Membrane with Chlorhexidine Gel
Annelyze Podolan Kloster, Natalino Lourenço Neto, Silgia Aparecida da Costa, Thais Marchini Oliveira, Rodrigo Cardoso de Oliveira, Maria Aparecida Andrade Moreira Machado
Brazilian Dental Journal.2018; 29(4): 354. CrossRef - How to improve root canal filling in teeth subjected to radiation therapy for cancer
Fabiana de Góes Paiola, Fabiane Carneiro Lopes, Jardel Francisco Mazzi-Chaves, Rodrigo Dantas Pereira, Harley Francisco Oliveira, Alexandra Mussolino de Queiroz, Manoel Damião de Sousa-Neto
Brazilian Oral Research.2018;[Epub] CrossRef - Assessment of toxicity and oxidative DNA damage of sodium hypochlorite, chitosan and propolis on fibroblast cells
Zeliha Uğur Aydin, Kerem Engin Akpinar, Ceylan Hepokur, Demet Erdönmez
Brazilian Oral Research.2018;[Epub] CrossRef - Recent developments in the use of nanoparticles for treatment of biofilms
Chendong Han, Nicholas Romero, Stephen Fischer, Julia Dookran, Aaron Berger, Amber L. Doiron
Nanotechnology Reviews.2017; 6(5): 383. CrossRef - Assessment of the Amount of Calcium Ions Released after the use of Different Chelating Agents and Agitation Protocols
Fábio Luis Miranda Pedro, Laura Maria Amorim Santana Costa, Gilberto Siebert Filho, Orlando Aguirre Guedes, Thiago Machado Pereira, Alvaro Henrique Borges
The Open Dentistry Journal.2017; 11(1): 133. CrossRef - Wettability and surface morphology of eroded dentin treated with chitosan
Mirian Saavedra Ururahy, Fabiana Almeida Curylofo-Zotti, Rodrigo Galo, Lucas Fabricio Bahia Nogueira, Ana Paula Ramos, Silmara Aparecida Milori Corona
Archives of Oral Biology.2017; 75: 68. CrossRef - Biophysical and biological characterization of intraoral multilayer membranes as potential carriers: A new drug delivery system for dentistry
Mariana dos Santos Silva, Natalino Lourenço Neto, Silgia Aparecida da Costa, Sirlene Maria da Costa, Thais Marchini Oliveira, Rodrigo Cardoso de Oliveira, Maria Aparecida Andrade Moreira Machado
Materials Science and Engineering: C.2017; 71: 498. CrossRef - Antibacterial Properties of Chitosan Nanoparticles and Propolis Associated with Calcium Hydroxide against Single- and Multispecies Biofilms: An In Vitro and In Situ Study
Aldo del Carpio-Perochena, Anil Kishen, Rafael Felitti, Anjali Y. Bhagirath, Manoj R. Medapati, Christopher Lai, Rodrigo S. Cunha
Journal of Endodontics.2017; 43(8): 1332. CrossRef - Analysis of the shelf life of chitosan stored in different types of packaging, using colorimetry and dentin microhardness
Antonio Miranda da Cruz-Filho, Angelo Rafael de Vito Bordin, Luis Eduardo Souza-Flamini, Débora Fernandes da Costa Guedes, Paulo César Saquy, Ricardo Gariba Silva, Jesus Djalma Pécora
Restorative Dentistry & Endodontics.2017; 42(2): 87. CrossRef - Does nanobiotechnology create new tools to combat microorganisms?
Marlena K. Zielińska-Górska, Ewa Sawosz, Konrad Górski, André Chwalibog
Nanotechnology Reviews.2017; 6(2): 171. CrossRef - New frontiers for anti-biofilm drug development
Suzana M. Ribeiro, Mário R. Felício, Esther Vilas Boas, Sónia Gonçalves, Fabrício F. Costa, Ramar Perumal Samy, Nuno C. Santos, Octávio L. Franco
Pharmacology & Therapeutics.2016; 160: 133. CrossRef - The effect of combined use of chitosan and PIPS on push-out bond strength of root canal filling materials
Ugur Aydin, Fatih Aksoy, Samet Tosun, Abdul Semih Ozsevik
Journal of Adhesion Science and Technology.2016; 30(18): 2024. CrossRef - Organic Nanomaterials and Their Applications in the Treatment of Oral Diseases
Maria Virlan, Daniela Miricescu, Radu Radulescu, Cristina Sabliov, Alexandra Totan, Bogdan Calenic, Maria Greabu
Molecules.2016; 21(2): 207. CrossRef
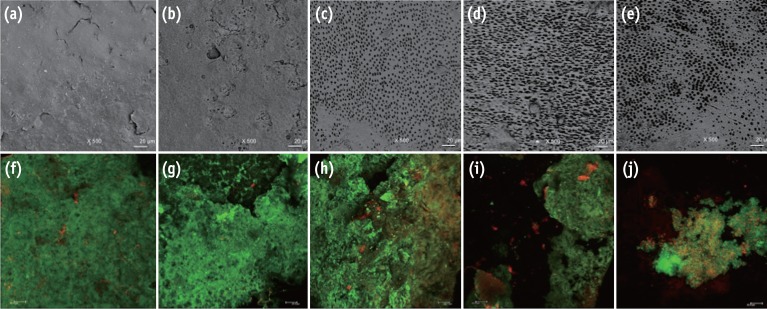
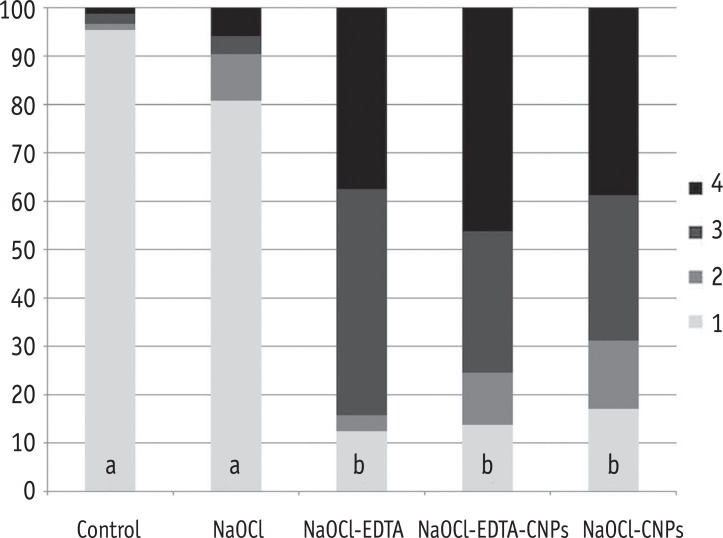
Figure 1
Figure 2
Medians (25 - 75 percentiles) of the total biovolume and the percentage of live cells of the comparisons among the groups
| Total Biovolume (µm3/µm2) | Percentage of live cells | |
|---|---|---|
| Control | 5.0 (4.0 - 5.4)a | 92 (90 - 96)a |
| NaOCl | 3.9 (3.1 - 5.1)ab | 90 (85 - 92)a |
| NaOCl-EDTA | 3.6 (2.8 - 5.1)ab | 91 (87 - 94)a |
| NaOCl-EDTA-CNPs | 2.6 (0.85 - 4.4)c | 73 (51 - 86)b |
| NaOCl-CNPs | 2.7 (1.8 - 4.2)bc | 77 (60 - 83)b |
*Different superscript letters in each column represent significant differences (p < 0.05).
NaOCl, Sodium hypochlorite; NaOCl-EDTA, Sodium hypochlorite-ethylenediaminetetraacetic acid; NaOCl-EDTA-CNPs, Sodium hypochlorite-ethylenediaminetetraacetic acid-chitosan nanoparticles; NaOCl-CNPs, Sodium hypochlorite-chitosan nanoparticles.
*Different superscript letters in each column represent significant differences ( NaOCl, Sodium hypochlorite; NaOCl-EDTA, Sodium hypochlorite-ethylenediaminetetraacetic acid; NaOCl-EDTA-CNPs, Sodium hypochlorite-ethylenediaminetetraacetic acid-chitosan nanoparticles; NaOCl-CNPs, Sodium hypochlorite-chitosan nanoparticles.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

