Articles
- Page Path
- HOME > Restor Dent Endod > Volume 39(2); 2014 > Article
- Research Article Pull-out bond strength of a self-adhesive resin cement to NaOCl-treated root dentin: effect of antioxidizing agents
- Maryam Khoroushi1, Marzieh Kachuei2
-
2014;39(2):-103.
DOI: https://doi.org/10.5395/rde.2014.39.2.95
Published online: March 21, 2014
1Dental Materials Research Center and Department of Operative Dentistry, Isfahan University of Medical Sciences School of Dentistry, Isfahan, Iran.
2Dental Students Research Center, Isfahan University of Medical Sciences School of Dentistry, Isfahan, Iran.
- Correspondence to Maryam Khoroushi, DDS, MS. Professor, Department of Operative Dentistry, Isfahan University of Medical Sciences School of Dentistry, Hezar Jerib St., Isfahan, Iran 81746-73461. TEL, +98-311-6687080; FAX, +98-311-6687080; khoroushi@dnt.mui.ac.ir
©Copyights 2014. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,869 Views
- 10 Download
- 15 Crossref
Abstract
-
Objectives This study evaluated the effect of three antioxidizing agents on pull-out bond strengths of dentin treated with sodium hypochlorite.
-
Materials and Methods Root canals of 75 single-rooted human teeth were prepared. Fifteen teeth were irrigated with normal saline for a negative control group, and the remaining 60 teeth (groups 2 - 5) with 2.5% NaOCl. The teeth in group 2 served as a positive control. Prior to post cementation, the root canals in groups 3 - 5 were irrigated with three antioxidizing agents including 10% rosmarinic acid (RA, Baridge essence), 10% hesperidin (HPN, Sigma), and 10% sodium ascorbate hydrogel (SA, AppliChem). Seventy-five spreaders (#55, taper .02, Produits Dentaires S.A) were coated with silica and silanized with the Rocatec system and ceramic bond. All the prepared spreaders were cemented with a self-adhesive resin cement (Bifix SE, Voco Gmbh) in the prepared canals. After storage in distilled water (24 h/37℃), the spreaders were pulled out in a universal testing machine at a crosshead speed of 1.0 mm/min. Pull-out strength values were analyzed by one-way ANOVA and Tukey's HSD test (α = 0.05).
-
Results There were significant differences between study groups (p = 0.016). The highest pull-out strength was related to the SA group. The lowest strength was obtained in the positive control group.
-
Conclusions Irrigation with NaOCl during canal preparation decreased bond strength of resin cement to root dentin. Amongst the antioxidants tested, SA had superior results in reversing the diminishing effect of NaOCl irrigation on the bond strength to root dentin.
Introduction
Materials and methods
Results
Discussion
Conclusions
Acknowledgement
- 1. Morgano SM. Restoration of pulpless teeth: application of traditional principles in present and future contexts. J Prosthet Dent 1996;75:375-380.ArticlePubMed
- 2. Ebert J, Leyer A, Günther O, Lohbauer U, Petschelt A, Frankenberger R, Roggendorf MJ. Bond strength of adhesive cements to root canal dentin tested with a novel pull-out approach. J Endod 2011;37:1558-1561.ArticlePubMed
- 3. Glazer B. Restoration of endodontically treated teeth with carbon fibre posts-a prospective study. J Can Dent Assoc 2000;66:613-618.PubMed
- 4. Malferrari S, Monaco C, Scotti R. Clinical evaluation of teeth restored with quartz fiber-reinforced epoxy resin posts. Int J Prosthodont 2003;16:39-44.
- 5. Amaral M, Santini MF, Wandscher V, Amaral R, Valandro LF. An in vitro comparison of different cementation strategies on the pull-out strength of a glass fiber post. Oper Dent 2009;34:443-451.ArticlePubMedPDF
- 6. Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater 2008;24:90-101.ArticlePubMed
- 7. Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials 2003;24:3795-3803.PubMed
- 8. Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res 2004;83:216-221.ArticlePubMedPDF
- 9. Carrilho MR, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, Pashley DH, Tjäderhane L. Chlorhexidine preserves dentin bond in vitro. J Dent Res 2007;86:90-94.ArticlePubMedPDF
- 10. Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res 2006;85:22-32.ArticlePubMedPDF
- 11. Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res 2005;84:741-746.ArticlePubMedPDF
- 12. Bedran-Russo AK, Yoo KJ, Ema KC, Pashley DH. Mechanical properties of tannic-acid-treated dentin matrix. J Dent Res 2009;88:807-811.ArticlePubMedPMCPDF
- 13. Macedo GV, Yamauchi M, Bedran-Russo AK. Effects of chemical cross-linkers on caries-affected dentin bonding. J Dent Res 2009;88:1096-1100.ArticlePubMedPMCPDF
- 14. Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res B Appl Biomater 2009;91:419-424.PubMedPMC
- 15. Wang R, Zhou W, Jiang X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J Agric Food Chem 2008;56:2694-2701.ArticlePubMed
- 16. Magalhães AC, Wiegand A, Rios D, Hannas A, Attin T, Buzalaf MA. Chlorhexidine and green tea extract reduce dentin erosion and abrasion in situ. J Dent 2009;37:994-998.ArticlePubMed
- 17. Cho YH, Kim JH, Sim GS, Lee BC, Pyo HB, Park HD. Inhibitory effects of antioxidant constituents from Melothria heterophylla on matrix metalloproteinase-1 expression in UVA-irradiated human dermal fibroblasts. J Cosmet Sci 2006;57:279-289.PubMed
- 18. Islam SM, Hiraishi N, Nassar M, Sono R, Otsuki M, Takatsura T, Yiu C, Tagami J. In vitro effect of hesperidin on root dentin collagen and de/re-mineralization. Dent Mater J 2012;31:362-367.ArticlePubMed
- 19. Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Antiinflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001;56:683-687.ArticlePubMed
- 20. Trzeciakiewicz A, Habauzit V, Mercier S, Barron D, Urpi-Sarda M, Manach C, Offord E, Horcajada MN. Molecular mechanism of hesperetin-7-O-glucuronide, the main circulating metabolite of hesperidin, involved in osteoblast differentiation. J Agric Food Chem 2010;58:668-675.ArticlePubMed
- 21. Nostro A, Cannatelli MA, Crisafi G, Musolino AD, Procopio F, Alonzo V. Modifications of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett Appl Microbiol 2004;38:423-427.ArticlePubMed
- 22. Hiraishi N, Sono R, Islam MS, Otsuki M, Tagami J, Takatsuka T. Effect of hesperidin in vitro on root dentine collagen and demineralization. J Dent 2011;39:391-396.ArticlePubMed
- 23. Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res 2005;25:3367-3374.PubMed
- 24. Benavente-García O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem 2008;56:6185-6205.ArticlePubMed
- 25. Miller EG, Peacock JJ, Bourland TC, Taylor SE, Wright JM, Patil BS, Miller EG. Inhibition of oral carcinogenesis by citrus flavonoids. Nutr Cancer 2008;60:69-74.ArticlePubMed
- 26. Wood N. Bound sugars in hepatic glycoproteins from male rats during dietary citrus bioflavonoid and/or ascorbic acid supplementation. J Med Food 2005;8:512-517.ArticlePubMed
- 27. Horcajada MN, Habauzit V, Trzeciakiewicz A, Morand C, Gil-Izquierdo A, Mardon J, Lebecque P, Davicco MJ, Chee WS, Coxam V, Offord E. Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J Appl Physiol 2008;104:648-654.ArticlePubMed
- 28. Choi EM, Kim YH. Hesperetin attenuates the highly reducing sugar-triggered inhibition of osteoblast differentiation. Cell Biol Toxicol 2008;24:225-231.ArticlePubMedPDF
- 29. Islam S, Hiraishi N, Nassar M, Yiu C, Otsuki M, Tagami J. Effect of natural cross-linkers incorporation in a self-etching primer on dentine bond strength. J Dent 2012;40:1052-1059.ArticlePubMed
- 30. Lyubimova T, Caglio S, Gelfi C, Righetti PG, Rabilloud T. Photopolymerization of polyacrylamide gels with methylene blue. Electrophoresis 1993;14:40-50.ArticlePubMed
- 31. Tirkeş S, Toppare L, Alkan S, Bakir U, Onen A, Yağci Y. Immobilization of glucose oxidase in polypyrrole/polytetrahydrofuran graft copolymers. Int J Biol Macromol 2002;30:81-87.PubMed
- 32. Hernández-Hernández E, Ponce-Alquicira E, Jaramillo-Flores ME, Guerrero Legarreta I. Antioxidant effect rosemary (Rosmarinus officinalis L.) and oregano (Origanum vulgare L.) extracts on TBARS and colour of model raw pork batters. Meat Sci 2009;81:410-417.ArticlePubMed
- 33. Apak R, Güçlü K, Ozyürek M, Bektas Oğlu B, Bener M. Cupric ion reducing antioxidant capacity assay for food antioxidants: vitamins, polyphenolics, and flavonoids in food extracts. Methods Mol Biol 2008;477:163-193.ArticlePubMed
- 34. Bowen RL. Adhesive bonding of various materials to hard tooth tissues. IV. Bonding to dentin, enamel, and fluorapatite improved by the use of a surface-active comonomer. J Dent Res 1965;44:906-911.ArticlePubMedPDF
- 35. Taniguchi G, Nakajima M, Hosaka K, Iwamoto N, Ikeda M, Foxton RM, Tagami J. Improving the effect of NaOCl pretreatment on bonding to caries-affected dentin using self-etch adhesives. J Dent 2009;37:769-775.ArticlePubMed
- 36. Prasansuttiporn T, Nakajima M, Kunawarote S, Foxton RM, Tagami J. Effect of reducing agents on bond strength to NaOCl-treated dentin. Dent Mater 2011;27:229-234.ArticlePubMed
- 37. da Cunha LF, Furuse AY, Mondelli RF, Mondelli J. Compromised bond strength after root dentin deproteinization reversed with ascorbic acid. J Endod 2010;36:130-134.ArticlePubMed
- 38. Weston CH, Ito S, Wadgaonkar B, Pashley DH. Effects of time and concentration of sodium ascorbate on reversal of NaOCl-induced reduction in bond strengths. J Endod 2007;33:879-881.ArticlePubMed
- 39. Moreira DM, de Andrade Feitosa JP, Line SR, Zaia AA. Effects of reducing agents on birefringence dentin collagen after use of different endodontic auxiliary chemical substances. J Endod 2011;37:1406-1411.ArticlePubMed
- 40. May LG, Salvia AC, Souza RO, Michida SM, Valera MC, Takahashi FE, Bottino MA. Effect of sodium ascorbate and the time lapse before cementation after internal bleaching on bond strength between dentin and ceramic. J Prosthodont 2010;19:374-380.ArticlePubMed
- 41. Bouillaguet S, Troesch S, Wataha JC, Krejci I, Meyer JM, Pashley DH. Microtensile bond strength between adhesive cements and root canal dentin. Dent Mater 2003;19:199-205.ArticlePubMed
- 42. Feilzer AJ, De Gee AJ, Davidson CL. Increased wall-to-wall curing contraction in thin bonded resin layers. J Dent Res 1989;68:48-50.ArticlePubMedPDF
- 43. Kremeier K, Fasen L, Klaiber B, Hofmann N. Influence of endodontic post type (glass fiber, quartz fiber or gold) and luting material on push-out bond strength to dentin in vitro. Dent Mater 2008;24:660-666.ArticlePubMed
- 44. Park JY, Kwon TY, Kim YK. Effective application duration of sodium ascorbate antioxidant in reducing microleakage of bonded composite restoration in intracoronally-bleached teeth. Restor Dent Endod 2013;38:43-47.ArticlePubMedPMC
- 45. Hikita K, Van Meerbeek B, De Munck J, Ikeda T, Van Landuyt K, Maida T, Lambrechts P, Peumans M. Bonding effectiveness of adhesive luting agents to enamel and dentin. Dent Mater 2007;23:71-80.ArticlePubMed
- 46. Kim DS, Park SH, Choi GW, Choi KK. The effect of bonding resin on bond strength of dual-cure resin cements. J Korean Acad Conserv Dent 2007;32:426-436.Article
- 47. Kim SR, Yum J, Park JK, Hur B, Kim HC. Comparison of push-out bond strength of post according to cement application methods. J Korean Acad Conserv Dent 2010;35:479-485.Article
- 48. Khoroushi M, Saneie T. Post-bleaching application of an antioxidant on dentin bond strength of three dental adhesives. Dent Res J (Isfahan) 2012;9:46-53.ArticlePubMedPMC
- 49. Khoroushi M, Aghelinejad S. Effect of postbleaching application of an antioxidant on enamel bond strength of three different adhesives. Med Oral Patol Oral Cir Bucal 2011;16:e990-e996.ArticlePubMed
- 50. Miguez PA, Pereira PN, Atsawasuwan P, Yamauchi M. Collagen cross-linking and ultimate tensile strength in dentin. J Dent Res 2004;83:807-810.ArticlePubMedPDF
REFERENCES
UDMA, urethane dimethacrylate; Bis-GMA, bisphenol A diglycidyl methacrylate; BHT, butylhydroxytoluene.
Tables & Figures
REFERENCES
Citations

- Effect of collagen crosslinkers on sodium hypochlorite treated dentin bond strength: a systematic review and meta-analysis
Weiqing Zhou, Shuting Feng, Xiaojun Chu, Shuaimei Xu, Xiongqun Zeng
Frontiers in Bioengineering and Biotechnology.2025;[Epub] CrossRef - The impact of passive ultrasonic irrigation on the bond strength of two different self-etch adhesives to human pulp chamber dentine: a laboratory investigation
Mohammed Turky, Jukka Matinlinna, Monika Lukomska-Szymanska, Venkateshbabu Nagendrababu, Paul M. H. Dummer, Ahmad Abdel Hamid Elheeny, Nermin Alsayed Mahmoud
BMC Oral Health.2025;[Epub] CrossRef - The influence of cavity design on the mechanical behavior of endo-crown restorations: an ex-vivo study
Mohamed Gomaa Altamimi, Omaima El Mahallawi, Monika Lukomska-Szymanska, Mohammed Turky
BMC Oral Health.2025;[Epub] CrossRef - In Vitro Bond Strength of Dentin Treated with Sodium Hypochlorite: Effects of Antioxidant Solutions
Guillermo Grazioli, Elisa de León Cáceres, Romina Tessore, Rafael Lund, Ana Monjarás-Ávila, Monika Lukomska-Szymanska, Louis Hardan, Rim Bourgi, Carlos Cuevas-Suárez
Antioxidants.2024; 13(9): 1116. CrossRef - Effect of Dentin Irrigants on Push-Out Bond Strength in Resin Cementation Protocols for Fiber Posts in Endodontically Treated Teeth: An In Vitro Study
Sandra García-Varela, João Carlos Ramos, María José Ginzo-Villamayor, Pablo Castelo-Baz, Ramón Méndez-Díaz, Marcos Aníbal Anache-D’Abate, Tania Gancedo-Gancedo, Manuel Ruíz-Piñón, Soledad Mareque-Bueno, Benjamín José Martín-Biedma
Materials.2024; 17(6): 1432. CrossRef - A facile method for rejuvenating the bonding efficacy of root canal sealer-smeared dentine
Wenqing Lin, Yuan Gao, Surong Chen, Yan Yang, Weihu Ye, Diana Tran, Brian E. Bergeron, Franklin R. Tay, Jingzhi Ma
Journal of Dentistry.2023; 136: 104591. CrossRef - The Effect of Antioxidants on Dentin Bond Strength after Application of Common Endodontic Irrigants: A Systematic Review
Regina Gascón, Leopoldo Forner, Carmen Llena
Materials.2023; 16(6): 2260. CrossRef - Effect of epigallocatechin-3-gallate and thermal cycling on the bond strength of resin cements to the root dentin
Danielle Cristine Messias, Moisés Franco Barbosa da Silva, Natália Spadini de Faria, Tatiane Rocco Dias-Arnez, Fuad Jacob Rached-Júnior, Ana Beatriz Silva Sousa
Odontology.2021; 109(4): 854. CrossRef - Effect of Er
Horieh Moosavi, Farzaneh Ahrari, Maryam Zanjani
Dental Research Journal.2021; 18(1): 17. CrossRef - Effect of sodium hypochlorite on adhesive charactersitics of dentin: A systematic review of laboratory-based testing
Ensanya A. Abou Neel, Jonathan C. Knowles, Laurent Bozec
International Journal of Adhesion and Adhesives.2019; 95: 102419. CrossRef - Effect of irrigant neutralizing reducing agents on the compromised dislocation resistance of an epoxy resin and a methacrylate resin-based root canal sealer in vitro
Pallavi Reddy, Prasanna Neelakantan, Kavitha Sanjeev, Jukka Pekka Matinlinna
International Journal of Adhesion and Adhesives.2018; 82: 206. CrossRef - Sodium Hypochlorite Irrigation and Its Effect on Bond Strength to Dentin
Tariq S. Abuhaimed, Ensanya A. Abou Neel
BioMed Research International.2017; 2017: 1. CrossRef - Test methods for bond strength of glass fiber posts to dentin: A review
F. C. Dos Santos, M. D. Banea, H. L. Carlo, S. De Barros
The Journal of Adhesion.2017; 93(1-2): 159. CrossRef - Impact of conditioning regimens and time on adhesion of a fiber post to root dentin using two resin cements
P. Neelakantan, R. Mohanraj, E. Chua, S. Belli
Journal of Adhesion Science and Technology.2015; 29(4): 337. CrossRef - Effect of antioxidants on push-out bond strength of hydrogen peroxide treated glass fiber posts bonded with two types of resin cement
Maryam Khoroushi, Hamid Mazaheri, Pardis Tarighi, Pouran Samimi, Navid Khalighinejad
Restorative Dentistry & Endodontics.2014; 39(4): 303. CrossRef
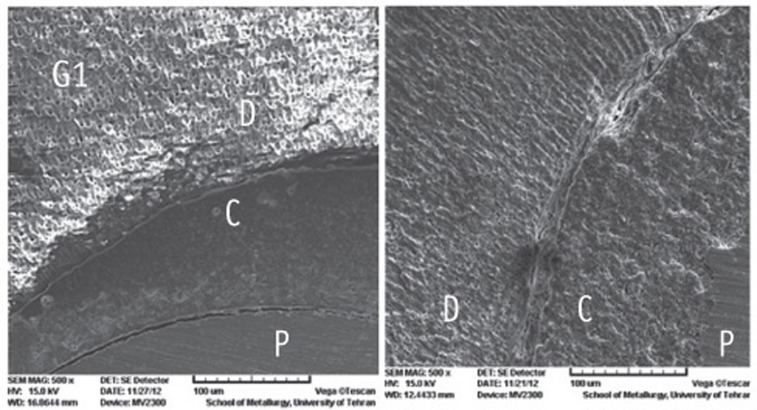
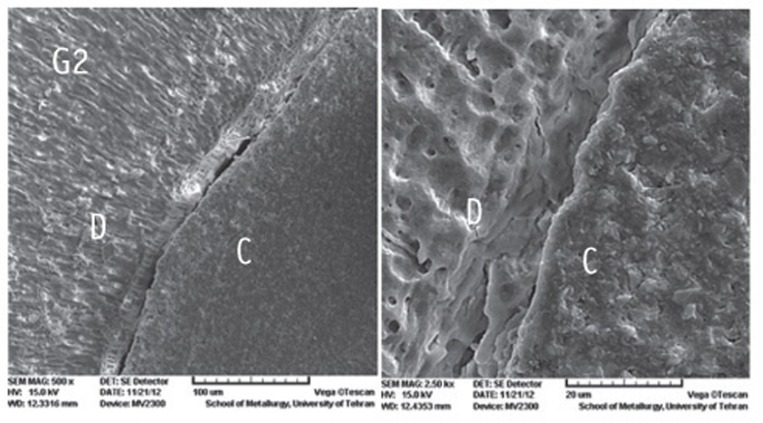
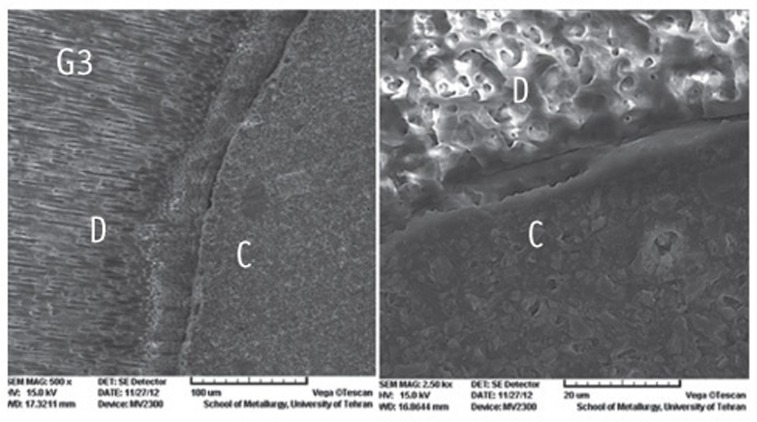
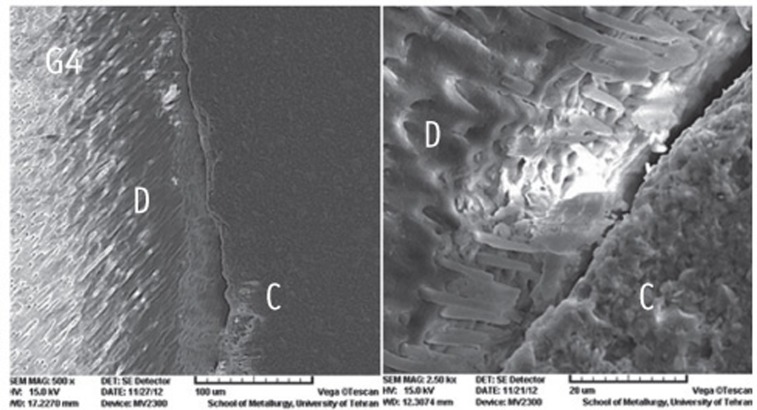
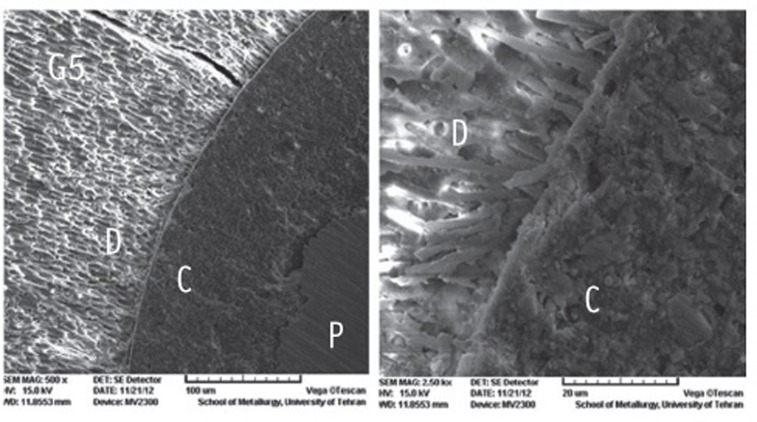
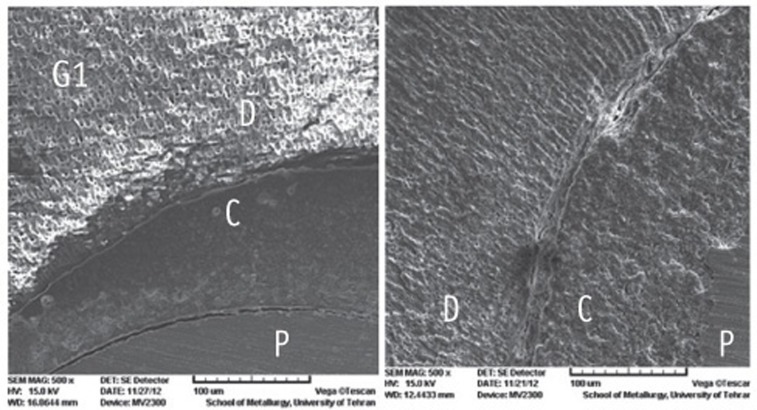
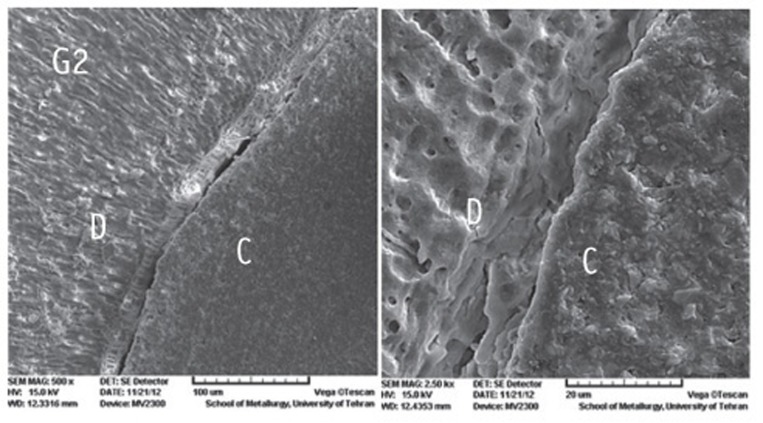
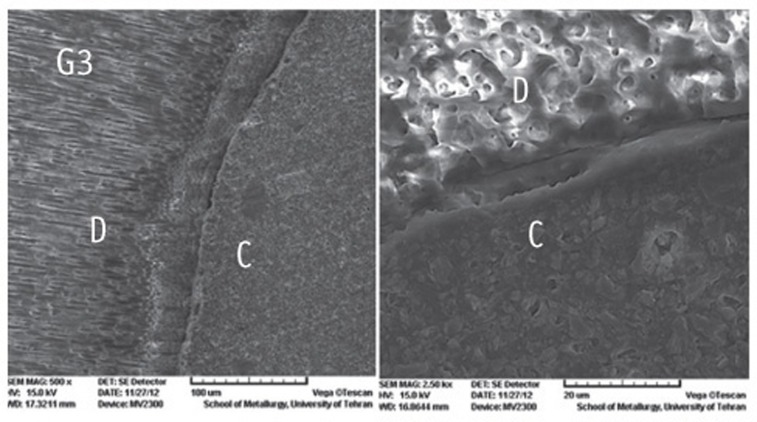
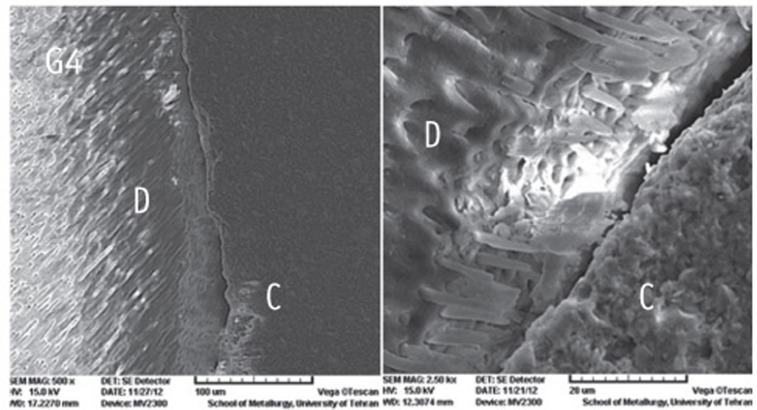
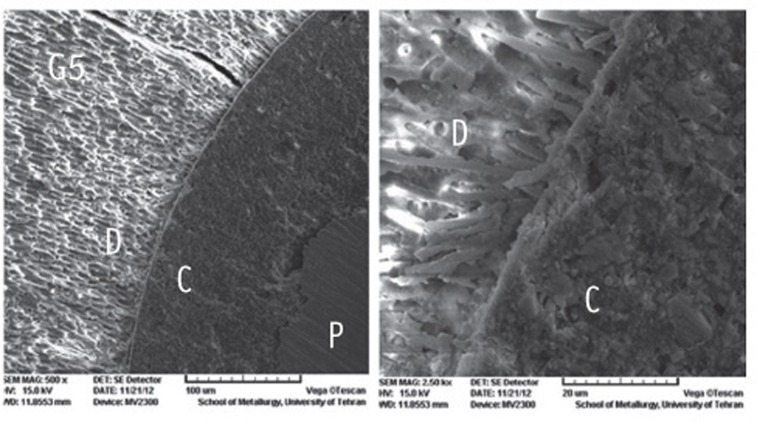
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Materials used in the study and the mode of their application according to the manufacturers' instructions

UDMA, urethane dimethacrylate; Bis-GMA, bisphenol A diglycidyl methacrylate; BHT, butylhydroxytoluene.
Pull-out bond strength of the specimens in the study groups (MPa)
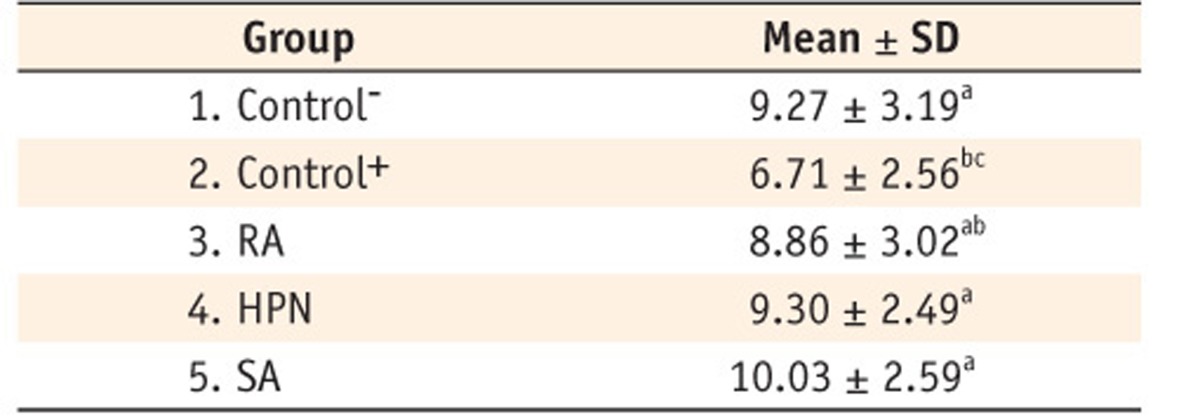
Groups with the same superscript are not statistically different (P > 0.05).
RA, rosemarinic acid; HPN, hesperidin; SA, sodium ascorbate gel; SD, standard deviation.
Distribution of different fracture modes in the study groups
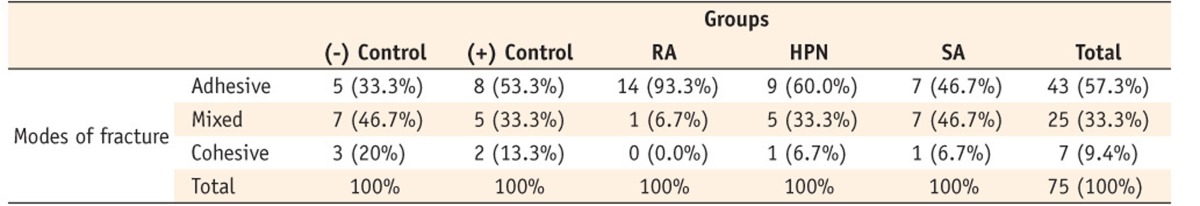
RA, rosemarinic acid; SA, sodium ascorbate; HPN, hesperidin.
UDMA, urethane dimethacrylate; Bis-GMA, bisphenol A diglycidyl methacrylate; BHT, butylhydroxytoluene.
Groups with the same superscript are not statistically different ( RA, rosemarinic acid; HPN, hesperidin; SA, sodium ascorbate gel; SD, standard deviation.
RA, rosemarinic acid; SA, sodium ascorbate; HPN, hesperidin.

 KACD
KACD


 ePub Link
ePub Link Cite
Cite

