Articles
- Page Path
- HOME > Restor Dent Endod > Volume 39(2); 2014 > Article
- Review Article Biologic response of local hemostatic agents used in endodontic microsurgery
- Youngjune Jang, Hyeon Kim, Byoung-Duck Roh, Euiseong Kim
-
2014;39(2):-88.
DOI: https://doi.org/10.5395/rde.2014.39.2.79
Published online: March 21, 2014
Microscope Center, Department of Conservative Dentistry and Oral Science Research Center, Yonsei University College of Dentistry, Seoul, Korea.
- Correspondence to Euiseong Kim, DDS, MSD, PhD. Professor, Microscope Center, Department of Conservative Dentistry and Oral Science Research Center, Yonsei University College of Dentistry, 50 Yonsei-ro, Seodaemun-gu, Seoul, Korea 120-752. TEL, +82-2-2228-3145; FAX, +82-2-313-7575; andyendo@yuhs.ac
©Copyights 2014. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,510 Views
- 14 Download
- 15 Crossref
Abstract
- Appropriate use of local hemostatic agent is one of the important factors on the prognosis of endodontic microsurgery. However, most investigations to date focus on the hemostatic efficacy of the agents, whereas their biologic characteristics have not received enough attention. The purpose of this paper was to review the biologic response of local hemostatic agents, and to provide clinical guidelines on their use during endodontic microsurgery. Electronic database (PUBMED) was screened to search related studies from 1980 to 2013, and 8 clinical studies and 18 animal studies were identified. Among the materials used in these studies, most widely-investigated and used materials, epinephrine, ferric sulfate (FS) and calcium sulfate (CS), were thoroughly discussed. Influence of these materials on local tissue and systemic condition, such as inflammatory and foreign body reaction, local ischemia, dyspigmentation, delayed or enhanced bone and soft tissue healing, and potential cardiovascular complications were assessed. Additionally, biological property of their carrier materials, cotton pellet and absorbable collagen, were also discussed. Clinicians should be aware of the biologic properties of local hemostatic agents and their carrier materials, and should pay attention to the potential complications when using them in endodontic microsurgery.
Introduction
Review
1. Hemostatic property
2. Biological response
1) Local tissue ischemia
2) Cardiovascular complications
3. Clinical suggestion
1. Hemostatic property
2. Biological response
3. Clinical suggestion
1. Hemostatic property
2. Biological response
3. Clinical suggestion
1. Cotton pellet
2. Absorbable collagen sponge
3. Clinical suggestion
Conclusions
- 1. Kim S, Rethnam S. Hemostasis in endodontic microsurgery. Dent Clin North Am 1997;41:499-511.ArticlePubMed
- 2. Witherspoon DE, Gutmann JL. Haemostasis in periradicular surgery. Int Endod J 1996;29:135-149.ArticlePubMed
- 3. Cho YW, Kim E. Is stopping of anticoagulant therapy really required in a minor dental surgery? - How about in an endodontic microsurgery? Restor Dent Endod 2013;38:113-118.ArticlePubMedPMC
- 4. Finn MD, Schow SR, Schneiderman ED. Osseous regeneration in the presence of four common hemostatic agents. J Oral Maxillofac Surg 1992;50:608-612.ArticlePubMed
- 5. Ibarrola JL, Bjorenson JE, Austin BP, Gerstein H. Osseous reactions to three hemostatic agents. J Endod 1985;11:75-83.ArticlePubMed
- 6. Johnson P, Fromm D. Effects of bone wax on bacterial clearance. Surgery 1981;89:206-209.PubMed
- 7. Papay FA, Morales L Jr, Ahmed OF, Neth D, Reger S, Zins J. Comparison of ossification of demineralized bone, hydroxyapatite, Gelfoam, and bone wax in cranial defect repair. J Craniofac Surg 1996;7:347-351.ArticlePubMed
- 8. Thaller SR, Kim JC, Kawamoto HK. Calvarial bone graft donor site: a histological study in a rabbit model. Ann Plast Surg 1989;23:390-395.ArticlePubMed
- 9. Nappi JF, Lehman JA Jr. The effects of Surgicel on bone formation. Cleft Palate J 1980;17:291-296.PubMed
- 10. Alkan A, Inal S, Yildirim M, Baş B, Ağar E. The effects of hemostatic agents on peripheral nerve function: an experimental study. J Oral Maxillofac Surg 2007;65:630-634.ArticlePubMed
- 11. Boyes-Varley JG, Cleaton-Jones PE, Lownie JF. Effect of a topical drug combination on the early healing of extraction sockets in the vervet monkey. Int J Oral Maxillofac Surg 1988;17:138-141.ArticlePubMed
- 12. Hjortdal O. The fate of resorbable hemostatic implants in rats. Acta Odontol Scand 1970;28:323-336.ArticlePubMed
- 13. De Leonardis D, Pecora GE. Augmentation of the maxillary sinus with calcium sulfate: one-year clinical report from a prospective longitudinal study. Int J Oral Maxillofac Implants 1999;14:869-878.PubMed
- 14. Pecora G, De Leonardis D, Ibrahim N, Bovi M, Cornelini R. The use of calcium sulphate in the surgical treatment of a 'through and through' periradicular lesion. Int Endod J 2001;34:189-197.ArticlePubMedPDF
- 15. Vickers FJ, Baumgartner JC, Marshall G. Hemostatic efficacy and cardiovascular effects of agents used during endodontic surgery. J Endod 2002;28:322-323.ArticlePubMed
- 16. Vy CH, Baumgartner JC, Marshall JG. Cardiovascular effects and efficacy of a hemostatic agent in periradicular surgery. J Endod 2004;30:379-383.ArticlePubMed
- 17. Csillag M, Nyiri G, Vag J, Fazekas A. Dose-related effects of epinephrine on human gingival blood flow and crevicular fluid production used as a soaking solution for chemo-mechanical tissue retraction. J Prosthet Dent 2007;97:6-11.ArticlePubMed
- 18. Scarano A, Carinci F, Cimorelli E, Quaranta M, Piattelli A. Application of calcium sulfate in surgical-orthodontic treatment of impacted teeth: a new procedure to control hemostasis. J Oral Maxillofac Surg 2010;68:964-968.ArticlePubMed
- 19. Scarano A, Artese L, Piattelli A, Carinci F, Mancino C, Iezzi G. Hemostasis control in endodontic surgery: a comparative study of calcium sulfate versus gauzes and versus ferric sulfate. J Endod 2012;38:20-23.ArticlePubMed
- 20. Lemon RR, Steele PJ, Jeansonne BG. Ferric sulfate hemostasis: effect on osseous wound healing. Left in situ for maximum exposure. J Endod 1993;19:170-173.PubMed
- 21. Jeansonne BG, Boggs WS, Lemon RR. Ferric sulfate hemostasis: effect on osseous wound healing. II. With curettage and irrigation. J Endod 1993;19:174-176.ArticlePubMed
- 22. Pecora G, Andreana S, Margarone JE 3rd, Covani U, Sottosanti JS. Bone regeneration with a calcium sulfate barrier. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;84:424-429.ArticlePubMed
- 23. Clokie CM, Moghadam H, Jackson MT, Sandor GK. Closure of critical sized defects with allogenic and alloplastic bone substitutes. J Craniofac Surg 2002;13:111-121.ArticlePubMed
- 24. Miller Q, Meekin G, Murdock C. The effect of topical epinephrine on peripheral nerve conduction. Laryngoscope 2002;112:1888-1891.ArticlePubMed
- 25. Murashima Y, Yoshikawa G, Wadachi R, Sawada N, Suda H. Calcium sulphate as a bone substitute for various osseous defects in conjunction with apicectomy. Int Endod J 2002;35:768-774.ArticlePubMed
- 26. Strocchi R, Orsini G, Iezzi G, Scarano A, Rubini C, Pecora G, Piattelli A. Bone regeneration with calcium sulfate: evidence for increased angiogenesis in rabbits. J Oral Implantol 2002;28:273-278.ArticlePubMed
- 27. Walsh WR, Morberg P, Yu Y, Yang JL, Haggard W, Sheath PC, Svehla M, Bruce WJ. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop Relat Res 2003;406:228-236.Article
- 28. Apaydin ES, Torabinejad M. The effect of calcium sulfate on hard-tissue healing after periradicular surgery. J Endod 2004;30:17-20.ArticlePubMed
- 29. Orsini G, Ricci J, Scarano A, Pecora G, Petrone G, Iezzi G, Piattelli A. Bone-defect healing with calcium-sulfate particles and cement: an experimental study in rabbit. J Biomed Mater Res B Appl Biomater 2004;68:199-208.ArticlePubMed
- 30. von Arx T, Jensen SS, Hänni S, Schenk RK. Haemostatic agents used in periradicular surgery: an experimental study of their efficacy and tissue reactions. Int Endod J 2006;39:800-808.ArticlePubMed
- 31. Jensen SS, Yazdi PM, Hjørting-Hansen E, Bosshardt DD, von Arx T. Haemostatic effect and tissue reactions of methods and agents used for haemorrhage control in apical surgery. Int Endod J 2010;43:57-63.ArticlePubMed
- 32. Azargoon H, Williams BJ, Solomon ES, Kessler HP, He J, Spears R. Assessment of hemostatic efficacy and osseous wound healing using HemCon dental dressing. J Endod 2011;37:807-811.ArticlePubMed
- 33. Katzung BG, Masters S, Trevor A. Basic and clinical pharmacology. 12th ed. Columbus, OH: McGraw-Hill Education; 2011. p. 129-149.
- 34. Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod 2006;32:601-623.ArticlePubMed
- 35. Chu WS, Park SH, Ahn DK, Kim SK. Effect of local anesthesia on pulpal blood flow in mechanically stimulated teeth. J Korean Acad Conserv Dent 2006;31:257-262.Article
- 36. Lee JS, Kim SK. The influence of epinephrine concentration in local anesthetics on pulpal and gingival blood flows. J Korean Acad Conserv Dent 2003;28:475-484.Article
- 37. Kim E, Song JS, Jung IY, Lee SJ, Kim S. Prospective clinical study evaluating endodontic microsurgery outcomes for cases with lesions of endodontic origin compared with cases with lesions of combined periodontal-endodontic origin. J Endod 2008;34:546-551.ArticlePubMed
- 38. Song M, Jung IY, Lee SJ, Lee CY, Kim E. Prognostic factors for clinical outcomes in endodontic microsurgery: a retrospective study. J Endod 2011;37:927-933.ArticlePubMed
- 39. Chowdhry S, Seidenstricker L, Cooney DS, Hazani R, Wilhelmi BJ. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg 2010;126:2031-2034.ArticlePubMed
- 40. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges 2005;3:195-199.ArticlePubMed
- 41. Lalonde D, Bell M, Benoit P, Sparkes G, Denkler K, Chang P. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am 2005;30:1061-1067.ArticlePubMed
- 42. Sylaidis P, Logan A. Digital blocks with adrenaline. An old dogma refuted. J Hand Surg Br 1998;23:17-19.PubMed
- 43. Denkler K. A comprehensive review of epinephrine in the finger: to do or not to do. Plast Reconstr Surg 2001;108:114-124.ArticlePubMed
- 44. Jang Y, Kim E. Cardiovascular effect of epinephrine in endodontic microsurgery: a review. Restor Dent Endod 2013;38:187-193.ArticlePubMedPMC
- 45. Troullos ES, Goldstein DS, Hargreaves KM, Dionne RA. Plasma epinephrine levels and cardiovascular response to high administered doses of epinephrine contained in local anesthesia. Anesth Prog 1987;34:10-13.PubMedPMC
- 46. Gogerty JH, Strand HA, Ogilvie AL, Dille JM. Vasopressor effects of topical epinephrine in certain dental procedures. Oral Surg Oral Med Oral Pathol 1957;10:614-622.ArticlePubMed
- 47. Wood M, Reader A, Nusstein J, Beck M, Padgett D, Weaver J. Comparison of intraosseous and infiltration injections for venous lidocaine blood concentrations and heart rate changes after injection of 2% lidocaine with 1:100,000 epinephrine. J Endod 2005;31:435-438.ArticlePubMed
- 48. Besner E. Systemic effects of racemic epinephrine when applied to the bone cavity during periapical surgery. Va Dent J 1972;49:9-12.
- 49. Ferry DR, Henry RL, Kern MJ. Epinephrine-induced myocardial infarction in a patient with angiographically normal coronary arteries. Am Heart J 1986;111:1193-1195.ArticlePubMed
- 50. Foster CA, Aston SJ. Propranolol-epinephrine interaction: a potential disaster. Plast Reconstr Surg 1983;72:74-78.PubMed
- 51. Lee JY, Hong SJ, Chon JY, Kwon SY. Cardiac arrest induced by submucosal injection of epinephrine in a patient with variant angina. Rhinology 2010;48:251-253.ArticlePubMed
- 52. Umino M, Ohwatari T, Shimoyama K, Nagao M. Unexpected atrial fibrillation during tooth extraction in a sedated elderly patient. Anesth Prog 1994;41:77-80.PubMedPMC
- 53. Monsel L. Proprieté hémostatique du sulfate de peroxyde de Fer. Rec Mem Med Milit 1856;17:424.
- 54. Fischer DE. Tissue management: a new solution to an old problem. Gen Dent 1987;35:178-182.PubMed
- 55. Smith NL, Seale NS, Nunn ME. Ferric sulfate pulpotomy in primary molars: a retrospective study. Pediatr Dent 2000;22:192-199.PubMed
- 56. von Arx T, Jensen SS, Hänni S. Clinical and radiographic assessment of various predictors for healing outcome 1 year after periapical surgery. J Endod 2007;33:123-128.ArticlePubMed
- 57. von Arx T, Jensen SS, Hänni S, Friedman S. Five-year longitudinal assessment of the prognosis of apical microsurgery. J Endod 2012;38:570-579.ArticlePubMed
- 58. Evans BE. Local hemostatic agents. N Y J Dent 1977;47:109-114.PubMed
- 59. Davis JR, Steinbronn KK, Graham AR, Dawson BV. Effects of Monsel's solution in uterine cervix. Am J Clin Pathol 1984;82:332-335.ArticlePubMed
- 60. Sawchuk WS, Friedman KJ, Manning T, Pinnell SR. Delayed healing in full-thickness wounds treated with aluminum chloride solution. A histologic study with evaporimetry correlation. J Am Acad Dermatol 1986;15:982-989.PubMed
- 61. Armstrong RB, Nichols J, Pachance J. Punch biopsy wounds treated with Monsel's solution or a collagen matrix. A comparison of healing. Arch Dermatol 1986;122:546-549.ArticlePubMed
- 62. Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. Clinical observations and experimentations. Am J Dermatopathol 1980;2:197-205.ArticlePubMed
- 63. Duray PH, Livolsi VA. Recurrent dysplastic nevus following shave excision. J Dermatol Surg Oncol 1984;10:811-815.ArticlePubMed
- 64. Horbett TA. Principles underlying the role of adsorbed plasma proteins in blood interactions with foreign materials. Cardiovasc Pathol 1993;2(Supplement):137-148.PubMed
- 65. Ziats NP, Pankowsky DA, Tierney BP, Ratnoff OD, Anderson JM. Adsorption of Hageman factor (factor XII) and other human plasma proteins to biomedical polymers. J Lab Clin Med 1990;116:687-696.PubMed
- 66. Thomas MV, Puleo DA. Calcium sulfate: properties and clinical applications. J Biomed Mater Res B Appl Biomater 2009;88:597-610.ArticlePubMed
- 67. Payne JM, Cobb CM, Rapley JW, Killoy WJ, Spencer P. Migration of human gingival fibroblasts over guided tissue regeneration barrier materials. J Periodontol 1996;67:236-244.ArticlePubMed
- 68. Guarnieri R, Pecora G, Fini M, Aldini NN, Giardino R, Orsini G, Piattelli A. Medical grade calcium sulfate hemihydrate in healing of human extraction sockets: clinical and histological observations at 3 months. J Periodontol 2004;75:902-908.ArticlePubMed
- 69. Larson PO. Topical hemostatic agents for dermatologic surgery. J Dermatol Surg Oncol 1988;14:623-632.PubMed
- 70. Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg 2008;34:431-445.ArticlePubMed
- 71. Scarano A, Degidi M, Iezzi G, Pecora G, Piattelli M, Orsini G, Caputi S, Perrotti V, Mangano C, Piattelli A. Maxillary sinus augmentation with different biomaterials: a comparative histologic and histomorphometric study in man. Implant Dent 2006;15:197-207.ArticlePubMed
- 72. Orsini M, Orsini G, Benlloch D, Aranda JJ, Lazaro P, Sanz M, De Luca M, Piattelli A. Comparison of calcium sulfate and autogenous bone graft to bioabsorbable membranes plus autogenous bone graft in the treatment of intrabony periodontal defects: a split-mouth study. J Periodontol 2001;72:296-302.ArticlePubMed
- 73. Aichelmann-Reidy ME, Heath CD, Reynolds MA. Clinical evaluation of calcium sulfate in combination with demineralized freeze-dried bone allograft for the treatment of human intraosseous defects. J Periodontol 2004;75:340-347.ArticlePubMed
- 74. Kim CK, Chai JK, Cho KS, Moon IS, Choi SH, Sottosanti JS, Wikesjo UM. Periodontal repair in intrabony defects treated with a calcium sulfate implant and calcium sulfate barrier. J Periodontol 1998;69:1317-1324.ArticlePubMed
- 75. Shaffer CD, App GR. The use of plaster of paris in treating infrabony periodontal defects in humans. J Periodontol 1971;42:685-690.ArticlePubMed
- 76. Kalbermatten DF, Kalbermatten NT, Hertel R. Cotton-induced pseudotumor of the femur. Skeletal Radiol 2001;30:415-417.ArticlePubMedPDF
- 77. Sexton CC, Lawson JP, Yesner R. Case report 174: 'Cottonballoma' of femur (due to retained surgical sponge with foreign body giant cell reaction). Skeletal Radiol 1981;7:211-213.ArticlePubMedPDF
- 78. Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: a case series. J Endod 2010;36:536-541.ArticlePubMed
- 79. Ferris DM, Baumgartner JC. Perforation repair comparing two types of mineral trioxide aggregate. J Endod 2004;30:422-424.ArticlePubMed
- 80. Stein MD, Salkin LM, Freedman AL, Glushko V. Collagen sponge as a topical hemostatic agent in mucogingival surgery. J Periodontol 1985;56:35-38.ArticlePubMed
- 81. Chvapil M. Collagen sponge: theory and practice of medical applications. J Biomed Mater Res 1977;11:721-741.ArticlePubMed
- 82. Mannai C, Leake D, Pizzoferrato A, Ciapetti G, Spangiogi C. Histological evaluation of purified bovine tendon collagen sponge in tooth extraction sites in dogs. Oral Surg Oral Med Oral Pathol 1986;61:315-323.PubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Expert consensus on apical microsurgery
Hanguo Wang, Xin Xu, Zhuan Bian, Jingping Liang, Zhi Chen, Benxiang Hou, Lihong Qiu, Wenxia Chen, Xi Wei, Kaijin Hu, Qintao Wang, Zuhua Wang, Jiyao Li, Dingming Huang, Xiaoyan Wang, Zhengwei Huang, Liuyan Meng, Chen Zhang, Fangfang Xie, Di Yang, Jinhua Yu
International Journal of Oral Science.2025;[Epub] CrossRef - A drug-carrying, multiscene, absorbable biological suture from fish swim bladder
Peng Sun, Hao Cui, Jinwei Zhang, Jingan Li, Changwei Ren, Yongqiang Lai
International Journal of Surgery.2025; 111(10): 6663. CrossRef - Assessing the efficacy of apicoectomy without retrograde filling in treating periapical inflammatory cysts
Jeong-Kui Ku, Woo-Young Jeon, Seung-O Ko, Ji-Young Yoon
Journal of the Korean Association of Oral and Maxillofacial Surgeons.2024; 50(3): 140. CrossRef - Functional and structural neurodegenerative activities of Ankaferd BloodStopper in a mouse sciatic nerve model
Ramazan Üstün, Elif Oğuz, Ayşe Şeker, Filiz Taspinar
Experimental and Therapeutic Medicine.2024;[Epub] CrossRef - Local and Systemic Hemostatic Agents: A Comprehensive Review
Bardia Jamali, Saeed Nouri, Salimeh Amidi
Cureus.2024;[Epub] CrossRef - PLGA Nanoparticle Rapamycin- or Necrostatin-1-Coated Sutures Inhibit Inflammatory Reactions after Arterial Closure in Rats
Liwei Zhang, Wang Wang, Boao Xie, Peng Sun, Shunbo Wei, Haoliang Wu, Cong Zhang, Jingan Li, Zhuo Li, Hualong Bai
ACS Applied Bio Materials.2022; 5(4): 1501. CrossRef - COMPARING THE CLINICAL AND RADIOGRAPHIC OUTCOMES OF PULPOTOMIES IN PRIMARY MOLARS USING BIOACTIVE ENDODONTIC MATERIALS AND FERRIC SULFATE – A SYSTEMATIC REVIEW AND META-ANALYSIS OF RANDOMIZED CLINICAL TRIALS
VELLORE KANNAN GOPINATH, SHAJU JACOB PULIKKOTIL, SAJESH K VEETTIL, LALLI DHARMARAJAN, PONNUDURAI SAMUEL GNANA PRAKASH, VINEET DHAR, JAYAKUMAR JAYARAMAN
Journal of Evidence-Based Dental Practice.2022; 22(4): 101770. CrossRef - Perioperative Antiplatelet and Anticoagulant Management with Endodontic Microsurgical Techniques
Anita Aminoshariae, Mark Donaldson, Michael Horan, James C. Kulild, Dale Baur
Journal of Endodontics.2021; 47(10): 1557. CrossRef - Effect of blood contamination and various hemostatic procedures on the push-out bond strength of Biodentine when used for furcation perforation repair
Shanthana Reddy, Ramya Shenoy, LohithReddy Mandadi, Ishani Saluja, ManuelS Thomas
Journal of Conservative Dentistry.2021; 24(3): 260. CrossRef - Endodontic Perforation Closure by Five Mineral Oxides Silicate-Based Cement with/without Collagen Sponge Matrix
Talal Al-Nahlawi, Maisour Ala Rachi, Amjad Abu Hasna, Zohaib Khurshid
International Journal of Dentistry.2021; 2021: 1. CrossRef - Hemostatic agents in periapical surgery: The systematic review
Z. S. Khabadze, D. A. Nazarova, E. S. Shilyaeva, A. P. Kotelnikova, Yu. A. Bakayev, S. M. Abdulkerimova, Kh. O. Omarova
Endodontics Today.2021; 19(3): 184. CrossRef - An Innovative Bioceramic Bone Graft Substitute for Bone Defect Treatment: In Vivo Evaluation of Bone Healing
Syamsiah Syam, Yung-Chieh Cho, Chung-Ming Liu, Mao-Suan Huang, Wen-Chien Lan, Bai-Hung Huang, Takaaki Ueno, Chi-Hsun Tsai, Takashi Saito, May-Show Chen, Keng-Liang Ou
Applied Sciences.2020; 10(22): 8303. CrossRef - Trial finds better haemostasis with aluminium chloride during periapical surgery
Niall Mc Goldrick, Carly Ross, James Nelson
Evidence-Based Dentistry.2017; 18(2): 50. CrossRef - Comparison of the Hemostatic Activity of Quercus persica Jaub. & Spach. (Oak) With Ferric Sulfate in Bony Crypts
Mohammad Reza Nabavizadeh, Arman Zargaran, Fariborz Moazami, Fatemeh Askari, Safoora Sahebi, Alireza Farhadpoor, Pouya Faridi
Journal of Evidence-Based Complementary & Alternative Medicine.2016; 21(1): 34. CrossRef - Effect of the plant-based hemostatic agent Ankaferd Blood Stopper® on the biocompatibility of mineral trioxide aggregate
Muzaffer Emir Dinçol, Hakan Ozbas, Bulent Yılmaz, Handan Ersev, Selcuk Gokyay, Vakur Olgac
BMC Oral Health.2016;[Epub] CrossRef
Clinical studies evaluated biologic response of materials used as local hemostatic agents
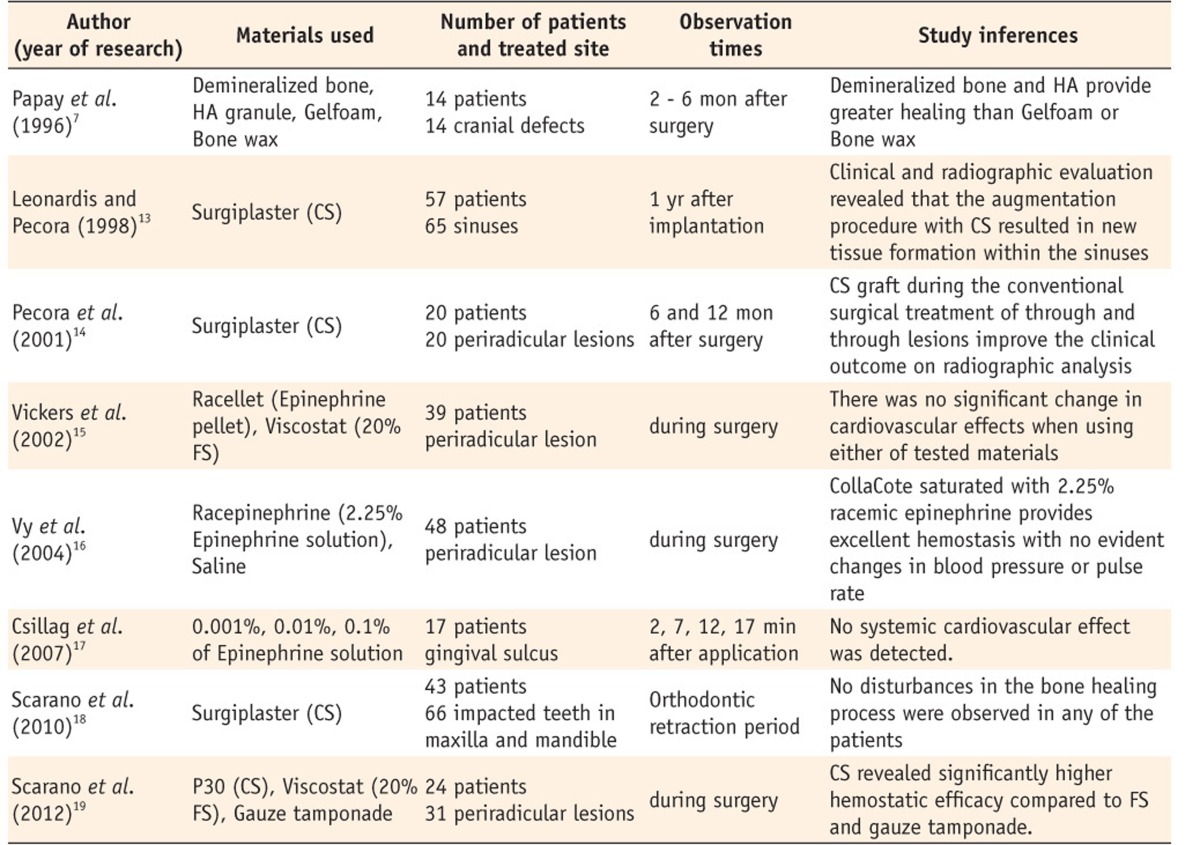
HA, hydroxyapatite; CS, calcium sulfate; FS, ferric sulfate.
Animal studies evaluated biologic response of materials used as local hemostatic agents
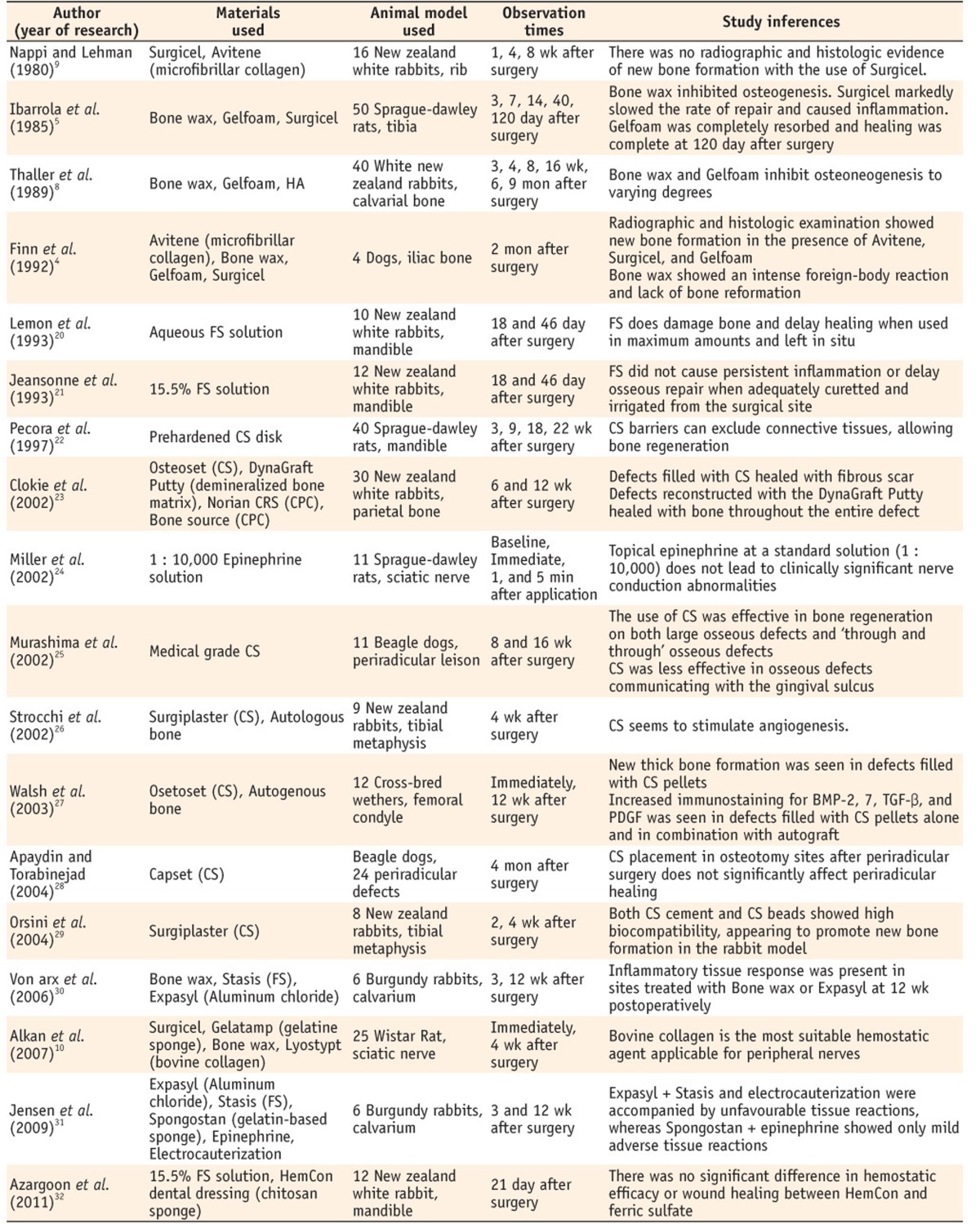
HA, hydroxyapatite; FS, ferric sulfate; CS, calcium sulfate; CPC, calcium phosphate cement.
Properties of epinephrine, ferric sulfate, and calcium sulfate as local hemostatic agent
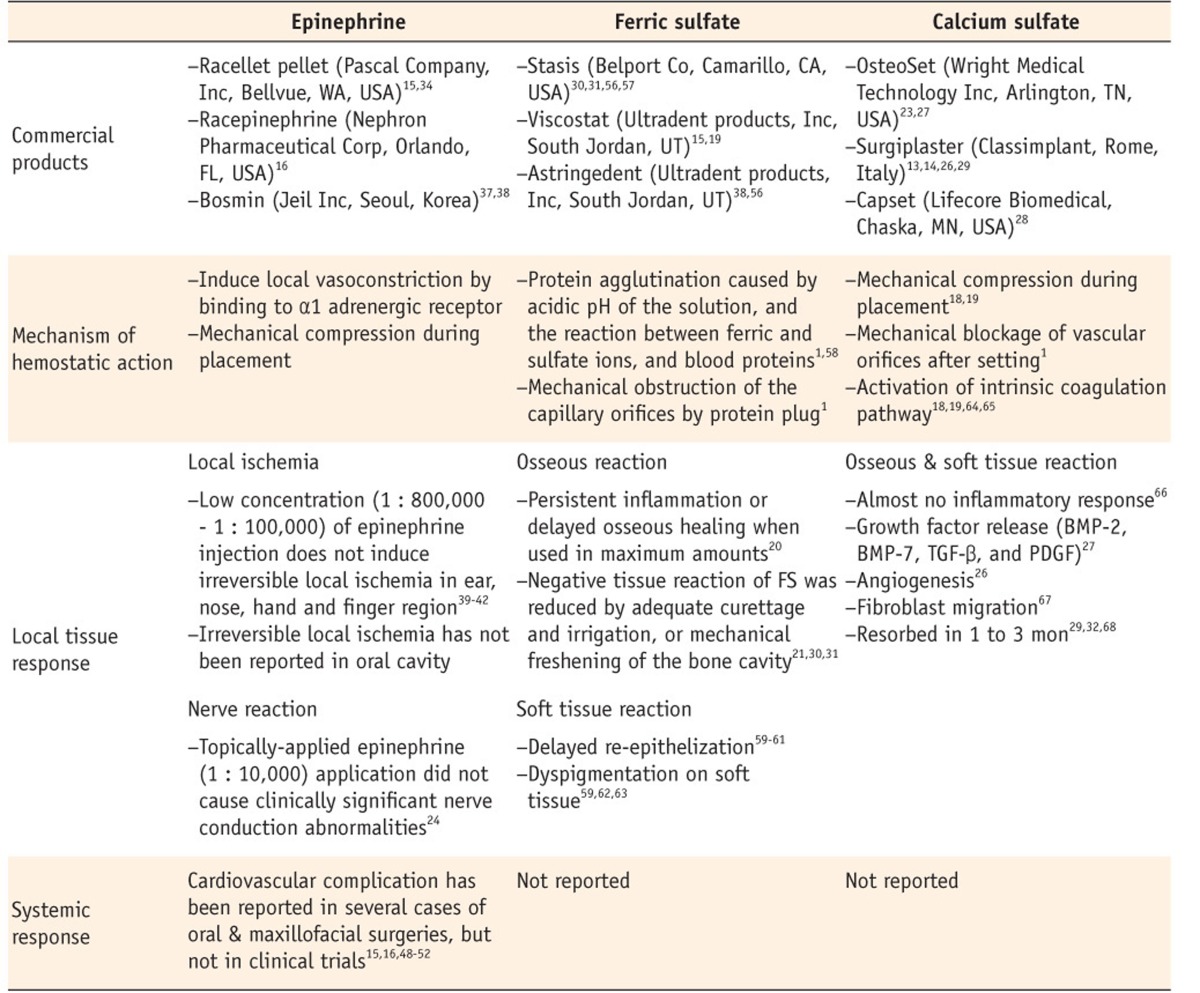
HA, hydroxyapatite; CS, calcium sulfate; FS, ferric sulfate.
HA, hydroxyapatite; FS, ferric sulfate; CS, calcium sulfate; CPC, calcium phosphate cement.

 KACD
KACD



 ePub Link
ePub Link Cite
Cite

