Articles
- Page Path
- HOME > Restor Dent Endod > Volume 39(1); 2014 > Article
- Case Report Clinical effectiveness of combining platelet rich fibrin with alloplastic bone substitute for the management of combined endodontic periodontal lesion
- Lata Goyal
-
2014;39(1):-55.
DOI: https://doi.org/10.5395/rde.2014.39.1.51
Published online: January 20, 2014
Department of Periodontics and Community Dentistry, Dr. Ziauddin Ahmad Dental College, Aligarh Muslim University, Aligarh, India.
- Correspondence to Lata Goyal, MDS. Ex Resident, Department of Periodontics and Community Dentistry, Dr. Ziauddin Ahmad Dental College, Aligarh Muslim University, University Road, Zakaullah Rd, Medical Colony, Uttar Pradesh 202002, Aligarh, India. TEL, +08475000284; latagoyal83@gmail.com
• Received: August 7, 2013 • Accepted: October 22, 2013
©Copyights 2014. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,709 Views
- 4 Download
- 18 Crossref
Abstract
- The term "endo-perio" lesion has been proposed to describe the destructive lesion resulting from inflammatory products found in varying degrees in both the periodontium and the pulpal tissues. In most of the cases, clinical symptoms disappear following successful endodontic therapy. However failure after conventional root canal treatment calls for surgical intervention. A 35 year old male patient with endo-perio lesion in right maxillary lateral incisor was treated with platelet rich fibrin (PRF) and alloplastic bone substitute after conventional endodontic therapy. At the end of 6 months there was gain in clinical attachment, increased radiographic bone fill and reduction in probing depth which was maintained till 18 month follow-up. Present case report aims to evaluate the efficacy of PRF and alloplastic bone substitute in the management of intrabony defect associated with endo-perio lesion in maxillary lateral incisor because the healing potential of PRF and bone graft has not been widely studied in endodontics. The use of PRF allows the clinician to optimize tissue remodelling, wound healing and angiogenesis by the local delivery of growth factors and proteins. The novel technique described here enables the clinician to be benefited from the full regenerative capacity of this autologous biologic material.
Introduction
The relationship between pulpal and periodontal disease was first described by Simring and Goldberg in 1964.1 In many cases it is easy to establish diagnosis, but there are certain cases, where situation becomes more complex, especially when it combines with periodontal disease. It becomes essential to correct periodontal defect simultaneously in these cases to prevent recurrence, and to improve functional status of the tooth.2 Various treatment modalities have been proposed earlier for the treatment of endo-perio involvement including open flap debridement, root resection and retrograde filling, where healing is by scar.3 Since this is not ideal, newer approaches such as regenerative procedures like guided tissue regeneration (GTR), bone grafts and growth factors that aim to restore lost tissue have been introduced. Platelet rich fibrin (PRF) enriched with platelets and growth factors promotes periapical tissue regeneration and healing. This case report presents an attempt to evaluate the healing kinetics of the combination of PRF and alloplastic bone substitute (Bioactive glass) as opposed to using these materials alone.
Case Report
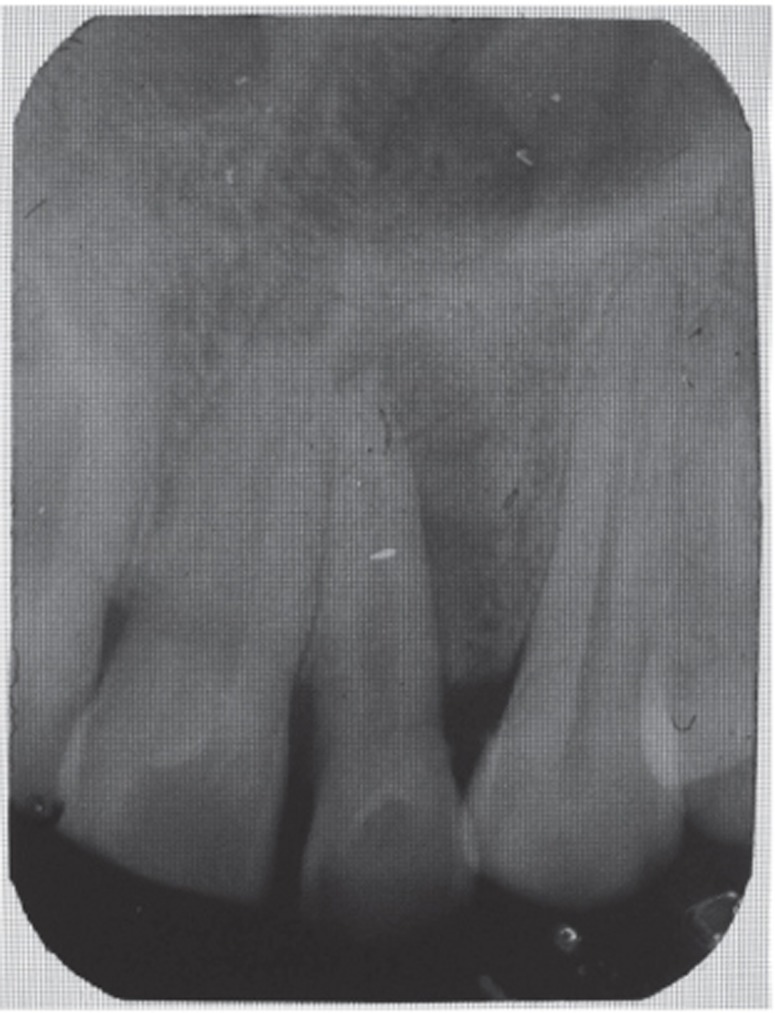
A 35 year old male patient reported to the Department of Periodontics with a complaint of pain in the upper left front tooth region associated with pus discharge for 2 months (Figure 1). He also gave history of trauma around 7 years back and noticed discoloration of tooth with time but he took no treatment as the tooth was asymptomatic. On intraoral examination there were no carious teeth. Intraoral periapical X-ray (IOPA) was taken which revealed radiolucency along the distal side of the entire root surface to the apex (Figure 2). Electric pulp testing was done to check the vitality of the tooth which confirmed that the tooth was non vital and the tooth was tender on percussion. Vertical probing depth was measured which was found to be 8 mm on distal side. Endodontic treatment was taken up first and then periodontal regenerative surgery was planned for treatment of defect.
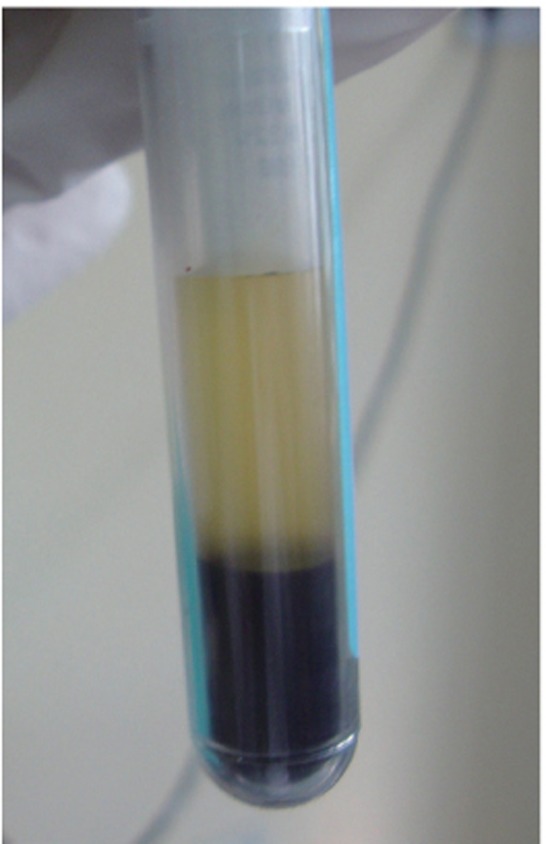
On the day of surgery, PRF was prepared. Exactly 10 mL of whole blood was drawn by venipuncture of the antecubital vein and collected in two 5 mL sterile glass tubes coated with an anticoagulant, acid-citrate-dextrose. Whole blood was initially centrifuged (3,000 rpm for 10 minutes, Figure 3).The plasma was then aspirated with a micropipette and subjected to a second centrifugation at 2,000 rpm for 10 minutes, which allowed the precipitation of the platelets (0.8 to 1.2 mL) to fall onto the bottom. Coagulated preparation of 0.3 mL of PRF was obtained by its combination with 0.1 g of calcium chloride (Figure 4). Then graft material was mixed with the coagulated platelet rich plasma (PRP) preparation.
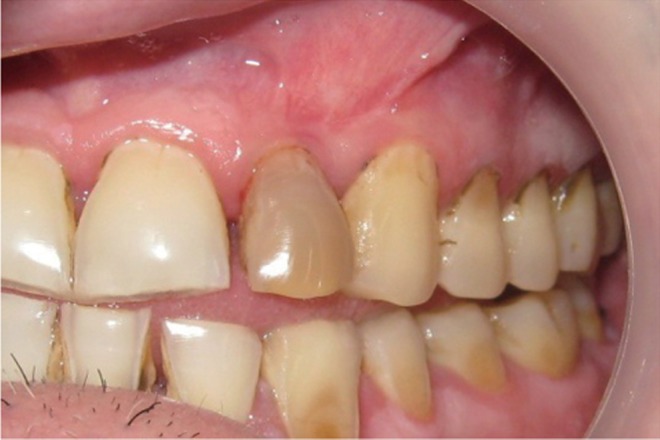
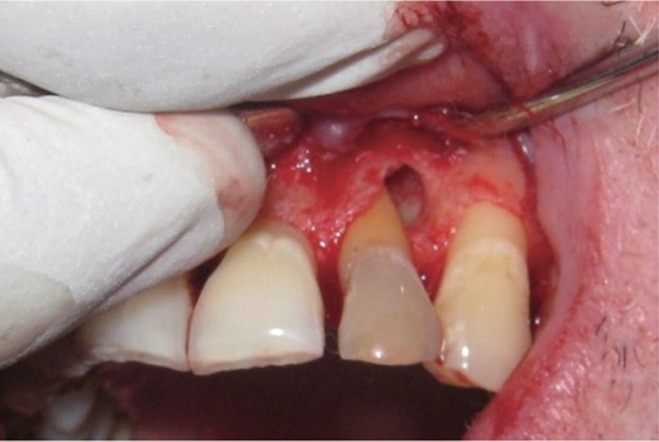
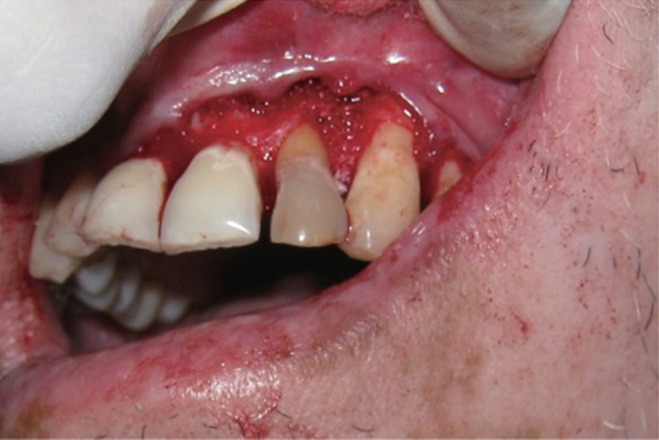
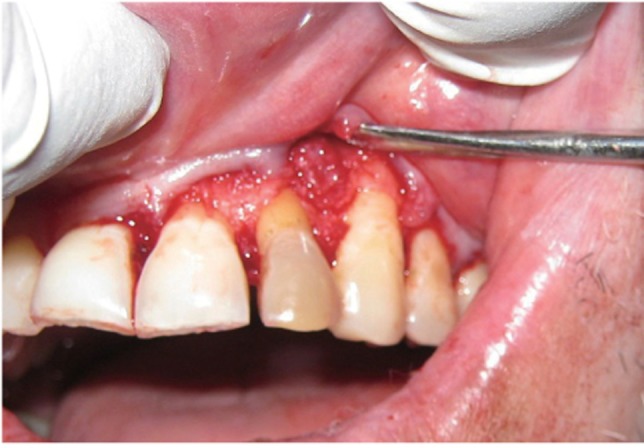
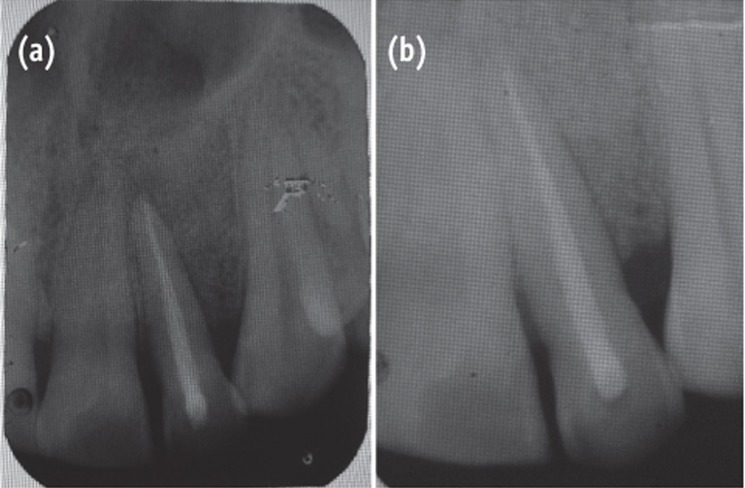
The area selected for surgery was anesthetized using xylocaine with adrenaline 1 : 200,000. A full thickness flap was raised at the labial aspect using intracrevicular incision (Figure 5). Thorough curettage of the defect area was done and a combination of PRF and bioactive glass was placed in the defect (Figures 6 and 7). Closure of soft tissue flap was done with non resorbable black silk (3-0) suture using interrupted suturing technique. The sutures were removed 10 days after surgery. The patient was scheduled for regular recall intervals at 3, 6, 9 and 12 months. PRF and bone graft resulted in substantial amount of bone fill (Figures 8a and 8b), and probing depth was reduced to 2 mm.
Discussion
Endo-perio lesions are common conditions that are difficult to diagnose. However, if patient's history is taken carefully and thorough clinical examination is done, these lesions can be treated completely to give favorable outcome. Data collected must include periapical radiographs, pulp vitality testing, cavity test, percussion, palpation, and pocket probing depth. In this report, history of trauma and the pulp vitality test which showed the nonvital nature of the tooth was a pivoting finding suggesting the endodontic involvement. Infrabony pocket of 8 mm on distal aspect of tooth indicated a secondary periodontal involvement requiring specific therapy to achieve success. The success rate of the endodontic-periodontal combined lesion without a concomitant regenerative procedure has been reported to a range from 27 to 37%.4 which suggests the need of surgical intervention. Recent advances in our understanding of cell regulation by growth factors present new options and provide additional treatment modalities for regenerating lost periodontal support.
The use of PRF is a recent and promising innovation in periodontal regenerative therapy. The positive impact of PRF on bone healing could be attributed to the angiogenic, proliferative and differentiating effects on osteoblasts of tissue growth factor β and platelet derived growth factor (TGF-β and PDGF) that are present in PRF in high concentrations.5 PRF has also been successfully used with different graft materials, with and without GTR, in the treatment of human periodontal infrabony defects.6-9 Bioactive glass enhances bone formation by ionic dissolution of ceramic material such that silica gel layer forms over the particles in contact with body fluids. Over the silica gel layer, a calcium phosphate layer forms which is quickly converted into hydroxycarbonate apatite layer.10 This apatite layer has been shown to identical with bone mineral and has provided surface for osteoblast cell attachment and bone dissolution.
PRP behaves like TGF-β to modulate cell proliferation in a specific manner which been shown to inhibit the epithelial cells while stimulating osteoblast and PDL cells.4 Fibrinogen converted to fibrin in combination with growth factors present in PRP may promote wound healing at the site of injury. As the blood clot stabilization is an important event in healing, PRF due to its high fibrin content may work as a hemostatic and stabilizing agent for the stability of the bone graft and blood clot.11 Attia has shown significantly favourable clinical improvement in periodontal infrabony defects with combination of PRF and tricalcium phosphate β (β-TCP) graft material.12
The healing potential of PRF combined with β-TCP has not been studied widely in endodontics. Kim et al. observed rapid bone formation, remodelling, and calcification in rabbits in the second week with combination of PRF with β-TCP than the β-TCP alone.13 In the present case, it was observed that at 3, 6, 9 and 12 months follow-up after the surgical treatment of large chronic periapical lesion, PRF combined with β-TCP resulted in significant clinical and radiographic bone regeneration.
Besides promoting wound healing, bone growth and maturation, PRF with bone graft have the advantages of graft stabilization, wound sealing, hemostasis and improved handling properties.14 However, like other case reports, this study also has limitations like short follow-up period of 12 months and a need for histological evaluation to confirm regeneration.
Conclusions
Endo-perio lesions are common conditions that are difficult to diagnose. However, if patient's history is taken carefully and thorough clinical examination is done, these lesions can be treated completely to give favorable outcome. The healing of an endodontic lesion is highly predictable, but the repair or regeneration of periodontal tissues is questionable if associated with it. Recent introduction of growth factors for periodontal treatment provide new opportunities for healing. This case report presents an attempt to evaluate the clinical effectiveness of the combination of PRF and β-TCP as opposed to using these materials alone. The use of autologous platelet preparations like PRF allows the clinician to optimize tissue remodelling, wound healing and angiogenesis by the local delivery of growth factors and proteins. The novel technique described enables the clinicians to gainfully harvest the full regenerative capacity of this autologous biologic material.
- 1. Simring M, Goldberg M. The pulpal pocket approach: retrograde periodontitis. J Periodontol 1964;35:22-48.Article
- 2. Aichelmann-Reidy ME, Yukna RA. Bone replacement grafts. The bone substitutes. Dent Clin North Am 1998;42:491-503.PubMed
- 3. Bashutski JD, Wang HL. Periodontal and endodontic regeneration. J Endod 2009;35:321-328.ArticlePubMed
- 4. Hirsch JM, Ahlström U, Henrikson PA, Heyden G, Peterson LE. Periapical surgery. Int J Oral Surg 1979;8:173-185.ArticlePubMed
- 5. Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, Wolff LF, Yoshie H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol 2003;74:849-857.PubMed
- 6. Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intra-bony defects treated with a natural bone mineral and a collagen membrane. J Clin Periodontol 2007;34:254-261.ArticlePubMed
- 7. Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intrabony defects treated with an anorganic bovine bone mineral and expanded polytetrafluoroethylene membranes. J Periodontol 2007;78:983-990.ArticlePubMed
- 8. Piemontese M, Aspriello SD, Rubini C, Ferrante L, Procaccini M. Treatment of periodontal intrabony defects with demineralized freeze-dried bone allograft in combination with platelet-rich plasma: a comparative clinical trial. J Periodontol 2008;79:802-810.ArticlePubMed
- 9. Pradeep AR, Shetty SK, Garg G, Pai S. Clinical effectiveness of autologous platelet-rich plasma and Peptide-enhanced bone graft in the treatment of intrabony defects. J Periodontol 2009;80:62-71.ArticlePubMed
- 10. Rose LF, Mealey BL, Genco RJ, Cohen DW. Periodontics: medicine, surgery and implants. St. Louis: Elsevier Mosby; 2004. p. 586.
- 11. Okuda K, Tai H, Tanabe K, Suzuki H, Sato T, Kawase T, Saito Y, Wolff LF, Yoshie H. Platelet-rich plasma combined with a porous hydroxyapaptite graft for the treatment of intrabony periodontal defects in humans: a comparative controlled clinical study. J Periodontol 2005;76:890-898.PubMed
- 12. Attia AM. Evaluation of beta-tricalcium phosphate and platelets rich plasma in management of intrabony defects: clinical and radiographic study. Egypt Dent J 2010;56:525-534.
- 13. Kim BJ, Kwon TK, Baek HS, Hwang DS, Kim CH, Chung IK, Jeong JS, Shin SH. A comparative study of the effectiveness of sinus bone grafting with recombinant human bone morphogenetic protein 2-coated tricalcium phosphate and platelet-rich fibrin-mixed tricalcium phosphate in rabbits. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;113:583-592.ArticlePubMed
- 14. Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: evolution of a second-generation platelet concentrate. Indian J Dent Res 2008;19:42-46.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- To Analyze the Efficacy of Platelet-rich Plasma in Contrast to Platelet-rich Fibrin along with Synthetic Nanocrystalline Hydroxyapatite and β-tricalcium Phosphate Bone Graft in Regeneration of Bony Defects in Children
Anshul Sharma, Sonali Saha, Amit Rai, Kavita Dhinsa, Nonie Marianne Koksi Sangma Shadap, Gunjan Yadav
International Journal of Clinical Pediatric Dentistry.2024; 16(6): 842. CrossRef - Regenerative Endodontic Management of an Immature Necrotic Premolar Using Advanced Platelet‐Rich Fibrin
Sepideh Hosseini, Nazanin Chitsaz, Mohammad Hassan Hamrah, Donya Maleki, Emad Taghizadeh, Hamdi Cem Gungor
Case Reports in Dentistry.2023;[Epub] CrossRef - Effect of biodentine coated with emdogain on proliferation and differentiation of human stem cells from the apical papilla
Hamed Karkehabadi, Erfan Ahmadyani, Rezvan Najafi, Elham Khoshbin
Molecular Biology Reports.2022; 49(5): 3685. CrossRef - Healing Assessment of Osseous Defects after Surgical Removal of Periapical Lesions in the Presence of Hydroxyapatite, Nanohydroxyapatite, and a Combination of Nanohydroxyapatite and Platelet-rich Fibrin: A Clinical Study
Amira Elkholly, Maged Negm, Reham Hassan, Nada Omar
Open Access Macedonian Journal of Medical Sciences.2022; 10(D): 406. CrossRef - Case report on combining PRF with alloplastic bone substitute in Endo-Perio lesion
Mansi Bansal, Manish Khatri, Komal Puri
Restorative Dentistry & Endodontics.2021;[Epub] CrossRef Treatment of an Endo-Perio Lesion with Ozone Gas in a Patient with Aggressive Periodontitis: A Clinical Case Report and Literature Review
Maria K Makeeva, Fatima Yu Daurova, Svetlana F Byakova, Anna Yu Turkina
Clinical, Cosmetic and Investigational Dentistry.2020; Volume 12: 447. CrossRef- Revisit to endo-perio lesion a review
Roopali Sharma, Akshita Gupta, K K. Gupta, Sarah Jameel, Rashmika Kapoor
IP International Journal of Periodontology and Implantology.2020; 5(2): 48. CrossRef - Autologous platelet-rich derivatives along with alloplastic bone substitute in the management of complex perio-endo cases
Lata Goyal, Namita Gupta, NarinderDev Gupta
Journal of Indian Society of Periodontology.2020; 24(2): 182. CrossRef - Platelet-Rich Fibrin as a Bone Graft Material in Oral and Maxillofacial Bone Regeneration: Classification and Summary for Better Application
Yiping Liu, Xiaolin Sun, Jize Yu, Jia Wang, Peisong Zhai, Siyu Chen, Manxuan Liu, Yanmin Zhou
BioMed Research International.2019; 2019: 1. CrossRef - Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo‐periodontal lesions
David Herrera, Belén Retamal‐Valdes, Bettina Alonso, Magda Feres
Journal of Clinical Periodontology.2018;[Epub] CrossRef - Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo‐periodontal lesions
David Herrera, Belén Retamal‐Valdes, Bettina Alonso, Magda Feres
Journal of Periodontology.2018;[Epub] CrossRef - Regenerative in endodontics: how, when and where
AL Ahmar Rima, Bassam Sanaa, Salloum Sarah, El Husseini Hassan, AL Ahmar Rima
Journal of Dental Health, Oral Disorders & Therapy.2018; 9(6): 531. CrossRef - Effect of Choukroun Platelet-Rich Fibrin Combined With Autologous Micro-Morselized Bone on the Repair of Mandibular Defects in Rabbits
Tian Zhou, Hua-Wei Yang, Zhuo-Wei Tian, Yang Wang, Xiao-Shan Tang, Jing-Zhou Hu
Journal of Oral and Maxillofacial Surgery.2018; 76(1): 221. CrossRef - Preliminary Results of Bone Regeneration in Oromaxillomandibular Surgery Using Synthetic Granular Graft
Noemi Mazzone, E. Mici, A. Calvo, M. Runci, S. Crimi, F. Lauritano, E. Belli
BioMed Research International.2018; 2018: 1. CrossRef - Treatment of endo-periodontal lesion using leukocyte- platelet- rich fibrin. A case report
Pablo Betancourt, Ricardo Elgueta, Ramon Fuentes
Colombia Medica.2017; 48(4): 204. CrossRef - The impact of autologous platelet concentrates on endodontic healing: a systematic review
Nastaran Meschi, Ana B. Castro, Katleen Vandamme, Marc Quirynen, Paul Lambrechts
Platelets.2016; 27(7): 613. CrossRef - A review of the regenerative endodontic treatment procedure
Bin-Na Lee, Jong-Wook Moon, Hoon-Sang Chang, In-Nam Hwang, Won-Mann Oh, Yun-Chan Hwang
Restorative Dentistry & Endodontics.2015; 40(3): 179. CrossRef - Platelet preparations in dentistry: How? Why? Where? When?
Luigi Fabrizio Rodella
World Journal of Stomatology.2015; 4(2): 39. CrossRef
Clinical effectiveness of combining platelet rich fibrin with alloplastic bone substitute for the management of combined endodontic periodontal lesion
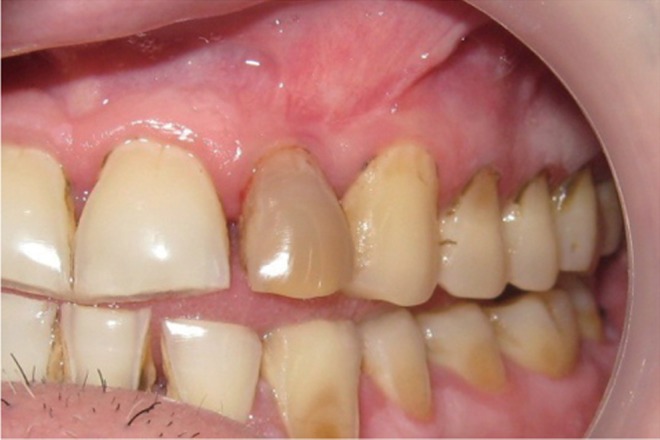
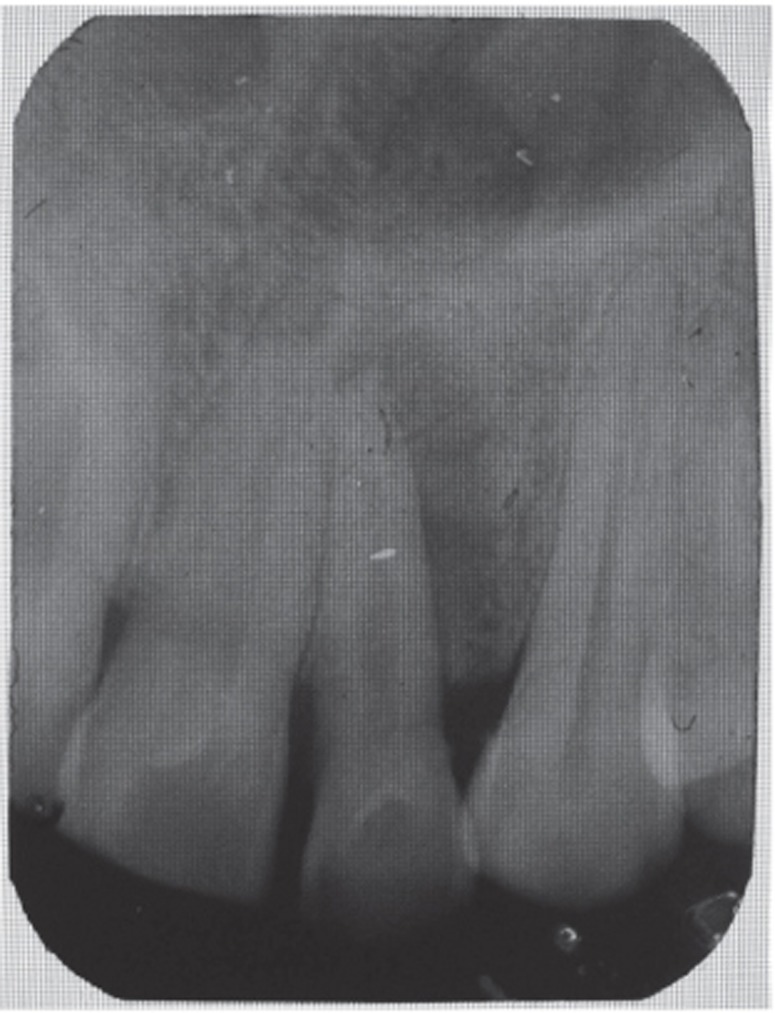
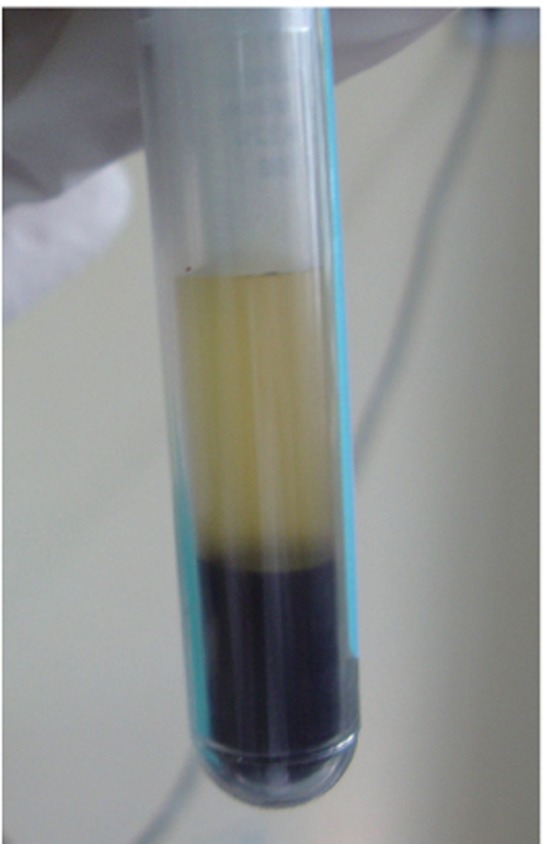

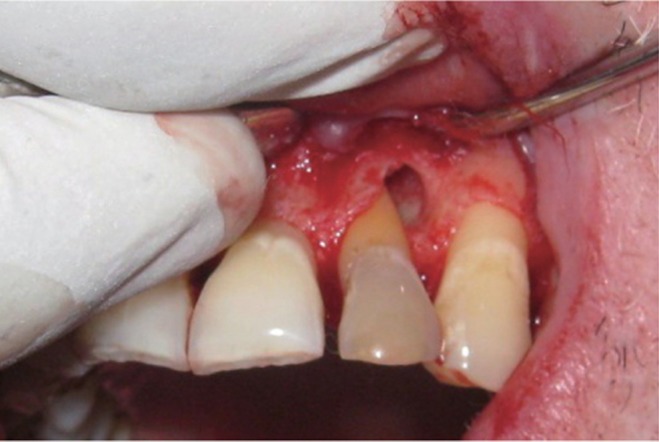
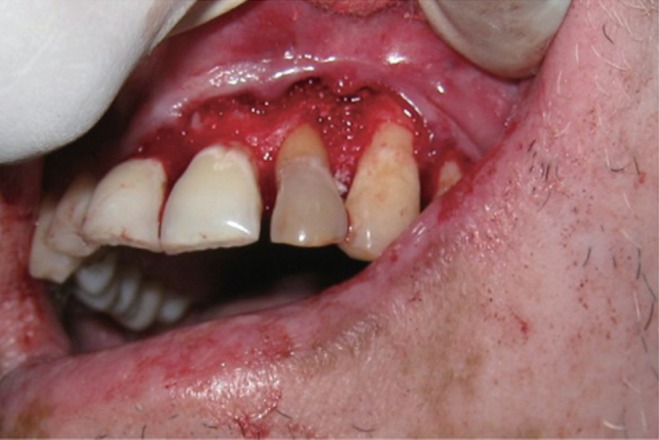
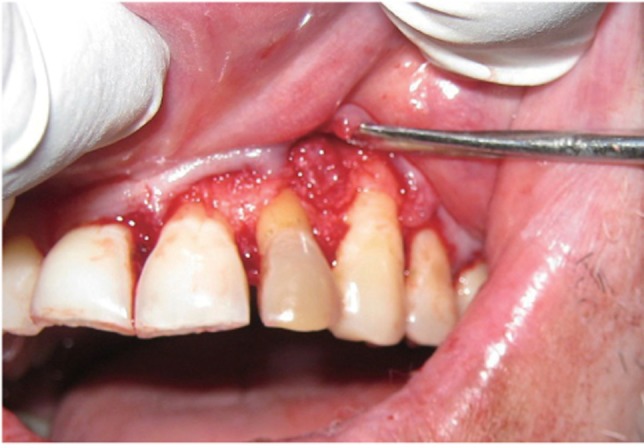
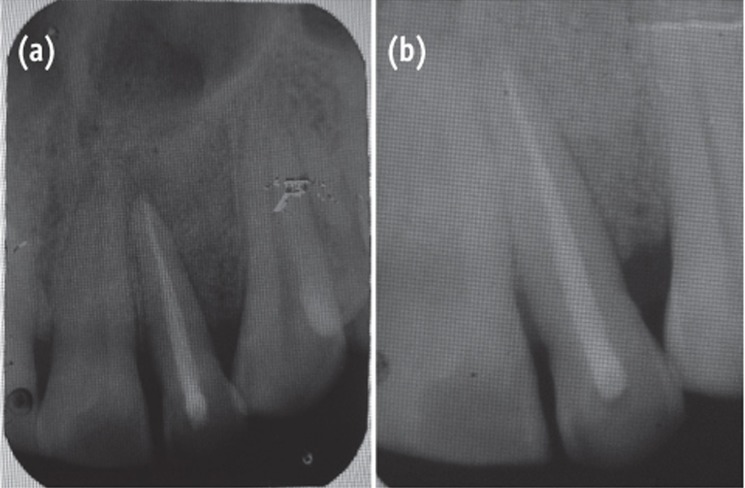
Figure 1 Preoperative view of discoloured maxillary lateral incisor.
Figure 2 Preoperative radiograph showing radiolucency along distal aspect of maxillary lateral incisor to the apex.
Figure 3 Separation of platelet rich plasma from the collected autologous blood.
Figure 4 Platelet rich fibrin after activation with calcium chloride.
Figure 5 Curettage of the defect after reflection of soft tissue flap using intracrevicular incision.
Figure 6 Placement of graft into defect.
Figure 7 Placement of PRF.
Figure 8 Follow-up radiographs (a) at 6 months, postoperative view showing bone fill; (b) at 12 months.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Clinical effectiveness of combining platelet rich fibrin with alloplastic bone substitute for the management of combined endodontic periodontal lesion

 KACD
KACD

 ePub Link
ePub Link Cite
Cite

