Abstract
-
Objectives
This study was designed to evaluate the synergistic antibacterial effect of xylitol and ursolic acid (UA) against oral biofilms in vitro.
-
Materials and Methods
S. mutans UA 159 (wild type), S. mutans KCOM 1207, KCOM 1128 and S. sobrinus ATCC 33478 were used. The susceptibility of S. mutans to UA and xylitol was evaluated using a broth microdilution method. Based on the results, combined susceptibility was evaluated using optimal inhibitory combinations (OIC), optimal bactericidal combinations (OBC), and fractional inhibitory concentrations (FIC). The anti-biofilm activity of xylitol and UA on Streptococcus spp. was evaluated by growing cells in 24-well polystyrene microtiter plates for the biofilm assay. Significant mean differences among experimental groups were determined by Fisher's Least Significant Difference (p < 0.05).
-
Results
The synergistic interactions between xylitol and UA were observed against all tested strains, showing the FICs < 1. The combined treatment of xylitol and UA inhibited the biofilm formation significantly and also prevented pH decline to critical value of 5.5 effectively. The biofilm disassembly was substantially influenced by different age of biofilm when exposed to the combined treatment of xylitol and UA. Comparing to the single strain, relatively higher concentration of xylitol and UA was needed for inhibiting and disassembling biofilm formed by a mixed culture of S. mutans 159 and S. sobrinus 33478.
-
Conclusions
This study demonstrated that xylitol and UA, synergistic inhibitors, can be a potential agent for enhancing the antimicrobial and anti-biofilm efficacy against S. mutans and S. sobrinus in the oral environment.
-
Keywords: Biofilm; Streptococcus mutans; Streptococcus sobrinus; Ursolic acid; Xylitol
Introduction
Dental caries is one of the most prevalent and costly infectious diseases worldwide. Dental plaque (a microbial biofilm) accumulation is a significant contributor to dental caries, leading to acidic demineralisation of the tooth enamel and dentin.
1,
2,
3 It is recognized that
Streptococcus spp. play key roles in cariogenesis, and
Streptococcus mutans and
Streptococcus sobrinus are considered the main cariogenic bacteria of human dental caries due to their remarkable abilities of surface colonization, carbohydrate metabolism, and lactic acid production in the oral environment.
4,
5 In the oral cavity, cariogenic bacteria adhere and auto-aggregate on the tooth surface to form dental plaque biofilms which produce lactic acid and cause tooth demineralization that often leads to dental caries.
In the past few decades, common anticaries agents such as sodium fluoride and chlorhexidine have been widely studied as prophylactic anticariogenic bacterial agents.
6,
7,
8 However, natural products have gained more and more applications in the field of dentistry. Among them, xylitol is one of the most widely used natural anticaries agents since it is a sugar substitute unlike other caries causing sweeteners.
9 It is commonly accepted that the presence of xylitol creates a starvation effect on the population of cariogenic bacteria since they cannot metabolize it, while long-term exposure of xylitol may create a permanent change in the oral population and lead to the emergence of xylitol-resistant (X
R)
S. mutans.
10,
11 Furthermore, it is a controversial issue that whether the X
R
S. mutans strains are less virulent or cariogenic than their xylitol-sensitive (Xs) counterparts.
12,
13,
14 Ursolic acid (UA) is a natural pentacyclic triterpenoid carboxylic acid that is often found in edible or medicinal plants known to have anti-inflammatory, antitumor and antibacterial activities.
15,
16
Previous studies have demonstrated that UA or xylitol has antibacterial activity.
17,
18,
19,
20 However, no study evaluated the synergistic effect of these two natural antimicrobials on anti-biofilm formation and biofilm disassembly. The advantage of the synergistic effect is that they not only produce a greater antibacterial effect at a lower dosage, but also reduce the chance of the emergence of antimicrobial resistant bacteria strains.
21 Thus, the aim of the present study was to investigate the inhibitory effects of xylitol and UA on
Streptococcus spp. and to determine whether these two natural antimicrobials had a synergistic effect on cariogenic bacterial growth, anti-biofilm formation and biofilm disassembly.
Materials and Methods
Bacterial strains and antimicrobial preparation
S. mutans UA 159 (wild type) and two clinical strains of S. mutans KCOM 1207, KCOM 1128 were obtained from the Korean Collection for Oral Microbiology (KCOM, Gwangju, Korea), and S. sobrinus ATCC 33478 was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All strains were routinely streaked on brain-heart infusion agar (BHIA, MB cell, Los Angeles, CA, USA) plates and grown at 37℃ under anaerobic conditions of 5% CO2 for 48 hours. Single bacterial colonies were inoculated into 3 mL of brain-heart infusion (BHI; BD, Franklin Lakes, NJ, USA), followed by overnight incubation. The culture was then refreshed in BHI broth in 1 : 20 ratio and incubated again until exponential phase (OD600 reached 0.5, approximately 6.5 × 107 CFU/mL). The culture served as the inoculums for each antimicrobial activity evaluation. BHI broth was used to dilute (1 : 100) the culture until the concentration reached approximately 1 × 106 CFU/mL. UA (U6753) and xylitol (X3375) were purchased from Sigma-Aldrich Chemicals Inc. (St. Louis, MO, USA) while the stock solutions of UA (10.24 mg/mL) and xylitol (40%) were prepared by dissolving in dimethylsulphoxide (DMSO, Sigma-Aldrich) and sterile water, respectively.
Antimicrobial susceptibility testing
The susceptibility of
S. mutans to UA and xylitol was evaluated using a broth microdilution method according to the Clinical Laboratory Standards Institute (CLSI) procedure with slight modification.
22 The stock solution of each drug was serially diluted with BHI. Approximately 1 × 10
6 CFU/mL of indicator bacteria (100 µL each) were inoculated into 96-well plates containing diluted drugs (100 µL) for final UA concentrations of 512, 256, 128, 64, 32, 16 µg/mL or final xylitol concentration of 20%, 10%, 5%, 2.5%, 1.25%, 0.625%. The final concentration of DMSO in 512 µg/mL condition was 5%. In order to eliminate the DMSO effect, BHI broth containing 5% DMSO was used as negative control. All plates were incubated for 18 hours at 37℃ under anaerobic conditions, followed by OD
600 analysis using Infinite F200 PRO (TECAN, Salzburg, Austria). The lowest concentration of UA and xylitol that inhibited visible growth was considered the minimal inhibitory concentration (MIC). For quantification of the minimal bactericidal concentration (MBC), 100 µL aliquots from the wells at the MIC value determined above were directly spread on BHI agar plates in a tenfold gradient dilution, followed by 48 hours incubation at 37℃ under anaerobic conditions of 5% CO
2. The MBC was defined as the lowest drug concentration that killed 99.9% of the initial inoculums in a given time as determined by counting viable cells.
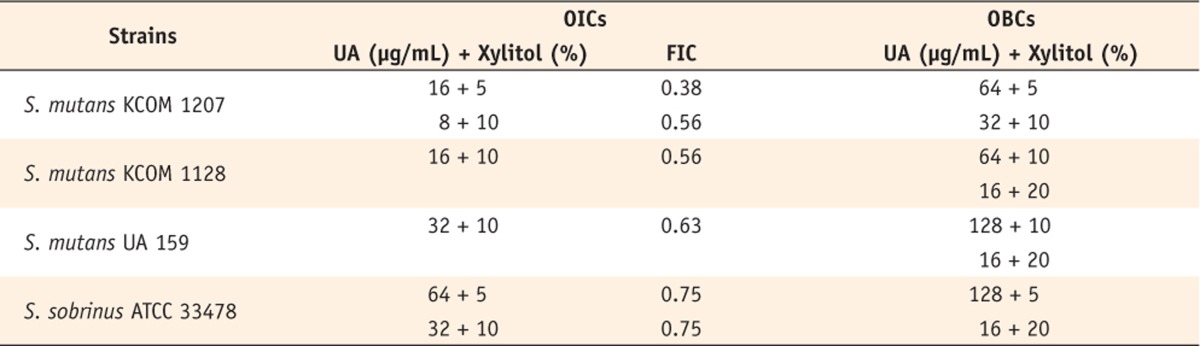
MICs from single antimicrobial susceptibility testing were used as starting points to prepare a series of mixtures of UA and xylitol with BHI broth in a 96-well plate. UA concentrations were 256, 128, 64, 32, 16, 8, and 4 µg/mL, and xylitol concentrations were 20%, 10%, and 5%. The indicator strains were inoculated into each well at approximately 1 × 106 CFU/mL followed by 24 hours incubation at 37℃ under anaerobic conditions of 5% CO2. Viable cell numbers were quantified by the plate counting method. Optimal inhibitory combinations (OICs) were defined as the combinations producing inhibitory activity that utilized the lowest concentration of one compound in combination with the other. Likewise, optimal bactericidal combinations (OBCs) were classified as the combinations producing bactericidal activity that utilized the lowest concentration of one compound in combination with the other.
Antimicrobial interaction assessment
Fractional inhibitory concentrations (FICs) were estimated by the following equation:
MICxylitol and MICUA are the MICs of xylitol and UA alone, and Cxylitol and CUA are the concentrations of xylitol and UA in combination, respectively. The antimicrobial interactions were classified as antagonistic (FIC > 1), no synergy/interaction (FIC = 1), or synergistic (FIC < 1).
Biofilm assay
The anti-biofilm activity of xylitol and UA on Streptococcus spp. was evaluated by growing cells in 24-well polystyrene microtiter plates using BHI medium containing 20 mM sucrose (BHIS). Briefly, a challenge plate was prepared, which contained a group of fixed combination of UA (1/8 MIC, 1/16 MIC, and 1/32 MIC) and xylitol (1/2 MIC, 1/4 MIC), as well as UA and xylitol alone. The strains (S. mutans UA 159 and S. sobrinus ATCC 33478 alone, and a mixture of both strains) were inoculated into each well approximately 1 × 106 CFU/mL within 1 mL BHIS medium followed by 18 hours incubation at 37℃ under anaerobic conditions of 5% CO2. The medium of each well was collected for pH determination by using a Mettler Toledo SevenEasy pH Meter. Meanwhile, each well was washed twice with PBS (pH 7.4) and re-suspended in 1mL PBS. The biofilm cells was detached by using a tip sonicator (Sonics Vibracell CV18, Woburn, MA, USA) and quantified by the plate counting method.
Moreover, biofilm disassembly ability of UA and xylitol combination was studied by using biofilm of different age. Biofilm cultivation was performed similar to anti-biofilm assay. In brief, strains (S. mutans UA 159 and S. sobrinus ATCC 33478 alone, and a mixture of both strains) were inoculated into each well at approximately 1 × 106 CFU/mL within 1 mL BHIS medium without xylitol or UA, followed 37℃ incubation. At a certain time point (3, 6, 12 and 24 hours), the planktonic cells of each well were removed by washing twice with PBS, then these cells were re-grown in UA alone (8, 16 and 32 µg/mL) or xylitol alone (20%), or combined with UA and xylitol, followed by 20 hours further incubation at 37℃ under anaerobic conditions of 5% CO2. Neither UA nor xylitol containing group was used as negative control. Biofilm cells collection and CFU counting were done using the previously described methods.
Statistical analysis
All analyses were performed in duplicate on three replicates. Data were analyzed using the SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). Significant mean differences among treatments or times were determined by Fisher's Least Significant Difference (LSD) at p < 0.05.
Results
Antimicrobial susceptibility testing
The MICs and MBCs of UA and xylitol against three species of
Streptococcus spp. are listed in
Table 1. The MICs of UA against four tested strains ranged from 128 to 256 µg/mL, and the MBC ranged from 256 to 512 µg/mL. The MIC of xylitol was 20% for all tested strains, while the MBC of xylitol was greater than 20%. Obviously, UA showed notable antimicrobial effect to four tested strains compared to xylitol. Especially, the strain of
S. mutans KCOM 1207 and
S. sobrinus ATCC 33478 showed more susceptibility to UA. In addition, all tested strains grown in BHI broth containing 5% DMSO showed similarity to those grown in BHI alone indicating that no DMSO effect was observed in the MICs or MBCs studies.
OICs for the UA and xylitol pairing are given in
Table 2.
S. mutans KCOM 1207 was most susceptible to the combination (FIC = 0.38), while
S. mutans KCOM 1128 and
S. mutans UA 159 only had one OIC, and
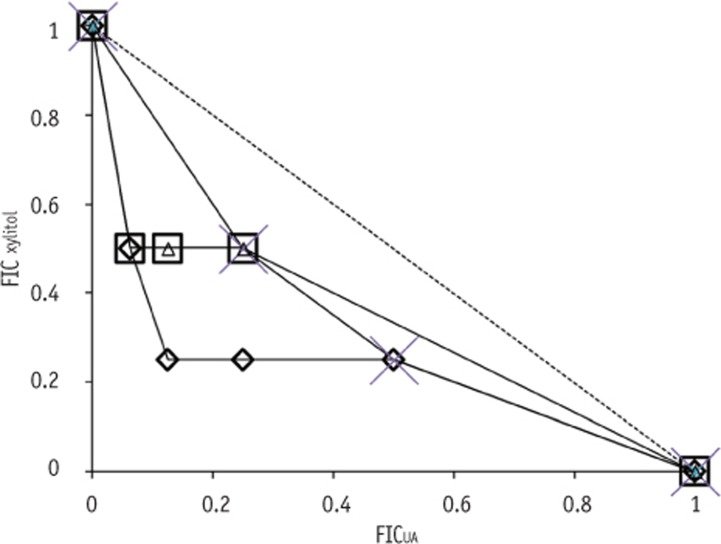
S. sobrinus ATCC 33478 had two OICs at the same FIC value. Meanwhile, a concave characteristic of isobologram (FIC < 1) was observed in
Figure 1, which indicates a synergistic interaction between UA and xylitol. OBCs had the same trend as OICs for the four tested strains. The antimicrobial activity of xylitol against selected strains was significantly enhanced by combining with UA, showing a strong bactericidal effect against all strains (
Tables 1 and
2).
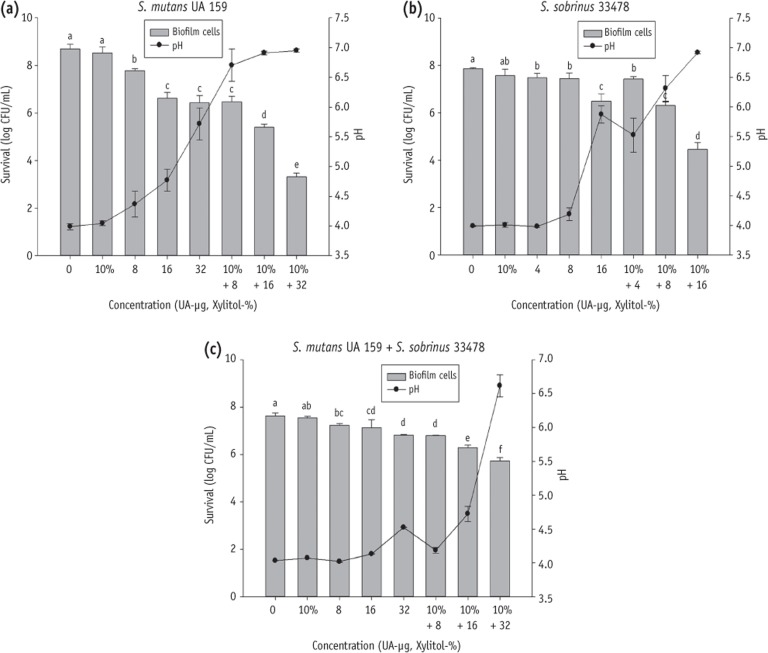
The combined effect was confirmed by exposing selected strains at the sublethal concentrations of UA (1/8 MIC = 32 or 16, 1/16 MIC = 16 or 8, 1/32 MIC = 8 or 4 µg/mL depending on the tested strains) and xylitol (10%) for 24 hours (
Figure 2). Xylitol (10%) alone did not inhibit the biofilm growth of
S. mutans UA 159 and
S. sobrinus ATCC 33478 alone or mixed culture in this study. UA alone showed biofilm inhibitory effect on
S. mutans,
S. sobrinus and mixed culture groups. However, the
S. mutans treated with a combination of UA (8, 16 and 32 µg/mL) and xylitol (10%) was decreased to 6.48, 5.40 and 3.31 log CFU mL
-1 in turn compared to xylitol alone treatment with 8.52 log CFU mL
-1 (
Figure 2a). Likewise, the
S. sobrinus and mixed culture was reduced to 7.43, 6.31, 4.45 and 6.79, 6.30, 5.72 log CFU mL
-1 respectively, compared to xylitol alone with 7.57 and 7.55 log CFU mL
-1 (
Figures 2b and 2c). Furthermore, the BHIS (initial pH = 7.2) medium containing both xylitol and UA controlled the pH reduction caused by the metabolism of selected stains more efficiently. This observation may be due to the synergistic effect which limited growth and metabolism of bacterial cells. All combination treatments showed higher pH than 5.5 in culture of
S. mutans and
S. sobrinus alone (
Figures 2a and 2b), while only one combination (10% + 32) had a pH value greater than 5.5 in mixed culture (
Figure 2c).
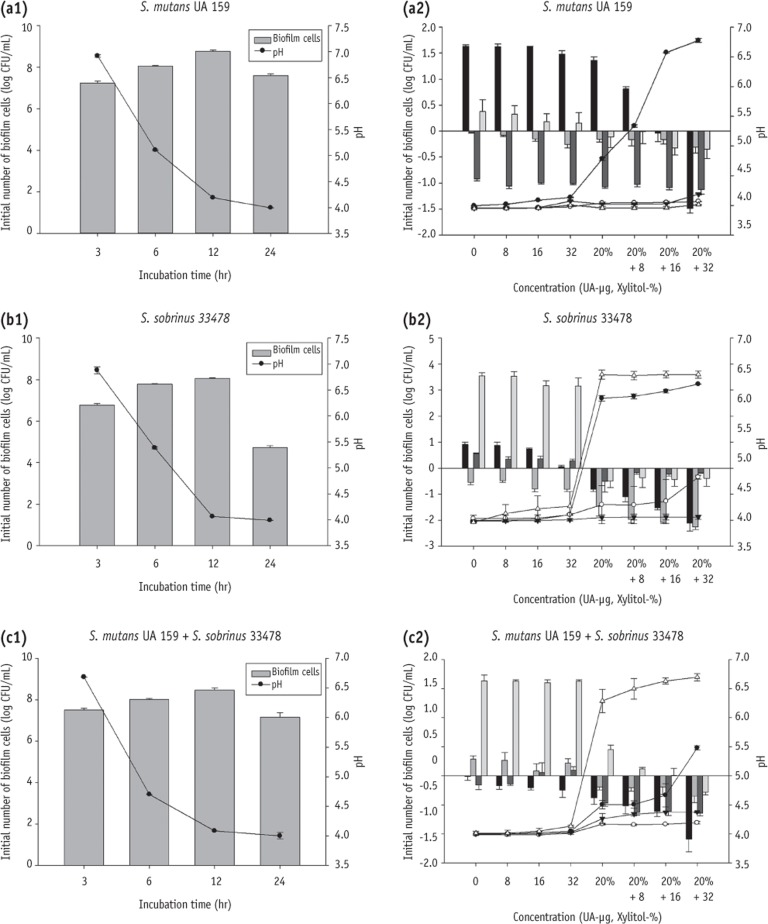
The numbers of biofilm cells was gradually increased within 12 hours, but a reduction was observed at 24 hours. Especially, only 4.73 log CFU mL
-1 was determined in
S. sobrinus after 24 hours incubation (
Figures 3a1, 3b1 and 3c1). The data of cell regrowth collected from different incubation times (3, 6, 12 and 24 hours) showed that the combined use of xylitol and UA were more effective to inhibit the biofilm cell growth (
Figures 3a2, 3b2 and 3c2). However, their effect was slightly different depending on the species and timing of collecting cells. At all ages of
S. mutans regrowth, the biofilm formed showed significant reduction when treated with 20% xylitol combined with 16 or 32 µg/mL UA, while treatment of UA alone or xylitol alone showed less biofilm reduction (
Figure 3a2). In
S. sobrinus and mixed groups, the combination of xylitol and UA, and xylitol alone led to biofilm reduction at all ages (
Figures 3b2 and 3c2).
After 6 hours incubation, all species showed a drop of pH below 5.5 (
Figures 3a1, 3b1 and 3c1). In
S. mutans regrowth groups, 20% xylitol plus 16 or 32 µg/mL UA treated group collected at 3 hours, showed a pH higher than 5.5 (
Figure 3a2). In
S. sobrinus groups, xylitol alone (20%) and all xylitol and UA combined groups showed a pH higher than 5.5 at 3 and 24 hours (
Figure 3b2). In mixed group, xylitol alone (20%) and all xylitol and UA combined groups showed a pH higher than 5.5 at 24 hours (
Figure 3c2).
Discussion
In the present study, we used several test conditions to quantitatively analyze the synergistic effect of xylitol and UA in planktonic and biofilm cell state. As oral biofilms or dental plaque formation is known as the most crucial factor to cause caries, the development of potential strategies to prevent oral biofilm formation is necessary.
The MICs of UA against
S. mutans observed in this study were similar to those found by other authors, while some difference was observed with MICs for xylitol.
16 This observation may due to the fact that both studies used different source of xylitol and
S. mutans strains.
23 The results of this experiment showed similar susceptibility for the three different
S. mutans strains, and more susceptibility for
S. sobrinus when exposed to UA. On the other hand, it showed uniform susceptibility for four strains when exposed to xylitol (
Table 1). When both antimicrobials were combined, they showed synergistic effect on the studied microorganisms and their effect was judged by FIC (
Figure 1). The antimicrobial activity of xylitol against selected
Streptococcus spp. was significantly enhanced in combination with UA (
Table 2). This synergistic activity against selected
Streptococcus spp. may attribute to the different mechanisms of action of xylitol and UA.
Following the FIC results, we selected two relatively resistant strains of
S. mutans UA 159 and
S. sobrinus 33478 for further biofilm studies. Comparing to other sugars, sucrose is the most cariogenic substance, as it can be fermented by cariogenic bacteria and produces glucans that promotes the adhesion, aggregation, and accumulation of cariogenic bacteria onto the smooth tooth surface, accelerating dental plaque development.
24 In order to determine whether the combined use can inhibit the biofilm formation of selected strains at optimal biofilm forming condition, we chose the most enriched medium (BHI) containing 20 mM sucrose for growing cariogenic bacteria. However, the combination of xylitol and UA also showed good synergistic effect to inhibit biofilm formation, as well as effective prevention of pH drop below the critical value of 5.5 (
Figure 2).
Tooth demineralization happens when the environment at the tooth-dental plaque interface tend to become acidic (pH 5.5 and below), following the bacterial metabolism of the acid.
25 The mixed culture of
S. mutans and
S. sobrinus was more resistant to the xylitol and UA combined treatment and kept the pH below 5.5 except for the 10% + 32 group (
Figure 2c). This enhanced antimicrobial resistance of mixed culture could increase the risk of cariogenicity and it was confirmed by a clinical study which showed that children harbouring both
S. mutans and
S. sobrinus had higher incidence of dental caries than those with
S. mutans alone.
26
Interestingly, the numbers of biofilm cells of
S. sobrinus showed a sharp decrease after 24 hours incubation (
Figure 3b1). This reduction may attribute to the two factors. One is rapid depletion of nutrient, thus leading to nutrient starvation, and the other one is the highly acidic environment (pH = 3.99) which reduced
S. sobrinus growth. The number of biofilm cells of mixed culture showed similarity with
S. mutans, but not with
S. sobrinus (
Figures 3a1 and 3c1). In general,
S. sobrinus is able to produce acids more rapidly than
S. mutans.
27,
28 In this study, we observed that a mixed culture caused more rapid pH drop than either strain alone. The observation confirmed the increased risk of cariogenicity when harbouring both
S. mutans and
S. sobrinus in the oral cavity. The initial numbers of
S. mutans biofilm at 3 hours was 7.24 log CFU mL
-1 and significant re-growth was seen after 20 hours of further incubation in controls (1.63 log CFU mL
-1 positive growth). Likewise, 24 hours
S. mutans biofilm showed a slightly positive growth (0.38 log CFU mL
-1) (
Figures 3a1 and 3a2). On the contrary, 3 hours
S. sobrinus biofilm presented an indistinctive positive growth (0.91 log CFU mL
-1) while the 24 hours biofilm (initial number = 4.73 log CFU mL
-1) expressed a remarkable positive growth (3.54 log CFU mL
-1) after 20 hours incubation (
Figures 3b1 and 3b2). Comparing the results of both strains, we further confirmed the previous hypothesis that the distinct reduction of
S. sobrinus number of biofilm cells at 24 hours is partly attributable to nutrient starvation and pH stress since significant positive growth implied that the cell numbers were far enough to reach the saturation state in the certain amount of medium at optimal culture condition.
Following the results of this study, we observed that the combined use of UA and xylitol could increase the antibacterial effect on the S. mutans. However, further studies to reveal the mechanism of this synergistic effect are needed so that we may develop better ways to control cariogenic bacteria.
Conclusions
This study demonstrated that xylitol and UA, synergistic inhibitors, can be a potential agent for enhancing the antimicrobial and anti-biofilm efficacy against S. mutans and S. sobrinus in the oral environment. The planktonic cells were highly sensitive to xylitol combined with UA than xylitol or UA alone, as were the biofilm formation and the biofilm disassembly. Furthermore, the xylitol combined with UA raised the pH above the critical value of 5.5 which enabled the prevention of tooth demineralization. However, the mixed culture of S. mutans and S. sobrinus was relatively resistant to combination treatment. This result illustrates new approaches to reduce the risk of cariogenicity in the oral cavity.
Acknowledgement
This research was supported by the grant of 2012 Yonsei university school of dentistry research funds (6-2012-0069).
-
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1. Seneviratne CJ, Zhang CF, Samaranayake LP. Dental plaque biofilm in oral health and disease. Chin J Dent Res 2011;14:87-94.PubMed
- 2. Rautemaa R, Ramage G. Oral candidosis-clinical challenges of a biofilm disease. Crit Rev Microbiol 2011;37:328-336.ArticlePubMed
- 3. Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res 2010;89:657-665.ArticlePubMedPDF
- 4. Nicolas GG, Lavoie MC. Streptococcus mutans and oral streptococci in dental plaque. Can J Microbiol 2011;57:1-20.ArticlePubMed
- 5. Mosci F, Perito S, Bassa S, Capuano A, Marconi PF. The role of Streptococcus mutans in human caries. Minerva Stomatol 1990;39:413-429.PubMed
- 6. Bowden GH. Mutans streptococci caries and chlorhexidine. J Can Dent Assoc 1996;62:700. 703-707.PubMed
- 7. Gluzman R, Katz RV, Frey BJ, McGowan R. Prevention of root caries: a literature review of primary and secondary preventive agents. Spec Care Dentist 2013;33:133-140.ArticlePubMed
- 8. Shen Y, Stojicic S, Haapasalo M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod 2011;37:657-661.ArticlePubMed
- 9. Ly KA, Milgrom P, Rothen M. Xylitol, sweeteners, and dental caries. Pediatr Dent 2006;28:154-163.PubMed
- 10. Trahan L, Bourgeau G, Breton R. Emergence of multiple xylitol-resistant (fructose PTS-) mutants from human isolates of mutans streptococci during growth on dietary sugars in the presence of xylitol. J Dent Res 1996;75:1892-1900.ArticlePubMedPDF
- 11. Trahan L, Mouton C. Selection for Streptococcus mutans with an altered xylitol transport capacity in chronic xylitol consumers. J Dent Res 1987;66:982-988.ArticlePubMedPDF
- 12. Assev S, Stig S, Scheie AA. Cariogenic traits in xylitol-resistant and xylitol-sensitive mutans streptococci. Oral Microbiol Immunol 2002;17:95-99.ArticlePubMedPDF
- 13. Trahan L, Söderling E, Dréan MF, Chevrier MC, Isokangas P. Effect of xylitol consumption on the plaque-saliva distribution of mutans streptococci and the occurrence and long-term survival of xylitol-resistant strains. J Dent Res 1992;71:1785-1791.ArticlePubMedPDF
- 14. Lee SH, Choi BK, Kim YJ. The cariogenic characters of xylitol-resistant and xylitol-sensitive Streptococcus mutans in biofilm formation with salivary bacteria. Arch Oral Biol 2012;57:697-703.ArticlePubMed
- 15. Leng S, Hao Y, Du D, Xie S, Hong L, Gu H, Zhu X, Zhang J, Fan D, Kung HF. Ursolic acid promotes cancer cell death by inducing Atg5-dependent autophagy. Int J Cancer 2013;133:2781-2790.ArticlePubMed
- 16. Zhou L, Ding Y, Chen W, Zhang P, Chen Y, Lv X. The in vitro study of ursolic acid and oleanolic acid inhibiting cariogenic microorganisms as well as biofilm. Oral Dis 2013;19:494-500.ArticlePubMedPDF
- 17. Söderling EM, Ekman TC, Taipale TJ. Growth inhibition of Streptococcus mutans with low xylitol concentrations. Curr Microbiol 2008;56:382-385.ArticlePubMedPDF
- 18. Assev S, Vegarud G, Rölla G. Growth inhibition of Streptococcus mutans strin OMZ 176 by xylitol. Acta Pathol Microbiol Scand B 1980;88:61-63.PubMed
- 19. do Nascimento PG, Lemos TL, Bizerra AM, Arriaga ÂM, Ferreira DA, Santiago GM, Braz-Filho R, Costa JG. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014;19:1317-1327.ArticlePubMedPMC
- 20. Kim S, Song M, Roh BD, Park SH, Park JW. Inhibition of Streptococcus mutans biofilm formation on composite resins containing ursolic acid. Restor Dent Endod 2013;38:65-72.ArticlePubMedPMC
- 21. Torella JP, Chait R, Kishony R. Optimal drug synergy in antimicrobial treatments. PLoS Comput Biol 2010;6:e1000796.ArticlePubMedPMC
- 22. CLSI. M07-A9 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Apprived standard. 9th ed. Wayne PA: Clinical and Laboratory Standards Institute; 2012. p. 1-63.
- 23. Misra S, Raghuwanshi S, Gupta P, Saxena RK. Examine growth inhibition pattern and lactic acid production in Streptococcus mutans using different concentrations of xylitol produced from Candida tropicalis by fermentation. Anaerobe 2012;18:273-279.ArticlePubMed
- 24. Forssten SD, Björklund M, Ouwehand AC. Streptococcus mutans, caries and simulation models. Nutrients 2010;2:290-298.ArticlePubMedPMC
- 25. Cura F, Palmieri A, Girardi A, Martinelli M, Scapoli L, Carinci F. Lab-Test(®) 4: dental caries and bacteriological analysis. Dent Res J (Isfahan) 2012;9:S139-S141.PubMedPMC
- 26. Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, Kozai K. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J Med Microbiol 2005;54:661-665.ArticlePubMed
- 27. de Soet JJ, van Loveren C, Lammens AJ, Pavicić MJ, Homburg CH, ten Cate JM, de Graaff J. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res 1991;25:116-122.ArticlePubMed
- 28. Martinez AR, Abranches J, Kajfasz JK, Lemos JA. Characterization of the Streptococcus sobrinus acid-stress response by interspecies microarrays and proteomics. Mol Oral Microbiol 2010;25:331-342.PubMedPMC
Figure 1
Isobologram for the antimicrobial interaction between UA and xylitol against Streptococcous mutans KCOM 1207 (◊), S. mutans KCOM 1128 (□), S. mutans UA 159 (△) and S. sobrinus ATCC 33478 (×). Dashed line indicates additive interactions.
FIC, fractional inhibitory concentrations; UA, ursolic acid.
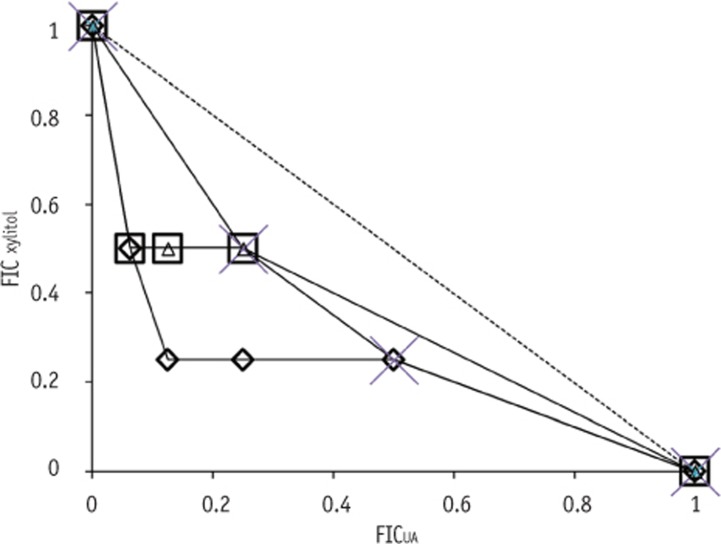
Figure 2Effect of xylitol or UA alone and xylitol-UA combined groups on the biofilm growth of S. mutans UA 159 (a) S. sobrinus 33478; (b) and mixture of S. mutans UA 159 with S. sobrinus 33478; (c) cultured in BHI containing 20 mM sucrose. UA, ursolic acid.
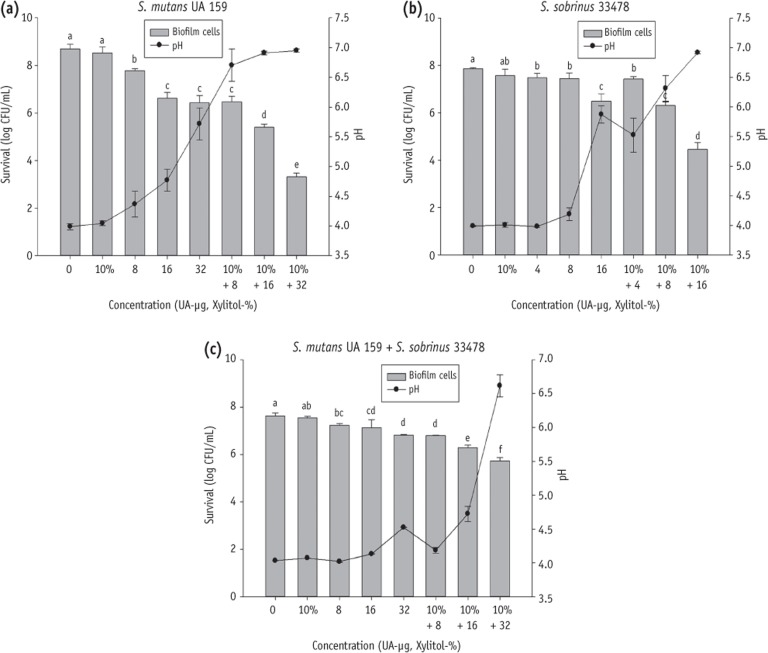
Figure 3The number of biofilm cells in S. mutans UA 159 (a1), S. sobrinus 33478 (b1), and co-culture of S. mutans UA 159 with S. sobrinus 33478 (c1) after certain incubation times (3, 6, 12, and 24 hours). Relative number of biofilm of different age (3 hours ■, 6 hours ▓, 12 hours ▒, 24 hours ░) grown at various treatment and pH change (3 hours ●, 6 hours ○, 12 hours ▼, 24 hours △) in S. mutans UA 159 (a2), S. sobrinus 33478 (b2), and co-culture of S. mutans UA 159 with S. sobrinus 33478 (c2). UA, ursolic acid.
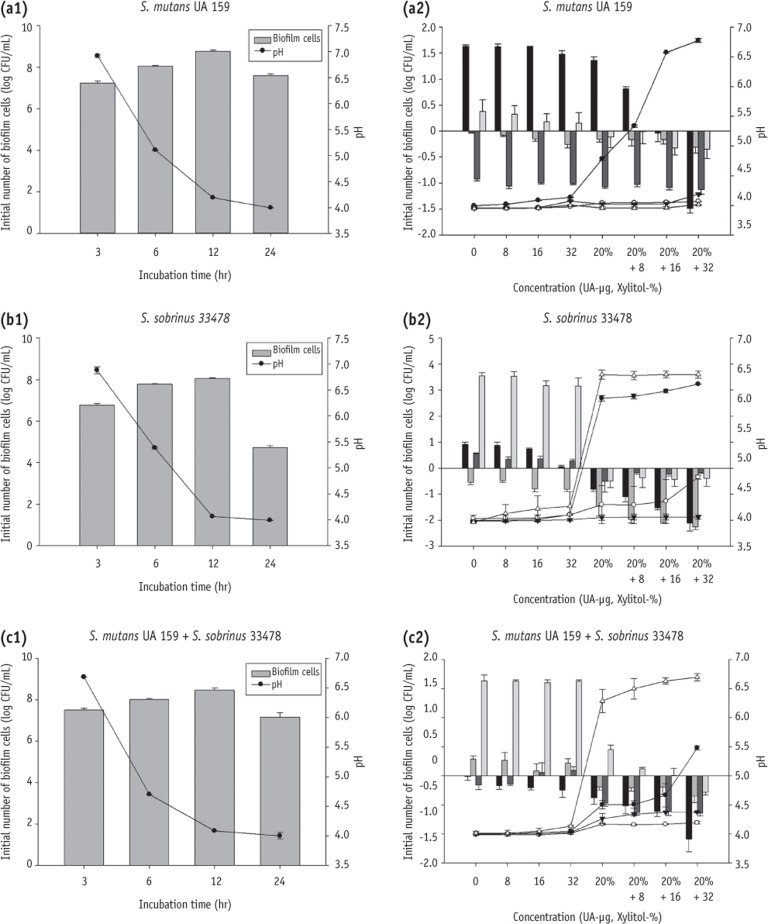
Table 1Antimicrobial effect of ursolic acid or xylitol against planktonic S. mutans
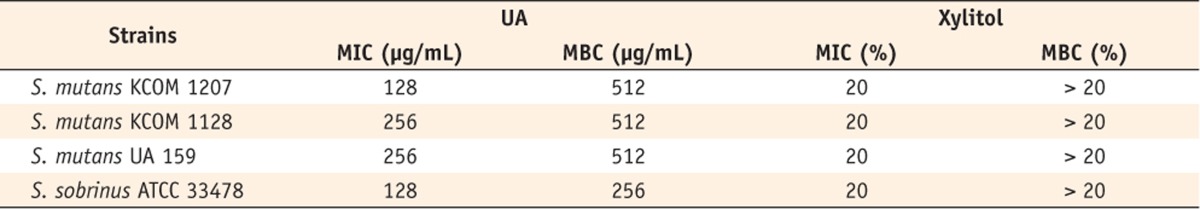
Table 2Optimal inhibitory combinations (OIC), fractional inhibitory concentration (FIC) indices, and optimal bactericidal combinations (OBC) of ursolic acid (UA) and xylitol against Streptococcus spp.








 KACD
KACD


 ePub Link
ePub Link Cite
Cite

