Articles
- Page Path
- HOME > Restor Dent Endod > Volume 38(3); 2013 > Article
- Review Article Biocompatibility of root-end filling materials: recent update
- Payal Saxena1, Saurabh Kumar Gupta1, Vilas Newaskar2
-
2013;38(3):-127.
DOI: https://doi.org/10.5395/rde.2013.38.3.119
Published online: August 23, 2013
1Department of Conservative Dentistry and Endodontics, Government College of Dentistry, Indore, Madhya Pradesh, India.
2Department of Oral and Maxillofacial Surgery, Government College of Dentistry, Indore, Madhya Pradesh, India.
- Correspondence to Payal Saxena, MDS. Assistant Professor, Department of Conservative Dentistry and Endodontics, Government College of Dentistry, Indore, Madhya Pradesh, India. TEL: +91-930-2793700, FAX: +91-731-2701608, payalmds@yahoo.co.in
©Copyights 2013. The Korean Academy of Conservative Dentistry.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,414 Views
- 26 Download
- 36 Crossref
Abstract
- The purpose of a root-end filling is to establish a seal between the root canal space and the periradicular tissues. As root-end filling materials come into contact with periradicular tissues, knowledge of the tissue response is crucial. Almost every available dental restorative material has been suggested as the root-end material of choice at a certain point in the past. This literature review on root-end filling materials will evaluate and comparatively analyse the biocompatibility and tissue response to these products, with primary focus on newly introduced materials.
Introduction
Review
1. Amalgam
2. Gutta-percha (GP)
3. Zinc oxide eugenol (ZOE) cement
4. Glass ionomer cement (GIC) and related materials
5. Composite resins and resin-ionomer hybrids
6. Diaket
7. Mineral trioxide aggregate (MTA)
8. Other MTA formulations
9. New materials under research
1) Endosequence root repair material (ERRM), putty and paste
2) Bioaggregate
3) iRoot BP Plus bioceramic putty
4) Novel root-end filling material
5) Experimental calcium aluminosilicate based materials
(1) EndoBinder
(2) Generex A
(3) Capasio
(4) Quick-Set
(5) Biodentine
6) Polymer nanocomposite (PNC) resin
7) Novel root-end filling material using epoxy resin and Portland cement (EPC)
8) Iron-free partially stabilized cement
1. In vitro studies and in vivo experimental studies
2. Clinical studies
Conclusions
- 1. Gatewood RS. Endodontic materials. Dent Clin North Am 2007;51:695-712.ArticlePubMed
- 2. Andreasen JO. Cementum repair after apicoectomy in humans. Acta Odontol Scand 1973;31:211-221.ArticlePubMed
- 3. Regan JD, Gutmann JL, Witherspoon DE. Comparison of Diaket and MTA when used as root-end filling materials to support regeneration of the periradicular tissues. Int Endod J 2002;35:840-847.ArticlePubMed
- 4. Dahl JE. Toxicity of endodontic filling materials. Endod Topics 2005;12:39-43.Article
- 5. Glickman GN, Hartwell GR. Chapter 33. Endodontic surgery. In: Ingle JI, Bakland LK, Baumgartner JC, editors. Ingle's Endodontics. 6th ed. Hamilton: BC Decker Inc; 2008. p. 1261-1294.
- 6. Chong BS, Pitt Ford TR. Root-end filling materials: rationale and tissue response. Endod Topics 2005;11:114-130.Article
- 7. Gutmann JL, Harrison JW. Posterior endodontic surgery: anatomical considerations and clinical techniques. Int Endod J 1985;18:8-34.ArticlePubMed
- 8. Friedman S. Retrograde approaches in endodontic therapy. Endod Dent Traumatol 1991;7:97-107.ArticlePubMed
- 9. In: Pitt Ford TR, editor. Harty's Endodontics in Clinical Practice. 5th ed. Edinburgh: Wright; 2004. Chapter 9, Surgical endodontics. p. 143-181.
- 10. In: Anusavice KJ, editor. Phillip's Science of Dental Materials. 11th ed. St Louis: Saunders; 2003. Chapter 8, Biocompatibility of dental materials. p. 171-202.
- 11. Pitt Ford TR, Andreasen JO, Dorn SO, Kariyawasam SP. Effect of IRM root end fillings on healing after replantation. J Endod 1994;20:381-385.ArticlePubMed
- 12. Pitt Ford TR, Andreasen JO, Dorn SO, Kariyawasam SP. Effect of various zinc oxide materials as root-end fillings on healing after replantation. Int Endod J 1995;28:273-278.ArticlePubMed
- 13. Chong BS, Ford TR, Kariyawasam SP. Tissue response to potential root-end filling materials in infected root canals. Int Endod J 1997;30:102-114.ArticlePubMed
- 14. Chong BS, Pitt Ford TR, Kariyawasam SP. Short-term tissue response to potential root-end filling materials in infected root canals. Int Endod J 1997;30:240-249.ArticlePubMed
- 15. Kimura JT. A comparative analysis of zinc and non-zinc alloys used in retrograde endodontic surgery. Part 1: apical seal and tissue reaction. J Endod 1982;8:359-363.ArticlePubMed
- 16. Kimura JT. A comparative analysis of zinc and nonzinc alloys used in retrograde endodontic surgery. Part 2: optical emission spectrographic analysis for zinc precipitation. J Endod 1982;8:407-409.ArticlePubMed
- 17. Pitt Ford TR, Andreasen JO, Dorn SO, Kariyawasam SP. Effect of super-EBA as a root end filling on healing after replantation. J Endod 1995;21:13-15.ArticlePubMed
- 18. Torabinejad M, Pitt Ford TR, McKendry DJ, Abedi HR, Miller DA, Kariyawasam SP. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod 1997;23:225-228.ArticlePubMed
- 19. Baek SH, Plenk H Jr, Kim S. Periapical tissue responses and cementum regeneration with amalgam, SuperEBA, and MTA as root-end filling materials. J Endod 2005;31:444-449.ArticlePubMed
- 20. Kaplan SD, Tanzilli JP, Raphael D, Moodnik RM. A comparison of the marginal leakage of retrograde techniques. Oral Surg Oral Med Oral Pathol 1982;54:583-585.ArticlePubMed
- 21. Pitt Ford TR, Andreasen JO, Dorn SO, Kariyawasam SP. Effect of various sealers with gutta-percha as root-end fillings on healing after replantation. Endod Dent Traumatol 1996;12:33-37.ArticlePubMed
- 22. Wälivaara DA, Abrahamsson P, Isaksson S, Salata LA, Sennerby L, Dahlin C. Periapical tissue response after use of intermediate restorative material, gutta-percha, reinforced zinc oxide cement, and mineral trioxide aggregate as retrograde root-end filling materials: a histologic study in dogs. J Oral Maxillofac Surg 2012;70:2041-2047.ArticlePubMed
- 23. Phillips RW, Love DR. The effect of certain additive agents on the physical properties of zinc oxide-eugenol mixtures. J Dent Res 1961;40:294-303.ArticlePDF
- 24. Weine FS. Endodontic Therapy. 4th ed. St Louis: Mosby; 1982. p. 498-502.
- 25. Torabinejad M, Walton RE. Principles and Practice of Endodontics. 3rd ed. Philadelphia: Saunders; 2002. p. 275-278.
- 26. Hendra LP. EBA cement. A practical system for all cementation. J Br Endod Soc 1970;4:28-32.ArticlePubMed
- 27. Oynick J, Oynick T. A study of a new material for retrograde fillings. J Endod 1978;4:203-206.ArticlePubMed
- 28. Jeng JH, Hahn LJ, Lu FJ, Wang YJ, Kuo MY. Eugenol triggers different pathobiological effects on human oral mucosal fibroblasts. J Dent Res 1994;73:1050-1055.ArticlePubMedPDF
- 29. Meryon SD, Jakeman KJ. The effects in vitro of zinc released from dental restorative materials. Int Endod J 1985;18:191-198.ArticlePubMed
- 30. Haglund R, He J, Jarvis J, Safavi KE, Spångberg LS, Zhu Q. Effects of root-end filling materials on fibroblasts and macrophages in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:739-745.ArticlePubMed
- 31. Al-Sabek F, Shostad S, Kirkwood KL. Preferential attachment of human gingival fibroblasts to the resin ionomer Geristore. J Endod 2005;31:205-208.ArticlePubMed
- 32. Al-Hiyasat AS, Al-Sa'Eed OR, Darmani H. Quality of cellular attachment to various root-end filling materials. J Appl Oral Sci 2012;20:82-88.ArticlePubMedPMC
- 33. Barkhordar RA, Pelzner RB, Stark MM. Use of glass ionomers as retrofilling materials. Oral Surg Oral Med Oral Pathol 1989;67:734-739.ArticlePubMed
- 34. Pissiotis E, Sapounas G, Spångberg LS. Silver glass ionomer cement as a retrograde filling material: a study in vitro. J Endod 1991;17:225-229.PubMed
- 35. Pissiotis E, Spângberg L. Reaction of bony tissue to implanted silver glass ionomer and a reinforced zinc oxide-eugenol cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:623-629.ArticlePubMed
- 36. Brook IM, Hatton PV. Glass-ionomers: bioactive implant materials. Biomaterials 1998;19:565-571.ArticlePubMed
- 37. Callis PD, Santini A. Tissue response to retrograde root filling in the ferret canine: a comparison of a glass ionomer cement and gutta-percha with sealer. Oral Surg Oral Med Oral Pathol 1987;64:475-479.PubMed
- 38. Geurtsen W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med 2000;11:333-355.PubMed
- 39. Andreasen JO, Rud J, Munksgaard EC. Retrograde root obturations using resin and a dentin bonding agent: a preliminary histologic study of tissue reactions in monkeys. Tandlaegebladet 1989;93:195-197.PubMed
- 40. Trope M, Lost C, Schmitz HJ, Friedman S. Healing of apical periodontitis in dogs after apicoectomy and retrofilling with various filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:221-228.ArticlePubMed
- 41. Camp MA, Jeansonne BG, Lallier T. Adhesion of human fibroblasts to root-end filling materials. J Endod 2003;29:602-607.ArticlePubMed
- 42. Gupta SK, Saxena P, Pant VA, Pant AB. Adhesion and biologic behavior of human periodontal fibroblast cells to resin ionomer Geristore: a comparative analysis. Dent Traumatol 2012 11 06 [Epub ahead of print]. DOI: 10.1111/edt.12016.Article
- 43. Williams SS, Gutmann JL. Periradicular healing in response to Diaket root-end filling material with and without tricalcium phosphate. Int Endod J 1996;29:84-92.ArticlePubMed
- 44. Kettering JD, Torabinejad M. Cytotoxicity of root canal sealers: a study using HeLa cells and fibroblasts. Int Endod J 1984;17:60-66.ArticlePubMed
- 45. Spångberg L. Biological effects of root canal filling materials. 7. Reaction of bony tissue to implanted root canal filling material in guineapigs. Odontol Tidskr 1969;77:133-159.PubMed
- 46. Olsson B, Wennberg A. Early tissue reaction to endodontic filling materials. Endod Dent Traumatol 1985;1:138-141.ArticlePubMed
- 47. Nencka D, Walia H, Austin BP. Histological evaluation of the biocompatibility of Diaket. J Dent Res 1995;74(Supplement 1):101.
- 48. Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod 2006;32:601-623.ArticlePubMed
- 49. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-Part III: Clinical applications, drawbacks, and mechanism of action. J Endod 2010;36:400-413.ArticlePubMed
- 50. Thomson TS, Berry JE, Somerman MJ, Kirkwood KL. Cementoblasts maintain expression of osteocalcin in the presence of mineral trioxide aggregate. J Endod 2003;29:407-412.ArticlePubMed
- 51. Pistorius A, Willershausen B, Briseño Marroquin B. Effect of apical root-end filling materials on gingival fibroblasts. Int Endod J 2003;36:610-615.ArticlePubMedPDF
- 52. Pelliccioni GA, Ciapetti G, Cenni E, Granchi D, Nanni M, Pagani S, Giunti A. Evaluation of osteoblast-like cell response to Proroot MTA (mineral trioxide aggregate) cement. J Mater Sci Mater Med 2004;15:167-173.ArticlePubMed
- 53. Huang TH, Yang CC, Ding SJ, Yan M, Chou MY, Kao CT. Biocompatibility of human osteosarcoma cells to root end filling materials. J Biomed Mater Res B Appl Biomater 2005;72:140-145.ArticlePubMed
- 54. Al-Rabeah E, Perinpanayagam H, MacFarland D. Human alveolar bone cells interact with ProRoot and tooth-colored MTA. J Endod 2006;32:872-875.ArticlePubMed
- 55. Souza NJ, Justo GZ, Oliveira CR, Haun M, Bincoletto C. Cytotoxicity of materials used in perforation repair tested using the V79 fibroblast cell line and the granulocyte-macrophage progenitor cells. Int Endod J 2006;39:40-47.ArticlePubMed
- 56. Yoshimine Y, Ono M, Akamine A. In vitro comparison of the biocompatibility of mineral trioxide aggregate, 4META/MMA-TBB resin, and intermediate restorative material as root-end filling materials. J Endod 2007;33:1066-1069.ArticlePubMed
- 57. Vajrabhaya LO, Korsuwannawong S, Jantarat J, Korre S. Biocompatibility of furcal perforation repair material using cell culture technique: Ketac Molar versus Pro-Root MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:e48-e50.ArticlePubMed
- 58. Gorduysus M, Avcu N, Gorduysus O, Pekel A, Baran Y, Avcu F, Ural AU. Cytotoxic effects of four different endodontic materials in human periodontal ligament fibroblasts. J Endod 2007;33:1450-1454.ArticlePubMed
- 59. Asrari M, Lobner D. In vitro neurotoxic evaluation of root-end filling materials. J Endod 2003;29:743-746.ArticlePubMed
- 60. Gomes-Filho JE, de Faria MD, Bernabé PF, Nery MJ, Otoboni-Filho JA, Dezan-Júnior E, de Moraes Costa MM, Cannon M. Mineral trioxide aggregate but not light-cure mineral trioxide aggregate stimulated mineralization. J Endod 2008;34:62-65.ArticlePubMed
- 61. Gomes-Filho JE, de Moraes Costa MM, Cintra LT, Duarte PC, Takamiya AS, Lodi CS, Bernabé PF. Evaluation of rat alveolar bone response to Angelus MTA or experimental light-cured mineral trioxide aggregate using fluorochromes. J Endod 2011;37:250-254.ArticlePubMed
- 62. Gomes-Filho JE, de Moraes Costa MT, Cintra LT, Lodi CS, Duarte PC, Okamoto R, Bernabé PF, Nery MJ, Cannon M. Evaluation of alveolar socket response to Angelus MTA and experimental light-cure MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:e93-e97.ArticlePubMed
- 63. Gomes-Filho JE, Rodrigues G, Watanabe S, Estrada Bernabé PF, Lodi CS, Gomes AC, Faria MD, Domingos Dos Santos A, Silos Moraes JC. Evaluation of the tissue reaction to fast endodontic cement (CER) and Angelus MTA. J Endod 2009;35:1377-1380.ArticlePubMed
- 64. Brasseler USA Co.. Endosequence root repair material paste, Information. updated 2010 Dec]. Available from: http://www.brasselerusa.com/display.cfm?pid=newproducts.
- 65. Alanezi AZ, Jiang J, Safavi KE, Spangberg LS, Zhu Q. Cytotoxicity evaluation of endosequence root repair material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:e122-e125.ArticlePubMed
- 66. Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair materials. J Endod 2011;37:793-798.ArticlePubMed
- 67. Damas BA, Wheater MA, Bringas JS, Hoen MM. Cytotoxicity comparison of mineral trioxide aggregates and EndoSequence bioceramic root repair materials. J Endod 2011;37:372-375.ArticlePubMed
- 68. Chung CR, Kim E, Shin SJ. Biocompatibility of bioaggregate cement on human pulp and periodontal ligament (PDL) derived cells. J Korean Acad Conserv Dent 2010;35:473-478.Article
- 69. Lee JH, Shon WJ, Lee W, Baek SH. The effect of several root-end filling materials on MG63 osteoblast-like cells. J Korean Acad Conserv Dent 2010;35:222-228.Article
- 70. Innovative BioCeramix, Inc Co.. Information. updated 2012 Jan]. Available from: http://www.ibioceramix.com/Publications.html.
- 71. De-Deus G, Canabarro A, Alves GG, Marins JR, Linhares AB, Granjeiro JM. Cytocompatibility of the ready-to-use bioceramic putty repair cement iRoot BP Plus with primary human osteoblasts. Int Endod J 2012;45:508-513.ArticlePubMed
- 72. Kim M, Ko H, Yang W, Lee Y, Kim S, Mante FK. A new resin-bonded retrograde filling material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e111-e116.ArticlePubMed
- 73. Yang WK, Ko HJ, Kim MR. Evaluation of the rat tissue reaction to experimental new resin cement and mineral trioxide aggregate cement. Restor Dent Endod 2012;37:194-200.ArticlePubMedPMC
- 74. Wei W, Qi YP, Nikonov SY, Niu LN, Messer RL, Mao J, Primus CM, Pashley DH, Tay FR. Effects of an experimental calcium aluminosilicate cement on the viability of murine odontoblast-like cells. J Endod 2012;38:936-942.ArticlePubMed
- 75. Aguilar FG, Roberti Garcia LF, Panzeri Pires-de-Souza FC. Biocompatibility of new calcium aluminate cement (EndoBinder). J Endod 2012;38:367-371.ArticlePubMed
- 76. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod 1999;25:197-205.ArticlePubMed
- 77. Ørstavik D, Nordahl I, Tibballs JE. Dimensional change following setting of root canal sealer materials. Dent Mater 2001;17:512-519.ArticlePubMed
- 78. Porter ML, Bertó A, Primus CM, Watanabe I. Physical and chemical properties of new-generation endodontic materials. J Endod 2010;36:524-528.ArticlePubMed
- 79. Bird DC, Komabayashi T, Guo L, Opperman LA, Spears R. In Vitro evaluation of dentinal tubule penetration and biomineralization ability of a new root-end filling material. J Endod 2012;38:1093-1096.ArticlePubMedPMC
- 80. Washington JT, Schneiderman E, Spears R, Fernandez CR, He J, Opperman LA. Biocompatibility and osteogenic potential of new generation endodontic materials established by using primary osteoblasts. J Endod 2011;37:1166-1170.ArticlePubMed
- 81. Laurent P, Camps J, De Méo M, Déjou J, About I. Induction of specific cell responses to a Ca3SiO5-based posterior restorative material. Dent Mater 2008;24:1486-1494.ArticlePubMed
- 82. Goldberg M, Pradelle-Plasse N, Tran X, Colon P, Laurent P, Aubut V, About I, Boukpessi T, Septier D. Emerging trends in (bio)material researches. In: Goldberg M, editor. Biocompatibility or cytotoxic effects of dental composites. Oxford: Coxmoor Publishing; 2009. p. 181-203.
- 83. Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J 2011;44:1081-1087.ArticlePubMed
- 84. Zhou HM, Shen Y, Wang ZJ, Li L, Zheng YF, Häkkinen L, Haapasalo M. In Vitro cytotoxicity evaluation of a novel root repair material. J Endod 2013;39:478-483.ArticlePubMed
- 85. Krishnan PS, Joshi M, Bhargava P, Valiyaveettil S, He C. Effect of heterocyclic based organoclays on the properties of polyimide-clay nanocomposites. J Nanosci Nanotechnol 2005;5:1148-1157.ArticlePubMed
- 86. Modareszadeh MR, Chogle SA, Mickel AK, Jin G, Kowsar H, Salamat N, Shaikh S, Qutbudin S. Cytotoxicity of set polymer nanocomposite resin root-end filling materials. Int Endod J 2011;44:154-161.ArticlePubMed
- 87. Lee SJ, Chung J, Na HS, Park EJ, Jeon HJ, Kim HC. Characteristics of novel root-end filling material using epoxy resin and Portland cement. Clin Oral Investig 2013;17:1009-1015.ArticlePubMedPDF
- 88. Ndong F, Sadhasivam S, Lin FH, Savitha S, Wen-Hsi W, Lin CP. The development of iron-free partially stabilized cement for use as dental root-end filling material. Int Endod J 2012;45:557-564.ArticlePubMed
- 89. Coon D, Gulati A, Cowan C, He J. The role of cyclooxygenase-2 (COX-2) in inflammatory bone resorption. J Endod 2007;33:432-436.ArticlePubMed
- 90. Hammad HM, Hamadah MA, Al-Omari WM. Histological evaluation of rat tissue response to GMTA, Retroplast, and Geristore retrograde filling materials. Aust Endod J 2011;37:18-25.ArticlePubMed
- 91. Song M, Kim E. A prospective randomized controlled study of mineral trioxide aggregate and super ethoxybenzoic acid as root-end filling materials in endodontic microsurgery. J Endod 2012;38:875-879.ArticlePubMed
- 92. Chong BS, Pitt Ford TR, Hudson MB. A prospective clinical study of mineral trioxide aggregate and IRM when used as root-end filling materials in endodontic surgery. Int Endod J 2003;36:520-526.PubMed
- 93. Lindeboom JA, Frenken JW, Kroon FH, van den Akker HP. A comparative prospective randomized clinical study of MTA and IRM as root-end filling materials in single-rooted teeth in endodontic surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:495-500.ArticlePubMed
- 94. Wälivaara DÅ, Abrahamsson P, Fogelin M, Isaksson S. Super-EBA and IRM as root-end fillings in periapical surgery with ultrasonic preparation: a prospective randomized clinical study of 206 consecutive teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:258-263.ArticlePubMed
- 95. Saunders WP. A prospective clinical study of periradicular surgery using mineral trioxide aggregate as a root-end filling. J Endod 2008;34:660-665.ArticlePubMed
- 96. Christiansen R, Kirkevang LL, Hørsted-Bindslev P, Wenzel A. Randomized clinical trial of root-end resection followed by root-end filling with mineral trioxide aggregate or smoothing of the orthograde gutta-percha root filling - 1-year follow-up. Int Endod J 2009;42:105-114.ArticlePubMed
- 97. Evidence-based review of clinical studies on the root apex. J Endod 2009;35:1158-1159.PubMed
- 98. Wälivaara DA, Abrahamsson P, Sämfors KA, Isaksson S. Periapical surgery using ultrasonic preparation and thermoplasticized gutta-percha with AH Plus sealer or IRM as retrograde root-end fillings in 160 consecutive teeth: a prospective randomized clinical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:784-789.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Bioceramics in Endodontics: Limitations and Future Innovations—A Review
Peramune Arachchilage Amila Saman Prasad Kumara, Paul Roy Cooper, Peter Cathro, Maree Gould, George Dias, Jithendra Ratnayake
Dentistry Journal.2025; 13(4): 157. CrossRef - Development of zinc partially-stabilized cement carrying growth factor and anti-inflammatory drug for vital pulp therapy
Tsao-Li Chuang, Chih-Chun Chang, Chun-Liang Yeh, Chun-Pin Lin
Journal of Dental Sciences.2025; 20(4): 2250. CrossRef - Structural and morphological characterization of silver nanoparticles intruded mineral trioxide aggregate admixture as a chair-side restorative medicament: an in vitro experimental study
H. Murali Rao, Rajkumar Krishnan, Chitra Shivalingam, Ramya Ramadoss
Restorative Dentistry & Endodontics.2025; 50(3): e30. CrossRef - MTA as modulator of periapical tissue healing in rat molar: A histological study
Christian Khoswanto, Ira Kusuma Dewi
Journal of Oral Biology and Craniofacial Research.2024; 14(2): 201. CrossRef - Effects of Three Retrograde Filling Materials on Production of Inflammatory Cytokines and Resorbing Mediators
Samaneh Arab, Marjan Bahraminasab, Masoumeh Motamedi, Jamshid Hadjati, Alaviye Vahid
Journal of Microbiota.2024;[Epub] CrossRef - An in vitro assessment of cytotoxicity and genotoxicity of root repair materials
Shreya A. Harti, M. S. Adarsha, N. Meena, N. S. Priya, L. Vijayalakshmi, Akshata J. Airsang
Journal of Oral and Maxillofacial Pathology.2023; 27(4): 700. CrossRef - In Vitro Comparison of Differences in Setting Time of Premixed Calcium Silicate-Based Mineral Trioxide Aggregate According to Moisture Content of Gypsum
Hyun-Jin Kim, Jun-Seok Lee, Dong-Hoon Gwak, Yong-Seok Ko, Chun-Il Lim, Seung-Youl Lee
Materials.2023; 17(1): 35. CrossRef - An in-vitro comparison of bond strength of three different root end filling materials with Universal testing machine
Vandana Goyal, Iyana Garg, Parminder Kaur, Ankita Tomar
IP Indian Journal of Conservative and Endodontics.2023; 8(4): 221. CrossRef - Push-out bond strength and intratubular biomineralization of a hydraulic root-end filling material premixed with dimethyl sulfoxide as a vehicle
Ju-Ha Park, Hee-Jin Kim, Kwang-Won Lee, Mi-Kyung Yu, Kyung-San Min
Restorative Dentistry & Endodontics.2023;[Epub] CrossRef - A Systematic Review on Comparison of Periapical Healing and Post-Operative Pain between Bioceramic and Epoxy Resin Based Sealers
Deepali Mahajan, Devansh Manocha, Priyesha Patel, Maulik B. Saraiya, Keral Chaniyara
Journal of Pharmacy and Bioallied Sciences.2023; 15(Suppl 2): S862. CrossRef - Periapical Healing following Root Canal Treatment Using Different Endodontic Sealers: A Systematic Review
Akshay Khandelwal, Krishnamachari Janani, KavalipurapuVenkata Teja, Jerry Jose, Gopi Battineni, Francesco Riccitiello, Alessandra Valletta, Ajitha Palanivelu, Gianrico Spagnuolo, Vincenzo Grassia
BioMed Research International.2022;[Epub] CrossRef - Confocal laser scanning microscopic evaluation of sealing ability of bone cement, mineral trioxide aggregate and biodentine as root-end filling materials
Shalin Ann Saji, Tony Mathew, Aditya Shetty, Gurmeen Kaur, Sunheri Bajpe
Endodontology.2022; 34(2): 86. CrossRef - Adhesive Ability of Different Oral Pathogens to Various Dental Materials: An In Vitro Study
Yan Tu, Shuli Deng, Yuan Wang, Xiaolong Lin, Zhenyu Yang, Tingtao Chen
Canadian Journal of Infectious Diseases and Medical Microbiology.2022; 2022: 1. CrossRef - Fast self-curing α-tricalcium phosphate/β-dicalcium silicate composites beneficial for root canal sealing treatment
Youyang Zheng, Xianyan Yang, Shuxin Liu, Siqi Bao, Yuyue Xu, Yunyi Wang, Feng Zhang, Zhongru Gou
Heliyon.2022; 8(9): e10713. CrossRef - Pleiotropic Effects of Eugenol: The Good, the Bad, and the Unknown
Oana M. Aburel, Ioana Z. Pavel, Maria D. Dănilă, Theia Lelcu, Alexandra Roi, Rodica Lighezan, Danina M. Muntean, Laura C. Rusu, M rcio Carocho
Oxidative Medicine and Cellular Longevity.2021;[Epub] CrossRef - Novel nanosystems to enhance biological activity of hydroxyapatite against dental caries
Nataliya Babayevska, Marta Woźniak-Budych, Jagoda Litowczenko, Barbara Peplińska, Marcin Jarek, Patryk Florczak, Grażyna Bartkowiak, Beata Czarnecka, Stefan Jurga
Materials Science and Engineering: C.2021; 124: 112062. CrossRef - Comparison of the sealing ability of various bioceramic materials for endodontic surgery
Benjamin Rencher, Ana M. Chang, Hanson Fong, James D. Johnson, Avina Paranjpe
Restorative Dentistry & Endodontics.2021;[Epub] CrossRef - Scanning electron microscopy analysis of marginal adaptation of mineral trioxide aggregate, tricalcium silicate cement, and dental amalgam as a root end filling materials
Lena Z. Jovanović, Branislav V. Bajkin
Microscopy Research and Technique.2021; 84(9): 2068. CrossRef - Assessment of bone healing after mineral trioxide aggregate and platelet-rich fibrin application in periapical lesions using cone-beam computed tomographic imaging
Nazife Begüm Karan, Banu Aricioğlu
Clinical Oral Investigations.2020; 24(2): 1065. CrossRef - Micro-computed tomographic evaluation of the flow and filling ability of endodontic materials using different test models
Fernanda Ferrari Esteves Torres, Juliane Maria Guerreiro-Tanomaru, Gisselle Moraima Chavez-Andrade, Jader Camilo Pinto, Fábio Luiz Camargo Villela Berbert, Mario Tanomaru-Filho
Restorative Dentistry & Endodontics.2020;[Epub] CrossRef - Investigating unset endodontic sealers’ eugenol and hydrocortisone roles in modulating the initial steps of inflammation
Charlotte Jeanneau, Thomas Giraud, Jean-Louis Milan, Imad About
Clinical Oral Investigations.2020; 24(2): 639. CrossRef - Tricalcium silicate cements: osteogenic and angiogenic responses of human bone marrow stem cells
Mohamed R. W. Ali, Manal Mustafa, Asgeir Bårdsen, Athanasia Bletsa
European Journal of Oral Sciences.2019; 127(3): 261. CrossRef - Systemic bone marker expression induced by grey and white mineral trioxide aggregate in normal and diabetic conditions
I. O. de Azevedo Queiroz, W. G. Mello, C. M. Martins, R. Dal Fabbro, L. G. Narciso, L. Massunari, L. T. A. Cintra, E. Ervolino, J. E. Gomes‐Filho
International Endodontic Journal.2018; 51(8): 889. CrossRef - The use of Bioceramics as root-end filling materials in periradicular surgery: A literature review
Sumaya M. Abusrewil, William McLean, J. Alun Scott
The Saudi Dental Journal.2018; 30(4): 273. CrossRef - Endodontic medicine: interrelationships among apical periodontitis, systemic disorders, and tissue responses of dental materials
Luciano Tavares Angelo Cintra, Carlos Estrela, Mariane Maffei Azuma, Índia Olinta de Azevedo Queiroz, Toshihisa Kawai, João Eduardo Gomes-Filho
Brazilian Oral Research.2018;[Epub] CrossRef - Comparative Evaluation of Marginal Integrity of Mineral Trioxide Aggregate and Biodentine as Retrograde Filling Materials-An In Vitro Study
Preneet Kaur, Muskan Behl
AMEI's Current Trends in Diagnosis & Treatment.2018; 2(2): 92. CrossRef - Osteogenic Response of Osteoblastic Cells to Root-End Filling Materials
Eui Ri Na, Jong Wook Moon, Young Joon Kim
Materials Science Forum.2018; 926: 95. CrossRef - Hard tissue reaction to mineral trioxide aggregate and experimental root-end filling material in guinea pig mandibles
Ali Akhavan, Peter Parashos, Sayed Mohammad Razavi, Amin Davoudi, Elham Shadmehr
Journal of Dental Sciences.2017; 12(2): 107. CrossRef - Management of extensive external apical root resorption leading to root perforation
Robia Ghafoor, Sadia Tabassum, Muhammad Hasan Hameed
BMJ Case Reports.2017; 2017: bcr-2017-220234. CrossRef - Factors affecting the periapical healing process of endodontically treated teeth
Roberto Holland, João Eduardo Gomes Filho, Luciano Tavares Angelo Cintra, Índia Olinta de Azevedo Queiroz, Carlos Estrela
Journal of Applied Oral Science.2017; 25(5): 465. CrossRef - Tissue Reaction to Different Types of Calcium Hydroxide Paste in Rat
Mina Zarei, Maryam Javidi, Maryam Gharechahi, Moaied Kateb, Reza Zare, Ziba Shirkhani Kelagari
The Bulletin of Tokyo Dental College.2016; 57(2): 57. CrossRef - Sealing Ability of Root-end Filling Materials
Alvaro Henrique Borges, Matheus Coelho Bandéca, Cyntia Rodrigues de Araújo Estrela, Octávio Amezcua, Álvaro Cruz Gonzalez, Carlos Estrela
The Journal of Contemporary Dental Practice.2015; 16(3): 210. CrossRef - Comparative analysis of physicochemical properties of root perforation sealer materials
Maura Cristiane Gonçales Orçati Dorileo, Fábio Luis Miranda Pedro, Matheus Coelho Bandeca, Orlando Aguirre Guedes, Ricardo Dalla Villa, Alvaro Henrique Borges
Restorative Dentistry & Endodontics.2014; 39(3): 201. CrossRef - Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials
Young-Eun Jang, Bin-Na Lee, Jeong-Tae Koh, Yeong-Joon Park, Nam-Eok Joo, Hoon-Sang Chang, In-Nam Hwang, Won-Mann Oh, Yun-Chan Hwang
Restorative Dentistry & Endodontics.2014; 39(2): 89. CrossRef - The Era of Endodontic Research…………Root-end Filling Materials
Nikhil Marwah
International Journal of Clinical Pediatric Dentistry.2014;[Epub] CrossRef - Rational design and fabrication of a β-dicalcium silicate-based multifunctional cement with potential for root canal filling treatment
Xianyan Yang, Min Liu, Yu Zhao, Hongyu Jia, Sanzhong Xu, Xigong Li, Xiaoyi Chen, Feng Zhang, Changyou Gao, Zhongru Gou
J. Mater. Chem. B.2014; 2(24): 3830. CrossRef
Recent comparative studies evaluating in vitro toxicity of various root end filling materials
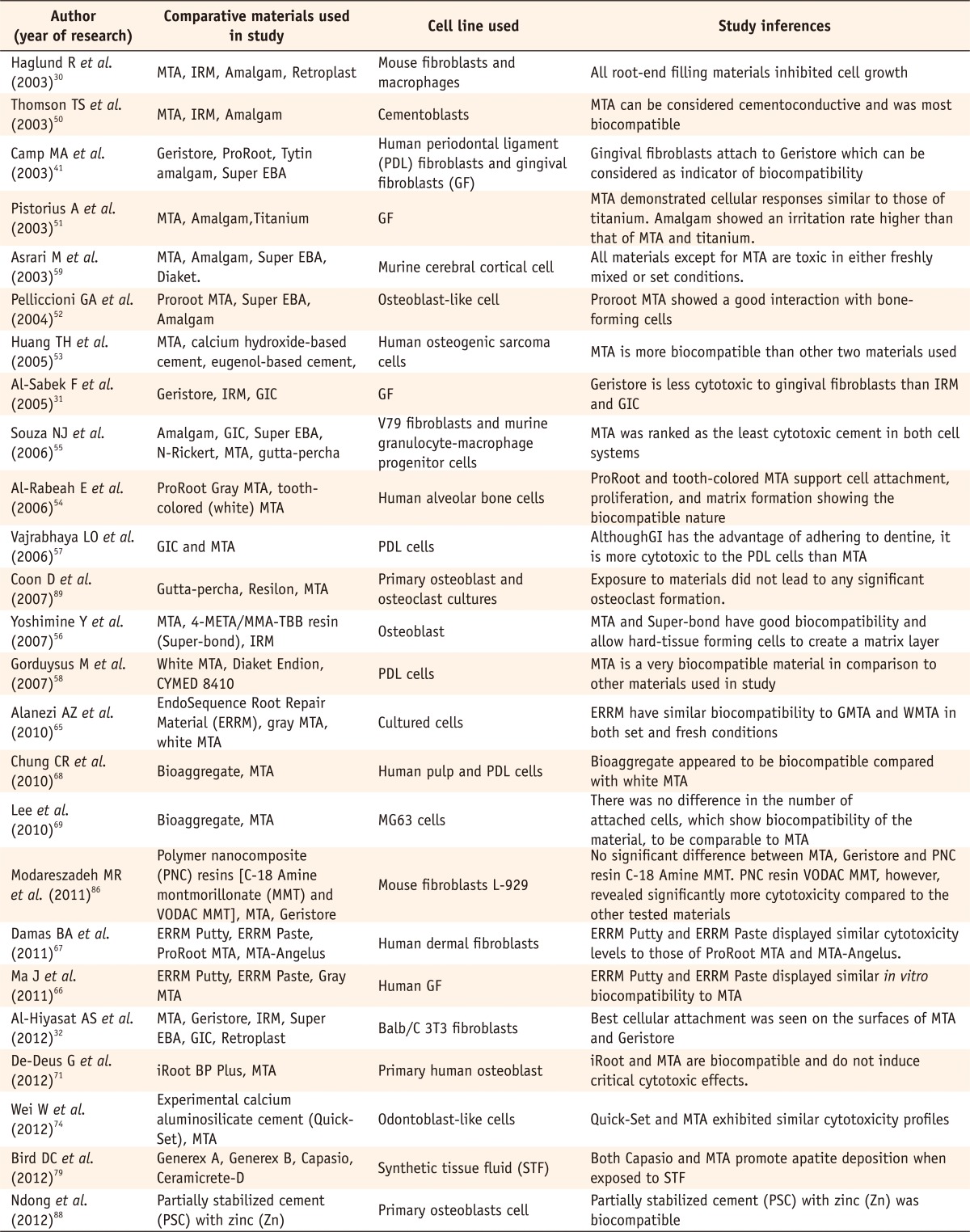
MTA, mineral trioxide aggregate; IRM, intermediate restorative material; EBA, ethoxy benzoic acid; GIC, glass ionomer cement; VODAC, vinylbenzyl octadecyldimethyl ammonium chloride.
Recent comparative studies evaluating in vivo toxicity of various root end filling materials
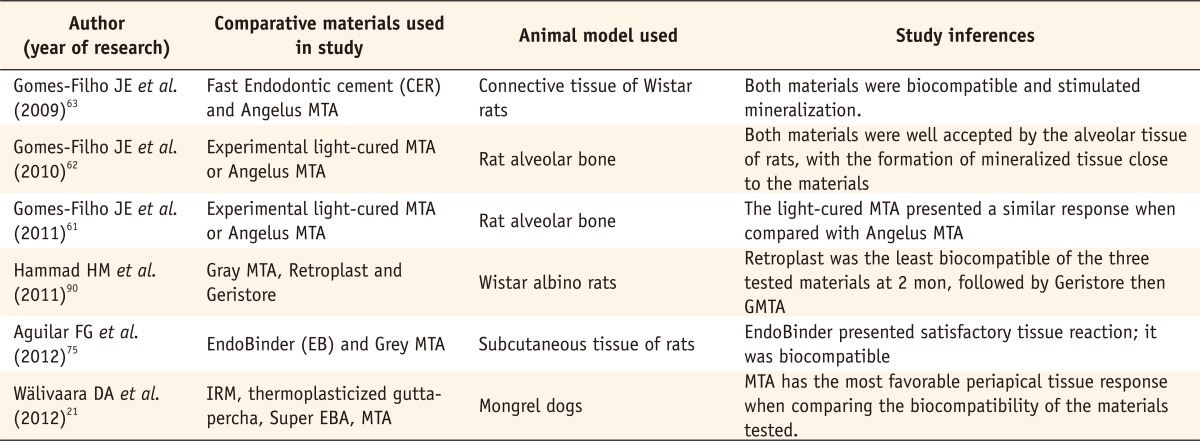
CER, Cimento Endodôntico Rápido; MTA, mineral trioxide aggregate; EBA, ethoxy benzoic acid.
MTA, mineral trioxide aggregate; IRM, intermediate restorative material; EBA, ethoxy benzoic acid; GIC, glass ionomer cement; VODAC, vinylbenzyl octadecyldimethyl ammonium chloride.
CER, Cimento Endodôntico Rápido; MTA, mineral trioxide aggregate; EBA, ethoxy benzoic acid.

 KACD
KACD


 ePub Link
ePub Link Cite
Cite

