Abstract
-
Objectives
This study aimed to evaluate the effect of pomegranate solution (Punica granatum) on eroded dentin through antioxidant action, shear bond strength (SBS) and interface morphology.
-
Materials and Methods
The 10% pomegranate peel extract was prepared by the lyophilization method. Punicalagin polyphenol was confirmed by high-performance liquid chromatography. Antioxidant activity was evaluated by capturing the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. For the SBS, 48 dentin fragments were divided into sound or eroded, and subdivided according to the pretreatment (n = 12): water or P. granatum. The surfaces were restored with self-etch adhesive and a bulk-fill resin (Ecosite; DMG). The SBS was done immediately (24 hours) and after thermal cycling + water storage (12 months). For scanning electron microscopy, 48 dentin fragments (24 sound and 24 eroded) received the same treatments as for SBS (n = 6), and they were analyzed after 24 hours and 12 months.
-
Results
The P. granatum had antioxidant action similar (p = 0.246) to the phenolic standard antioxidants. After 24 hours, eroded dentin had lower SBS than sound dentin (p < 0.001), regardless of the pretreatment. After 12 months, P. granatum maintained the SBS of sound dentin (13.46 ± 3.42 MPa) and eroded dentin (10.96 ± 1.90 MPa) statistically similar. The lowest values were found on eroded dentin treated with water (5.75 ± 1.65 MPa) (p < 0.001). P. granatum on eroded dentin caused peritubular demineralization and hybrid layer with resin tags.
-
Conclusions
The pomegranate extract had antioxidant action and preserved the adhesive interface of the eroded dentin.
-
Keywords: Antioxidant; Dentin-bonding agent; Punica granatum
INTRODUCTION
Erosive tooth wear is related to acidic foods, erosive products, reduced salivary flow, and gastrointestinal disorders [
1,
2,
3,
4]. It is a chronic and irreversible disease associated with the loss of dental tissues (enamel or dentin) without bacterial involvement [
1,
5].
The bond between restorative resin and dentin is associated with adhesive infiltration into the collagen matrix, which is exposed through acid etching or using an acidic adhesive/primer [
6,
7]. In the process of erosion, the tooth structure is in an acidic environment, causing demineralization of the tooth surface. This process causes mineral loss and the collapse of the collagen meshwork, which makes it difficult for the adhesive to penetrate the dentinal tubules and consequently adhesion to this substrate [
7,
8].
The endogenous proteases of oral saliva and dentin matrices are denominated matrix metalloproteinases (MMPs) [
9]. In erosive conditions, salivary buffers have a lower pH, which can activate the host's proteases [
10]. Upon demineralization, the collagen fibers of the organic matrix are exposed, activating MMPs that will degrade collagen fibers [
10]. When triggered by a self-etching system, type I collagen fibrils' degradation influences the bond strength procedure [
11].
Most of the natural extracts have antioxidant activity that plays an important role in the inhibition of MMPs. The antioxidant action of the natural extracts and their metabolites is related to their ability to scavenge superoxide radicals [
12,
13]. The free radicals come from metabolites produced by MMPs through the digestion of extracellular matrix proteins [
12]. The role of antioxidants is related to the ability to eliminate free radicals locally [
12,
14].
In Restorative Dentistry, antioxidants have been used to inhibit MMPs and improve collagen stability prior to the restorative procedure [
15]. For this reason, therapeutic strategies with compounds containing natural agents are widely studied to improve the wettability of dental substrate, modifying the surface for adhesive procedures [
16,
17].
Among the natural products used in dentistry,
Punica granatum has an inhibitory effect on MMPs and antimetastatic potential against oral cancer cells, anti-inflammatory effect and antioxidant potential [
18,
19,
20,
21,
22]. These favorable properties are due to flavonoids (catechins and anthocyanins) and tannins (punicalin, punicalagin, punic, gallic, and ellagic acids) present in the extract [
23]. The high concentration of phenolic compounds is important for cellular protection against the harmful effects caused by free radicals that are responsible for inflammatory diseases, cellular aging, and alterations in the biological systems [
22,
24,
25,
26].
Studies on dental substrates bleached with high-concentrated hydrogen peroxide reported that the
P. granatum solution could fully or partially restore the bond strength of the restorative material to enamel and dentin [
13,
27,
28]. Unbleached dentin treated with 20% pomegranate solution increased the bond strength compared to the untreated control [
29]. In addition, Peng
et al. [
18] demonstrated that the inhibitory migration of pomegranate extract in oral cells can be mediated by the inactivation of MMPs.
Although some studies clarify the benefits of
P. granatum in Dentistry, only a few used
P. granatum extract to treat tooth surfaces before resin composite application, mainly in bleached surfaces [
13,
18,
20,
27,
28,
30,
31,
32]. Besides, to the best of our knowledge, there is only 1 that investigated the
P. granatum on eroded dentin and 2 that assessed the bond strength of resin composite to dentin pretreated with
P. granatum extract [
17,
29,
33]. Incorporating a pomegranate extract in the demineralized dentin could inactivate matrix MMPs by the antioxidant action, increase the mechanical strength and raise the degradation time of the adhesive interface.
This study investigates the effect of P. granatum 10% on the shear bond strength (SBS) and interface morphology of the resin composite to eroded dentin substrate. The alternative hypotheses were: 1) After interface degradation, the SBS of the specimens treated with P. granatum will be higher than those treated only with water, regardless of the substrate (sound or eroded); 2) Surface pretreatment with P. granatum will provide a better interface morphology that surface that received water, mainly on eroded dentin.
MATERIALS AND METHODS
Estimation of sample size
A pilot study with n = 3 was done for the SBS test to estimate the number of dental specimens required to find a difference among at least 1 experimental group. Power analysis was done using G*Power software with the following parameters: 2-tailed test, α error = 0.05, power (1−β) = 0.80, and effect size = 0.5. A minimum of 12 specimens was found for each group.
Experimental design
In the antioxidant action test, pomegranate peel patterns were used and tested in triplicate, in different models modified to MOHA (Sigma-Aldrich, St. Louis, MO, USA). The variable response was tested by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical capture method. For the SBS, 48 bovine dentin fragments were divided into 2 levels: sound (n = 12) and eroded (n = 12), then subdivided into 2 subgroups according to surface pre-treatment: distilled water (control) and P. granatum (12 teeth for each subgroup), the same sample was used for the analysis after aging. An immediate analysis was performed (24 hours after the restorative protocol) and a long-term analysis was performed after thermocycling and water storage for 12 months. In the morphological analysis, 48 dentin fragments (n = 6) were used, subdivided, and treated in the same way as the bond strength.
Sample selection and solution preparation
Granatum solutions were prepared from pomegranate fruits obtained from the same plantation. Approximately 10 ripe fruits were harvested and sanitized with running filtered water and a brush. Each fruit was cut in half and the peel and pulp (seed and juice) were separated. The pulp was sieved until all the juice was extracted from the seed. The juice extract was not used due to its high sugar content to avoid changes in the result.
The peel samples were weighed on an analytical balance (Shimadzu, AUW, Baureri, SP, Brazil) and dried by lyophilization method (Nova analytical, São Paulo, SP, Brazil) at −80ºC for 48 hours. The diluent used to prepare the extract (50% ethanol) was previously analyzed for antimicrobial activity at several hydroalcoholic concentrations, and 50% ethanol did not inhibit microbial growth [
34].
After extraction, the samples were taken to total evaporation by controlled heating and vacuum. The pure powder of the fruit peel was obtained, identified and stored at 4ºC.
The solutions were prepared from 1g of powder, diluted in 10 mL of 50% ethanol (Merck KGaA, Darmstadt, HE, Germany) + distilled water (ethanol and water 1:1), thus obtaining a 10% hydroalcoholic extract. The concentration was determined in a previous study of our group, in which the 10% pomegranate peel extract had lower microbial growth [
34].
The samples were then subjected to an ultrasonic bath (Cientec, Belo Horizonte, MG, Brazil) for 60 minutes at 15,000 rpm. The extract was filtered in a rotary evaporator (ATX, Odontobras, Ribeirão Preto, SP, Brazil) until obtaining a clear aspect.
Detecting the punicalagin tannin in the extract by the high-performance liquid chromatography (HPLC) technique
The extract was characterized by HPLC. The tannin punicalagin was chosen, which is a polyphenol classified as a high molecular weight hydrolyzable tannin (ellagitannin) with recognized antioxidant, anti-inflammatory and antimicrobial activities, standing out as a promising molecule with several beneficial health functions [
23].
The samples were analyzed in an Acquity UPLC H-Class system (Waters Corporation, Milford, MA, USA) of ultra-performance liquid chromatography coupled to diode array detectors (Diode Array Detector) and the Xevo TQ-S mass spectrometer (Waters Corporation) with Z-Spray ionization source in negative analysis mode.
A 5 µL volume of sample was injected onto an Ascentis® Express C18 column (100 × 4.6 mm, 2.7 µm particle diameter) (Supelco-53829U MSDS). The mobile phase consisted of 0.1% formic acid (solvent A) and methanol + 0.1% formic acid (solvent B). Separation of compounds started in gradient mode with 10% methanol, then increased to 90% methanol in 4 minutes, remaining there for 6 minutes, and returning to initial conditions with 10% methanol within the next 6 minutes. The total analysis time was 12 minutes with a flow rate of 500 μL/min.
The operating parameters used in the Z-spray source were: capillary voltage = 2.50 kV, cone voltage = 40 V, source temperature = 150°C, desolvation gas temperature = 400°C, and dissolving gas = 900 L/h. The analysis was performed in selected ion recording mode. The concentration of punicalagin in the experimental extract was compared with that of the standard punicalagin (Sigma-Aldrich, Louis, MO, USA). These extracts were stored in closed flasks and refrigerated at 4°C. The pH of the extract was measured at 4.75 [
34].
The standard curves for the identification of punicalagin (α and β isomers) were constructed with concentrations based on estimates of the levels of the fruit. Seven dilutions were made from a stock solution in methanol containing punicalagin, starting from a concentration of 1,000 µg/mL to a concentration of 0.05 µg/mL. For the P. granatum solution, 6 dilutions were made, starting from 1,000 µg/mL to 0.1 µg/mL.
It was verified that the peaks of the isomers of the punicalagin standard (α = 3.05 µg/mL and β =3.25 µg/mL) and of the experimental extract of P. granatum (α = 3.05 µg/mL and β =3.26 µg/mL) were similar. The total amount of punicalagin was 6.31 µg/mL (α + β) for the experimental extract.
Antioxidant potential of the experimental extract
The evaluation of the antioxidant potential was performed by the DPPH free radical scavenging method [
34]. 10 μL of hydroalcoholic extract of pomegranate peel was used, which were tested in triplicate at different concentrations (10, 15, 20, 30, 40, and 50 μg/mL). Then, to the extracts, 1.0 mL of 0.1 M acetate buffer (pH 5.5), 1.0 mL of PA ethanol and 0.5 mL of 250 μM DPPH ethanolic solution were added. (Merck KGaA).
The change in absorbance was measured after 20 minutes at 515 nm at 25°C. The antioxidant activity of the specimens was compared with synthetic standards that prevent oxidation and scavenge free radicals. The standards used were 2(3)-tert-butyl-4-hydroxyanisole (BHA) and butylhydroxytoluene (BHT).
Results were expressed as IC50 (μg/mL), which indicates the concentration of solution required to produce a 50% reduction in DPPH free radical and presented as the mean and standard deviation obtained in triplicate measurements. Free radical scavenging was calculated by the formula: %AA = 100 – [(A (control) – A (sample) / A (control)] × 100, where A (control) represents the absorbance of the DPPH solution without the sample and A (sample) represents the absorbance of the sample with the DPPH [
34].
Bovine incisors preserved in 0.1% thymol solution at 9°C were washed in running water for 24 hours to remove residues of thymol solution and examined macroscopically with the aid of a stereoscopic magnifying glass (Leica Microsystems, Wetzlar, HE, Germany) at 20× magnification. Sixty incisors without fracture lines or crown fissures were selected. The bovine teeth were submitted to prophylaxis with a pumice stone for 60 seconds, transversely sectioned 2 mm at the cemento-enamel junction in a cutting machine (Isomet 1000, Buehler, Düsseldorf, Germany). The specimens were standardized in dimensions (5 × 5 × 3 mm). Subsequently, they were polished using #1200 sandpaper (Panambra, São Paulo, SP, Brazil) for 60 seconds.
Each specimen was immersed in 20 mL of 0.3% citric acid (pH = 3.2) individually and placed on the shaking table (CT155, Cientec) under constant agitation at 50 rpm for 2 hours to induce erosion. After, the specimens were washed with distilled water and stored individually in Eppendorf tubes containing artificial saliva at 37ºC for 24 hours. Artificial saliva was composed of methylparaben (2.0 g), sodium carboxymethyl cellulose (10.0 g), KCl (0.625 g), MgCl 2.6H
2O (0.059 g), CaCl
2.2H
2O (0.166 g), K
2HPO
4 (0.804 g), and KH
2PO
4 (0.326 g) in 1L of distilled water [
35].
The experimental extract was applied according to the protocol: drip 3 mL for 2 minutes, actively rub with a microbrush for 10 minutes, and lightly dry with absorbent paper [
34]. Then, self-etching adhesive (Ecosite Bond DMG, Hamburg, Germany) was applied and light-cured according to the manufacturer’s instructions. The dentin surfaces were restored with Ecosite Bulk Fill resin composite (Universal shade, Ecosite Bond DMG) using Teflon molds (3 mm in diameter and internal 4 mm in height), stabilized with wax, to obtain resin composite fragments with the measurements mentioned above. The resin composite was included in increments (3 mm to 4 mm) and polymerized using a light-emitting diode source (Ultradent Products Inc., South Jordan, UT, USA) for 20 seconds. Then, the wax was removed with a scalpel, the split matrix was opened and additional light curing was performed. The dentin/restorative material set was stored in relative humidity at 37°C for 24 hours.
Half of the specimens from each group were submitted to thermal and hydrolytic degradation of the adhesive interface. The samples were subjected to water storage in relative humidity for 12 months (37°C) and thermal cycling in a thermocycler machine (Ética Odontológica, São Paulo, SP, Brazil) at 5°C and 55°C in water. The immersion time in each bath was 30 seconds and the transfer time between baths was 2 seconds. The samples were subjected to 1,000 thermal cycles per month (twice a month) and were kept in distilled water at 37°C in intervals, totaling 12,000 cycles and 12 months of water storage. Distilled water was changed every week [
28].
After 24 hours, the samples were positioned in a universal test machine (Instron Corporation, Canton, MA, USA) with a load cell of 2 kN. The force was applied with a rectangular stainless steel tip at a speed of 0.5 mm/min until the displacement of the restoration.
Failure patterns were analyzed using a ×40 stereomicroscope (Leica Microsystems) and were classified: adhesive, when the dentin surface was covered by a thin layer of adhesive material; cohesive of the material, when the area was covered with resin composite; cohesive of the substrate, when the failure occurred in dentin; and mixed, a combination of adhesive and cohesive.
Morphological surface assessment by scanning electron microscopy (SEM)
Forty-eight samples were prepared for adhesive interface analysis, half destined for each period: immediately after 24 hours or aged after 12 months (n = 6). The dentin fragment (sound or eroded) was treated with different protocols. The specimens were restored as previously described for the shear strength test. The sections were ground with 600 and 1,200-grit sandpapers (Norton, Lorena, SP, Brazil) for 30 seconds each and with alumina 0.3 μm and 0.05 μm for 5 minutes. After polished, the sections received 37% phosphoric acid (Villevie, Joinvile, SC, Brazil) for 20 seconds, followed by rinsing and ultrasonically cleaned for 10 minutes. Then, the samples were dehydrated in 25%, 50%, 75%, and 95% ethanol by immersion for 20 minutes in each solution and then immersed in 100° ethanol for 1 hour and finally, were on hexamethyldisilazane for 10 minutes. They were fixed in metallic stubs (SCD 050 Bal-Tec, New York, NY, USA) and analyzed under a scanning electron microscope (Zeiss EVO 50, Carl Zeiss, Cambridge, MA, England). The most representative areas were photographed.
Statistical analysis
Statistical analysis was conducted using Statistical Package for Social Science software (SPSS 25, IBM Corp., Armonk, NY, USA) with a pre-set alpha of 0.05. Antioxidant action data (µg/mL) were analyzed by the non-parametric chi-square test. SBS (MPa) were subjected to normality and homogeneity tests, showing normal (Shapiro-Wilk test) and homogeneous (Levene test) distribution. Then, they were analyzed by 3-way analysis of variance, considering the substrate (sound or eroded), treatment (experimental or control) and time (immediate or aged) as independent factors. Failures were analyzed using the chi-square test. Photomicrographs were analyzed qualitatively by 3 calibrated examiners (kappa ≥ 0.8).
RESULTS
Antioxidant potential of the P. granatum solution
The experimental extraction was performed with the synthetic standards BHA and the antioxidant expression of the extract (%AA). There was no significant difference in the values of BHA, BHT, and
P. granatum 10% (
p = 0.246) (
Table 1), evidencing the antioxidant potential of the experimental extract.
Table 1Means and standard deviations (triplicates) of the inhibitory concentration of the 2,2-diphenyl-1-picrylhydrazyl radical (%AA) for the antioxidant activity of the 2(3)-tert-butyl-4-hydroxyanisole (BHT), butylhydroxytoluene (BHA), and P. granatum extract standards
|
Samples |
BHT |
BHA |
P. granatum 10% |
|
1 |
88.20 ± 0.24 |
85.81 ± 0.82 |
83.05 ± 0.52 |
|
2 |
81.69 ± 1.29 |
83.40 ± 0.66 |
83.60 ± 1.44 |
|
3 |
73.26 ± 1.67 |
76.30 ± 1.38 |
80.79 ± 2.65 |
|
4 |
64.67 ± 0.63 |
60.24 ± 1.26 |
67.47 ± 4.98 |
|
5 |
42.78 ± 1.68 |
35.46 ± 0.38 |
45.85 ± 9.96 |
|
6 |
27.78 ± 1.80 |
21.93 ± 3.46 |
32.41 ± 5.40 |
|
Total |
63.06 ± 0.64*
|
60.52 ± 1.10*
|
65.52 ± 3.42*
|
The IC50 (μg/mL) of each solution was calculated, which represents the amount of a substance needed to inhibit a given biological process by 50% (in this study, the DPPH radical). The respective IC50 values for BHA, BHT, and P. granatum were: 2.97 µg/mL, 3.01 µg/mL and 3.49 µg/mL.
SBS analysis
Data analysis showed a significant difference for the 3 tested factors: substrate (p < 0.001), treatment (p = 0.008), and time (p < 0.001). The sound dentin had higher SBS than the eroded dentin. Dentin pretreatment with P. granatum provided higher SBS than water pretreatment (control). The immediate SBS data was higher than the 12-months data, thus the degradation of the adhesive interface decreased the SBS.
The interactions between treatment × time (p < 0.001) and treatment × time × substrate (p < 0.001) also had significant differences. No significant interactions were found for treatment × substrate (p = 0.183) and substrate × time (p = 0.084).
Table 2 shows the means and standard deviations of the immediate SBS, considering the substrate and surface treatment. In the immediate analysis, eroded dentin had lower SBS than sound dentin, regardless of the dentin pretreatment (
P. granatum or water).
Table 2Shear bond strength means and standard deviations (MPa) of the restorative material to dentin, considering substrate and surface treatment (immediate analysis)
|
Pretreatment |
Sound dentin |
Eroded dentin |
|
Water (control) |
(17.70 ± 2.40) Aa |
(12.27 ± 2.28) Ab |
|
10% P. granatum
|
(16.95 ± 4.60) Aa |
(11.96 ± 3.24) Ab |
Table 3 shows the means and standard deviations of the SBS after 12 months, considering the substrate and surface treatment. After degradation, the
P. granatum applied on the eroded dentin kept the bond strength stable. The lower adhesion values were found after 12 months on eroded dentin treated with water.
Table 3Shear bond strength means and standard deviations (MPa) of the restorative material to dentin, considering substrate and surface treatment (after 12 months)
|
Pretreatment |
Sound dentin |
Eroded dentin |
|
Water (control) |
(11.47 ± 1.82) Aa |
(5.75 ± 1.65) Bb |
|
10% P. granatum
|
(13.46 ± 3.42) Aa |
(10.96 ± 1.90) Aa |
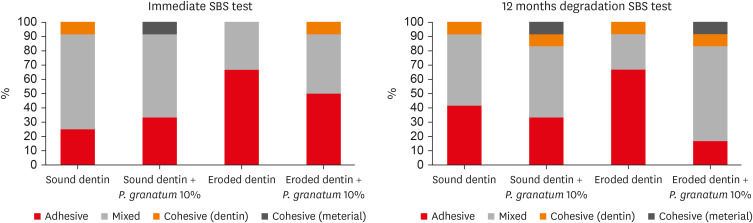
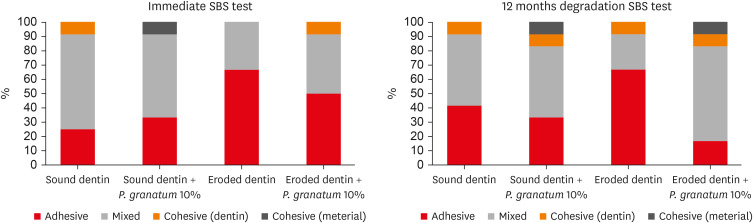
Failure modes
Mixed failures (combination of adhesive and cohesive) were found in the immediate analysis (24 hours) for sound dentin, regardless of treatment. Adhesive failures were more significant in eroded dentin, regardless of the treatment. The number of cohesive failures (dentin and material) was low. After aging, there was a predominance of mixed failures in sound dentin pretreated with water or
P. granatum and in the eroded dentin pretreated with
P. granatum. However, the predominance of adhesive failures in eroded dentin that received only water was observed (
Figure 1).
Figure 1
Failure modes after immediate bonding test and adhesive interface aging.
SBS, shear bond strength.
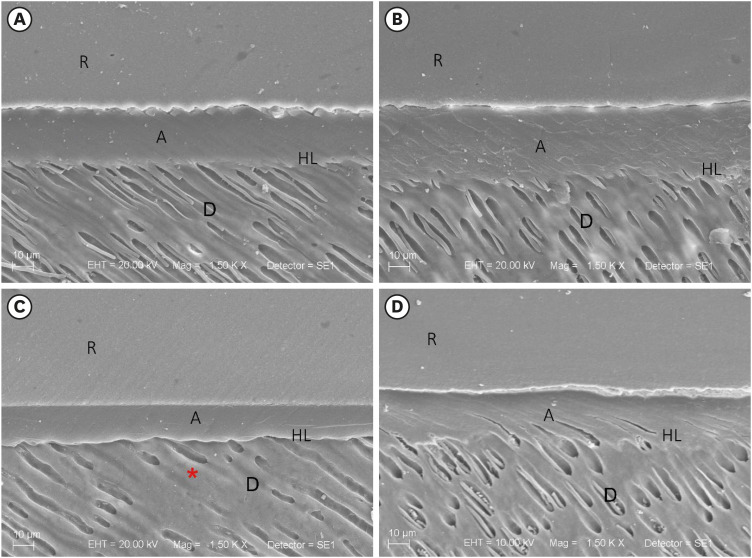
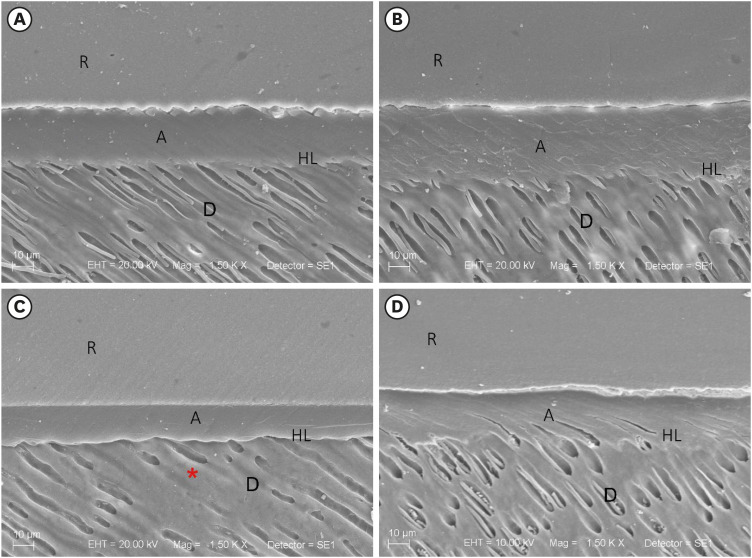
Surface morphology by SEM
Overall, both pretreatments (water and
P. granatum) provided a uniform adhesive layer. The immediate analysis of the sound dentin pretreated with water or
P. granatum had resin tags and a good adhesive interface. A reduction in resin tags, peritubular and intertubular demineralization was observed in the eroded dentin pretreated with water. (
Figure 2).
Figure 2
SEM of the interface of the restorative procedure (no degradation after the restorative procedure). (A) Sound dentin (control), (B) sound dentin + 10% P. granatum, (C) eroded dentin, and (D) eroded dentin + 10% P. granatum. Demineralized dentin without resin tags (red asterisk).
R, resin; A, adhesive; HL, hybrid layer; D, dentin; SEM, scanning electron microscopy.
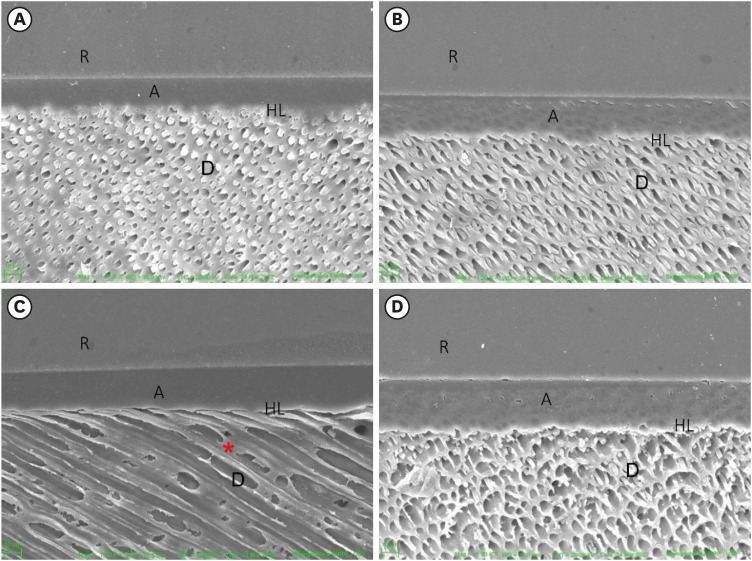
After 12 months, the hybrid layer, resin tags and good adhesive interface were observed in sound dentin (control) pretreated with water (
Figure 3A), as well as in the dentin pretreated with
P. granatum (
Figure 3B).
Figure 3
SEM of the interface after degradation of the adhesive interface (12 months). (A) Sound dentin (control), (B) sound dentin + 10% P. granatum, (C) eroded dentin, (D) eroded dentin + P. granatum 10%. Demineralized dentin without resin tags (red asterisk).
R, resin; A, adhesive; HL, hybrid layer; D, dentin; SEM, scanning electron microscopy.
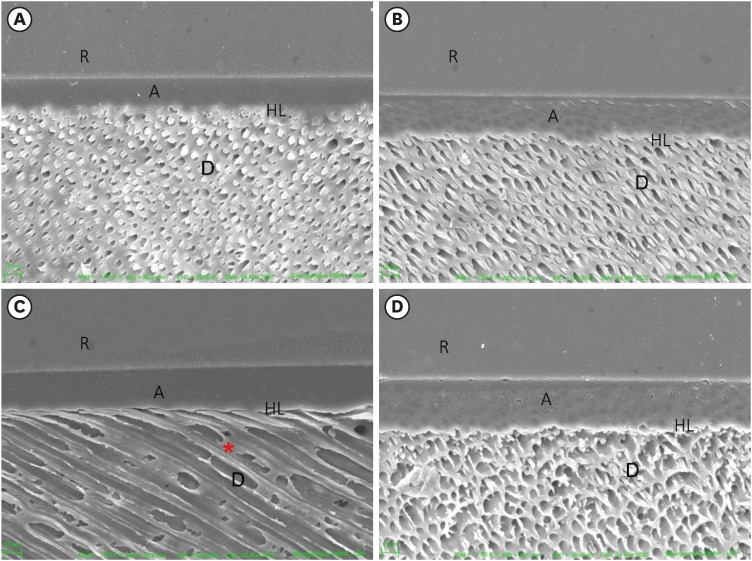
In eroded dentin, it was observed peritubular and intertubular demineralization (
Figure 3C and 3D). The number of resin tags was reduced in the eroded dentin treated with water and
P. granatum.
DISCUSSION
Exposed dentin from erosive wear needs restorative treatment, protecting dentinal tubules to reduce sensitivity and restoring severe wear through major reconstructions or indirect composites [
36]. However, the bond strength of the resin composite to the eroded substrate over time has been described as low [
37]. This fact can be explained by the degradation process of the hybrid layer mainly due to the action of MMPs, which remain trapped inside the extracellular matrix of dentin in an inactive form and are activated by the acid challenge [
38].
Pretreatment with modifying substances, such as natural extracts, has been suggested to improve bond strength on the dentin surface, as they decrease the degradation of collagen fibrils [
16,
17,
28].
In vitro study demonstrated antioxidant activity and a high concentration of flavonoids in the hydroalcoholic
P. granatum [
14].
This study identified the antioxidant potential of an experimental hydroalcoholic extract of 10%
P. granatum, and analyzed its effect on the adhesive strength of the resin material to the eroded dentin substrate. The lyophilization method was used to prepare the hydroalcoholic extract of
P. granatum. This process consists of removing water from the material by sublimation at low temperatures without chemical change (loss of volatile constituents) by heat [
39]. The lyophilized product is resistant to microorganisms due to the minimal residual water concentration and allows proper handling of the concentrations necessary for the experiment [
39].
Lyophilized powder from the fruit peel was used [
40,
41]. The concentration of the experimental extract was standardized at 10% to potentiate the effects of flavonoids since the pomegranate solution at low concentrations did not provide promising results in bond strength [
17,
28].
The punicalagin was quantified in the pomegranate peel extract by HPLC [
42]. This technique performs quantitative analysis of compounds from various samples with high efficiency [
41,
42]. This study found that the peaks of punicalagin isomers from the
P. granatum extract and the standard were close, evidencing the presence of this antioxidant tannin in the experimental extract.
DPPH is a violet-colored stable nitrogen-free radical with 515 and 520 nm absorption. The reduction of the DPPH radical is monitored by the decrease in absorbance during the reaction [
43]. Furthermore, the DPPH radical scavenging method is used to evaluate the antioxidant activity of extracts and has been widely used in the food industry [
44]. In our study,
P. granatum extract was shown to be as effective in scavenging DPPH free radicals as the synthetic standards BHA and BHT. This process can be explained by tannins such as punicalagin in the fruit peel [
40,
41]. Besides, punicalagin in
P. granatum has DPPH free radical scavenging activity, and antioxidants stabilize electrons so that the surface to be restored is uniform and non-porous, favoring adhesive procedures [
13,
24,
45,
46].
The first alternative hypothesis was accepted since the experimental pomegranate-based solution increased the SBS compared to the control without treatment. The pretreatment of eroded dentin with
P. granatum maintained the SBS after aging. This fact can be explained as flavonoids in natural extracts can induce collagen molecular bonds, improve dentin's mechanical properties, inhibit MMPs and prevent early degradation of the adhesive interface [
15,
17,
47]. Natural bioactive compounds improve the wettability of the dental substrate and leave the surface chemically prepared for adhesive procedures [
35].
A previous study of our research group did not find promising results with the pomegranate solution, probably because we used the standard method of preparing a solution (commercial powder heated in water), which caused residues of the solution in the dentin (checked by SEM) [
17]. Besides, we used pomegranate solution at 0.5%, a low concentration to achieve the desired effect. Sajana
et al. [
29] verified that
P. granatum solution at 20% increased bond strength of resin in sound dentin.
Regarding failures, the adhesive type was predominant in the immediate analysis of eroded dentin treated with water or
P. granatum. These findings agree with previous studies that reported a higher prevalence of adhesive failures in eroded teeth, suggesting fragility in the bond between the material and substrate [
37,
48]. In the 12-month analysis, a higher rate of mixed failures was observed in sound dentin pretreated with water, and in both dentin (sound and eroded) pretreated with
P. granatum. A higher percentage of mixed failures represents a better adhesive interface, which reflects superior bond strength values [
49].
The adhesive interface was qualitatively analyzed by SEM [
50]. The erosive process caused demineralization of the peri and intertubular dentin, and the matrix degradation probably reduced the resin tags in the specimens treated only with water. These findings agree with previous studies on dentin, which suggest that morphological changes caused by erosion may reflect lower values of bond strength of the restorative material [
10,
51].
The reduction of resin tags in the eroded groups reinforces the idea that the organic dentin matrix subjected to an erosive process makes it difficult for the adhesive system to penetrate due to the fragility of the fibers, which impairs hybridization and accelerates the degradation of the interface [
8]. The specimens generally showed a good adhesive interface, suggestive of a uniform hybrid layer and with resinous tags. The favorable adhesive interface observed in this study corroborates the high bond strength obtained in the SBS. Considering the above-mentioned facts, the second alternative hypothesis cannot be accepted.
Overall, this study elucidated some advantages of the 10% P. granatum solution applied to eroded dentin, such as antioxidant potential and durability of resin bonds over time. The main limitation is that this solution was not tested as a dental pretreatment in a clinical trial. Besides, its light brown color could stain the tooth surface. Our study supports new laboratory and randomized clinical research to clarify the benefits of using P. granatum in the dental substrate.
CONCLUSIONS
Based on our results, it can be concluded that the experimental extract of P. granatum showed antioxidant potential compared to synthetic standards BHT and BHA, adhesive interface with resin tags, preserved the integrity of the adhesive interface on eroded dentin after thermocycling and 12-months storage.
ACKNOWLEDGEMENTS
The authors are thankful to the financial support of the National Council for Scientific and Technological Development (CNPq # 140877/2019-3). We would also like to acknowledge the DMG Dental Company (Germany) for providing the resin and adhesive system used in this research.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Cortez TV, Gallas JA.
Data curation: Cortez TV, Souza-Gabriel AE, Corona SAM.
Formal analysis: Cortez TV, Souza-Gabriel AE, Oliveira WP, Corona SAM.
Funding acquisition: Cortez TV, Souza-Gabriel AE.
Investigation: Cortez TV, Souza-Gabriel AE, Oliveira WP, Corona SAM.
Methodology: Cortez TV, Souza-Gabriel AE, Gallas JA, Corona SAM.
Project administration: Souza-Gabriel AE, Oliveira WP, Corona SAM.
Resources: Cortez TV, Souza-Gabriel AE.
Software: Cortez TV, Souza-Gabriel AE, Oliveira WP.
Supervision: Souza-Gabriel AE, Oliveira WP, Corona SAM.
Validation: Souza-Gabriel AE, Corona SAM.
Visualization: Souza-Gabriel AE, Corona SAM.
Writing - original draft: Cortez TV, Cerqueira NM,.
Writing - review & editing: Cortez TV, Cerqueira NM.
REFERENCES
- 1. Chan AS, Tran TTK, Hsu YH, Liu SYS, Kroon J. A systematic review of dietary acids and habits on dental erosion in adolescents. Int J Paediatr Dent 2020;30:713-733.ArticlePubMedPDF
- 2. Methuen M, Kangasmaa H, Alaraudanjoki VK, Suominen AL, Anttonen V, Vähänikkilä H, et al. Prevalence of erosive tooth wear and associated dietary factors among a group of Finnish adolescents. Caries Res 2022;56:477-487.ArticlePubMedPMCPDF
- 3. Tulek A, Mulic A, Refsholt Stenhagen K, Galtung HK, Saeed M, Utheim TP, et al. Dental erosion in mice with impaired salivary gland function. Acta Odontol Scand 2020;78:390-400.ArticlePubMed
- 4. Kawar N, Park SG, Schwartz JL, Callahan N, Obrez A, Yang B, et al. Salivary microbiome with gastroesophageal reflux disease and treatment. Sci Rep 2021;11:188-196.ArticlePubMedPMCPDF
- 5. Wiegand A, Lechte C, Kanzow P. Adhesion to eroded enamel and dentin: systematic review and meta-analysis. Dent Mater 2021;37:1845-1853.ArticlePubMed
- 6. Frassetto A, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, et al. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability--a literature review. Dent Mater 2016;32:e41-e53.ArticlePubMed
- 7. Kanniappan G, Hari P, Jujare RH. Comparative evaluation of resin dentin interface using universal and total- etch adhesive systems on sound and eroded dentin: in vitro study. Eur J Dent 2022;16:153-160.ArticlePubMedPMC
- 8. Belmar da Costa M, Delgado AHS, Pinheiro de Melo T, Amorim T, Mano Azul A. Analysis of laboratory adhesion studies in eroded enamel and dentin: a scoping review. Biomater Investig Dent 2021;8:24-38.ArticlePubMedPMC
- 9. Shen J, Xie H, Wang Q, Wu X, Yang J, Chen C. Evaluation of the interaction of chlorhexidine and MDP and its effects on the durability of dentin bonding. Dent Mater 2020;36:1624-1634.ArticlePubMed
- 10. Siqueira FSF, Cardenas AM, Ocampo JB, Hass V, Bandeca MC, Gomes JC, et al. Bonding performance of universal adhesives to eroded dentin. J Adhes Dent 2018;20:121-132.PubMed
- 11. Jacobsen JA, Fullagar JL, Miller MT, Cohen SM. Identifying chelators for metalloprotein inhibitors using a fragment-based approach. J Med Chem 2011;54:591-602.ArticlePubMedPMC
- 12. Grimm T, Schäfer A, Högger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (pycnogenol). Free Radic Biol Med 2004;36:811-822.ArticlePubMed
- 13. Suratno IR, Dwiandhono I, Purnama RB. Pomegranate (Punica granatum L.) gel extract as an antioxidant on the shear bond strength of a resin composite post-bleaching application with 40% hydrogen peroxide. Dent Jour (Gigi) 2021;54:87-91.
- 14. Hanafy SM, Abd El-Shafea YM, Saleh WD, Fathy HM. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J Genet Eng Biotechnol 2021;19:80-90.PubMedPMC
- 15. Baena E, Cunha SR, Maravić T, Comba A, Paganelli F, Alessandri-Bonetti G, et al. Effect of chitosan as a cross-linker on matrix metalloproteinase activity and bond stability with different adhesive systems. Mar Drugs 2020;18:263.ArticlePubMedPMC
- 16. Fakhri E, Eslami H, Maroufi P, Pakdel F, Taghizadeh S, Ganbarov K, et al. Chitosan biomaterials application in dentistry. Int J Biol Macromol 2020;162:956-974.ArticlePubMed
- 17. Ziotti IR, Palma-Dibb RG, Cornona SAM, Souza-Gabriel AE. Viability of using natural extracts in dental restorative treatment. Eur J Pharm Med Res 2016;3:53-61.
- 18. Peng SY, Hsiao CC, Lan TH, Yen CY, Farooqi AA, Cheng CM, et al. Pomegranate extract inhibits migration and invasion of oral cancer cells by downregulating matrix metalloproteinase-2/9 and epithelial-mesenchymal transition. Environ Toxicol 2020;35:673-682.ArticlePubMedPDF
- 19. Ghavipour M, Sotoudeh G, Tavakoli E, Mowla K, Hasanzadeh J, Mazloom Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in rheumatoid arthritis patients. Eur J Clin Nutr 2017;71:92-96.ArticlePubMedPDF
- 20. Chau TP, Veeraragavan GR, Narayanan M, Chinnathambi A, Alharbi SA, Subramani B, et al. Green synthesis of zirconium nanoparticles using Punica granatum (pomegranate) peel extract and their antimicrobial and antioxidant potency. Environ Res 2022;209:112771.PubMed
- 21. Eghbali S, Askari SF, Avan R, Sahebkar A. Therapeutic effects of Punica granatum (pomegranate): an updated review of clinical trials. J Nutr Metab 2021;2021:5297162.PubMedPMC
- 22. Shaygannia E, Bahmani M, Zamanzad B, Rafieian-Kopaei M. A review study on Punica granatum L. J Evid Based Complementary Altern Med 2016;21:221-227.ArticlePubMedPDF
- 23. BenSaad LA, Kim KH, Quah CC, Kim WR, Shahimi M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum
. BMC Complement Altern Med 2017;17:47.PubMedPMC
- 24. Hosseini A, Razavi BM, Hosseinzadeh H. Protective effects of pomegranate (Punica granatum) and its main components against natural and chemical toxic agents: a comprehensive review. Phytomedicine 2023;109:154581.ArticlePubMed
- 25. Danesi F, Ferguson LR. Could pomegranate juice help in the control of inflammatory diseases? Nutrients 2017;9:1-23.ArticlePubMedPMC
- 26. Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, et al. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res 2019;33:2221-2243.ArticlePubMedPDF
- 27. Abodahb SM, Nizay MA, ElKorashy ME. Effect of pomegranate and banana extracts on microshear bond strength of resin composite to bleached bovine enamel: an in vitro study. Alexandria Dent J 2022;9:251-263.
- 28. Souza-Gabriel AE, Sousa-Neto MD, Scatolin RS, Corona SAM. Durability of resin on bleached dentin treated with antioxidant solutions or lasers. J Mech Behav Biomed Mater 2020;104:103647.ArticlePubMed
- 29. Sajana EM, Akhil YB, Lekshmy SD, Sreeja S, Lakshmi A. Effect of pomegranate peel extract on the shear bond strength of composite resin to dentin using etch& rinse adhesive system. J Med Sci Clin Res 2020;08:123-129.
- 30. Peyro Mousavi SF, Ganjovi A, Eskandarizadeh A, Saidi AR, Isaei E. Evaluating the antibacterial effect of synthesized herbal toothpastes and their efficacy for dentine tubule occlusion: scanning electron microscopy and energy-dispersive X-ray spectroscopy analysis. Microsc Res Tech 2022;85:19-27.ArticlePubMedPDF
- 31. Nirmal GD, Sai Sankar AJ, Sridevi E, Sridhar M, Sankar KS, Satish PR. Comparative evaluation of chelating efficacy of nano-chitosan, pomegranate extract, and ethylenediaminetetraacetic acid on primary radicular dentin: an in vitro study. J Indian Soc Pedod Prev Dent 2022;40:201-207.ArticlePubMed
- 32. Sharafeddin F, Farshad F. The effect of aloe vera, pomegranate peel, grape seed extract, green tea, and sodium ascorbate as antioxidants on the shear bond strength of composite resin to home-bleached enamel. J Dent (Shiraz) 2015;16:296-301.PubMedPMC
- 33. Sarialioglu Gungor A, Donmez N. Dentin erosion preventive effects of various plant extracts: an in vitro atomic force microscopy, scanning electron microscopy, and nanoindentation study. Microsc Res Tech 2021;84:1042-1052.PubMed
- 34. Gallas JA, Pelozo LL, Oliveira WP, Salvador SL, Corona SM, Souza-Gabriel AE. Characterization, antimicrobial activity, and antioxidant efficacy of a pomegranate peel solution against persistent root canal pathogens. Cureus 2023;15:e43142.ArticlePubMedPMC
- 35. Ururahy MS, Curylofo-Zotti FA, Galo R, Nogueira LF, Ramos AP, Corona SA. Wettability and surface morphology of eroded dentin treated with chitosan. Arch Oral Biol 2017;75:68-73.ArticlePubMed
- 36. Mortensen D, Mulic A, Pallesen U, Twetman S. Awareness, knowledge and treatment decisions for erosive tooth wear: a case-based questionnaire among Danish dentists. Clin Exp Dent Res 2021;7:56-62.ArticlePubMedPMCPDF
- 37. Milosevic A, Burnside G. The survival of direct composite restorations in the management of severe tooth wear including attrition and erosion: A prospective 8-year study. J Dent 2016;44:13-19.ArticlePubMed
- 38. Favetti M, Schroeder T, Montagner AF, Correa MB, Pereira-Cenci T, Cenci MS. Effectiveness of pre-treatment with chlorhexidine in restoration retention: a 36-month follow-up randomized clinical trial. J Dent 2017;60:44-49.ArticlePubMed
- 39. Rodriguez ES, Julio LM, Henning C, Diehl BW, Tomás MC, Ixtaina VY. Effect of natural antioxidants on the physicochemical properties and stability of freeze-dried microencapsulated chia seed oil. J Sci Food Agric 2019;99:1682-1690.ArticlePubMedPDF
- 40. Akhtar S, Ismail T, Fraternale D, Sestili P. Pomegranate peel and peel extracts: chemistry and dietary characteristics. Food Chem 2015;174:417-425.PubMed
- 41. Russo M, Fanali C, Tripodo G, Dugo P, Muleo R, Dugo L, et al. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: application to different Italian varieties. Anal Bioanal Chem 2018;410:3507-3520.ArticlePubMedPDF
- 42. Salwe KJ, Sachdev DO, Bahurupi Y, Kumarappan M. Evaluation of antidiabetic, hypolipedimic and antioxidant activity of hydroalcoholic extract of leaves and fruit peel of Punica granatum in male Wistar albino rats. J Nat Sci Biol Med 2015;6:56-62.PubMedPMC
- 43. Prior RL, Cao G.
In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med 1999;27:1173-1181.PubMed
- 44. Lee J, Sung JM, Cho HJ, Woo SH, Kang MC, Yong HI, et al. Natural extracts as inhibitors of microorganisms and lipid oxidation in emulsion sausage during storage. Food Sci Anim Resour 2021;41:1060-1077.ArticlePubMedPMCPDF
- 45. Jalali A, Kiafar M, Seddigh M, Zarshenas MM.
Punica granatum as a source of natural antioxidant and antimicrobial agent: a comprehensive review on related investigations. Curr Drug Discov Technol 2021;18:207-224.PubMed
- 46. Zhao X, Yuan Z. Anthocyanins from pomegranate (Punica granatum L.) and their role in antioxidant capacities in vitro
. Chem Biodivers 2021;18:e2100399.PubMed
- 47. Paik Y, Kim JH, Yoo KH, Yoon SY, Kim YI. Dentin biomodification with flavonoids and calcium phosphate ion clusters to improve dentin bonding stability. Materials (Basel) 2022;15:1494-1507.ArticlePubMedPMC
- 48. Frattes FC, Augusto MG, Torres CRG, Pucci CR, Borges AB. Bond strength to eroded enamel and dentin using a universal adhesive system. J Adhes Dent 2017;19:121-127.PubMed
- 49. Fang M, Liu R, Xiao Y, Li F, Wang D, Hou R, et al. Biomodification to dentin by a natural crosslinker improved the resin-dentin bonds. J Dent 2012;40:458-466.ArticlePubMed
- 50. Paken G, Çömlekoğlu ME, Sonugelen M. Detection of the hybrid layer biodegradation initiation factor with a scanning electron microscope. Microsc Res Tech 2021;84:2166-2175.ArticlePubMedPDF
- 51. Giacomini C, Maurina R, Tomazoni F, Bellan MC, Alessandretti R, Galafassi D. Bond strength of restorations made using universal adhesive systems in teeth subjected to acid erosion. JOI 2019;8:7-17.
 , Nathália Mancioppi Cerqueira1
, Nathália Mancioppi Cerqueira1 , Julia Adornes Gallas1
, Julia Adornes Gallas1 , Wanderley Pereira Oliveira2
, Wanderley Pereira Oliveira2 , Silmara Aparecida Milori Corona1
, Silmara Aparecida Milori Corona1 , Aline Evangelista Souza-Gabriel1
, Aline Evangelista Souza-Gabriel1





 KACD
KACD
 ePub Link
ePub Link Cite
Cite

