Articles
- Page Path
- HOME > Restor Dent Endod > Volume 35(4); 2010 > Article
- Basic Research Analysis of para-chloroaniline after chemical interaction between alexidine and sodium hypochlorite using mass spectrometry: A preliminary study
- Hyeon-Sik Kim1, Seung-Hyun Han2,3,4, Soram Oh1, Sang-Min Lim1, Yu Gu1,3,4, Kee-Yeon Kum1,3,4
-
2010;35(4):-301.
DOI: https://doi.org/10.5395/JKACD.2010.35.4.295
Published online: July 31, 2010
1Department of Conservative Dentistry, School of Dentistry, Seoul National University, Seoul, Korea.
2Department of Oral Microbiology and Immunology, School of Dentistry, Seoul National University, Seoul, Korea.
3Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea.
4BK21 Program, School of Dentistry, Seoul National University, Seoul, Korea.
- Corresponding Author: Kee-Yeon Kum. Department of Conservative Dentistry, Dental Research Institute, and BK21 Program, School of Dentistry, Seoul National University, 25-9 Yongon-Dong, Chongno-Gu, Seoul, 110-749, Korea. Tel: +82-2-2072-2651 Fax: +82-2-2072-3859, kum6139@snu.ac.kr
• Received: June 29, 2010 • Revised: July 7, 2010 • Accepted: July 7, 2010
Copyright © 2010 Korean Academy of Conservative Dentistry
- 1,694 Views
- 4 Download
- 1 Crossref
Abstract
- The purposes of this study were firstly to investigate the any formation of precipitate after interaction between ALX and NaOCL and secondarily to analyze the PCA formation by using time of flight secondary ion mass (TOF-SIM) spectrometry. Mass spectrometry analysis was performed for the mixture of 0.5% ALX and 5.25% NaOCl. As controls, 2.5% CHX with 5.25% NaOCl and 1% PCA solutions were used. Any formation of precipitates in 10 tested solutions was evaluated by naked eye. Results of mass spectrum showed that the typical peak of PCA was not detected in mixed solution of ALX and NaOCl, whereas CHX/NaOCl mixture showed the same peak that found in the PCA spectrum. Precipitate formation was only observed in CHX/NaOCL mixture. The present TOF-SIM spectrometry results indicated that ALX can be a useful root canal irrigant combined with NaOCl during canal instrumentation. Further study is necessary to confirm the antimicrobial effect of ALX against endodontic pathogen before its clinical application as an endodontic irrigant.
-
※This study was supported by the grants from the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (MEST) (No.2009-0086835), Republic of Korea.
- 1. Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004;30: 785-787.ArticlePubMed
- 2. Park JH. The effect of solvent action of sodium hypochlorite solution on pulp tissue. J Korean Acad Conserv Dent. 1982;8: 115-122.
- 3. Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifacio KC, Ito IY. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25: 167-171.ArticlePubMed
- 4. Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24: 472-476.ArticlePubMed
- 5. Jeansonne MJ, White RR. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994;20: 276-278.ArticlePubMed
- 6. Ferguson JW, Hatton JF, Gillespie MJ. Effectiveness of intracanal irrigants and medications against the yeast Candida albicans. J Endod. 2002;28: 68-71.ArticlePubMed
- 7. White RR, Hays GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod. 1997;23: 229-231.ArticlePubMed
- 8. Jeansonne MJ, White RR. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994;20: 276-278.ArticlePubMed
- 9. Ohara P, Torabinejad M, Kettering JD. Antibacterial effects of various endodontic irrigants on selected anaerobic bacteria. Endod Dent Traumatol. 1993;9: 95-100.ArticlePubMed
- 10. Delany GM, Patterson SS, Miller CH, Newton CW. The effect of chlorhexidine gluconate irrigation on the root canal flora of freshly extracted nectoric teeth. Oral Surg Oral Med Oral Pathol. 1982;53: 518-523.PubMed
- 11. Kim HJ, Park SH, Cho KM, Kim JW. Evaluation of time-dependent antimicrobial effect of sodium duchloroisocyanurate (NaDCC) on E. faecalis in the root canal. J Korean Acad Conserv Dent. 2007;32: 121-129.Article
- 12. Lee JK, Baik JE, Yun CH, Lee K, Han SH, Lee W, et al. Chlorhexidine gluconate attenuates the ability of lipoteichoic acid from Enterococcus faecalis to stimulate toll-like receptor 2. J Endod. 2009;35: 212-215.PubMed
- 13. Rosenthal S, Spaångberg L, Safavi K. Chlorhexidine substantivity in root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(4):488-492.ArticlePubMed
- 14. Basrani BR, Manek S, Sodhi RN, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007;33(8):966-969.ArticlePubMed
- 15. Chhabra RS, Huff JE, Haseman JK, Elwell MR, Peters AC. Carcinogenicity of p-chloroaniline in rat and mice. Food Chem Toxicol. 1991;29: 119-124.PubMed
- 16. Burkhardt-Holm P, Oulmi Y, Schroeder A, Storch V, Braunbeck T. Toxicity of 4-chloroaniline in early life stages if Zebrafish(Danio Rerio): II. Cytopathology and regeneration of liver and gills after prolonged exposure to waterborne 4 chloaniline. Arch Environ Contam Toxicol. 1999;37: 85-102.PubMed
- 17. Bui TB, Baumgartner JC, Mitchell JC. Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. J Endod. 2008;34: 181-185.ArticlePubMed
- 18. Zehnder M. Root canal irrigants. J Endod. 2006;32: 389-398.ArticlePubMed
- 19. Rasimick B, Nekich M, Hladek M, Musikant B, Beutch A. Interaction between chlorhexidine digluconate and EDTA. J Endod. 2008;34: 1521-1523.ArticlePubMed
- 20. Choi MS, Park SH, Cho KM, Kim JW. The comparison of different canal irrigation methods to prevent reaction precipitate of sodium hypochlorite and chlorhexidine. J Korean Acad Conserv Dent. 2010;35: 80-87.Article
- 21. McDonnell , Russell AD. Antiseptics and disinfectants: activity, action and resistance. Clin Microbiol Rev. 1999;12: 147-179.ArticlePubMedPMCPDF
- 22. Zorko M, Jerala R. Alexidine and chlorhexidine bind to lipopolysaccharide and lipoteichoic acid and prevent cell activation by antibiotics. J Antimicrob Chemother. 2008;62: 730-737.ArticlePubMed
- 23. Prestidge CA, Barnes TJ, Skinner W. Time-of-flight secondary-ion mass spectrometry for the surface characterization of solid-state pharmaceuticals. J Pharm Pharmacol. 2007;59(2):251-259.ArticlePubMedPDF
- 24. Thomas J, Sem D. An in vitro spectroscopic analysis to determine whether para-chloroaniline is produced from mixing sodium hypochlorite and chlorhexidine. J Endod. 2010;36: 315-317.ArticlePubMed
- 25. Basrani BR, Manek S, Mathers D, Fillery E, Sodhi RN. Determination of 4-chloroaniline and its derivatives formed in the interaction of sodium hypochlorite and chlorhexidine by using gas chromatography. J Endod. 2010;36(2):312-314.ArticlePubMed
- 26. Barbin LE, Saquy PC, Guedes DF, Sousa-Neto MD, Estrela C, Pécora JD. Determination of para-chloroaniline and reactive oxygen species in chlorhexidine and chlorhexidine associated with calcium hydroxide. J Endod. 2008;34(12):1508-1514.ArticlePubMed
- 27. Weatherford TW 3rd, Finn SB, Jamison HC. Effects of an alexidine mouthwash on dental plaque and gingivitis in humans over a six-month period. J Am Dent Assoc. 1977;94: 528-536.ArticlePubMed
- 28. Spolsky VW, Forsythe AB. Effects of alexidine.2HCL mouthwash on plaque and gingivitis after six months. J Dent Res. 1977;56(11):1349-1358.ArticlePubMedPDF
- 29. Roberts WR, Addy M. Comparison of the bisbiguanide antiseptics alexidine and chlorhexidine. I. Effect on plaque accumulation and salivary bacteria. J Clin Periodontol. 1981;8(3):213-219.ArticlePubMed
- 30. Addy M, Roberts WR. Comparison of the bisbiguanide antiseptics alexidine and chlorhexidine. II. Clinical and in vitro staining properties. J Clin Periodontol. 1981;8(3):220-230.ArticlePubMed
- 31. Chawner JA, Gilbert P. A comparative study of the bactericidal and growth inhibitory activities of the bis-biguanides alexidine and chlorhexidine. J Appl Bacteriol. 1989;66(3):243-252.ArticlePubMed
- 32. Baker PJ, Coburn RA, Genco RJ, Evans RT. Structural determinants of activity of chlorhexidine and alkyl bis-biguanides against the human oral flora. J Dent Res. 1987;66(6):1099-1106.ArticlePubMedPDF
- 33. Yip KW, Ito E, Mao X, Au PY, Hedley DW, Mocanu JD, et al. Potential use of alexidine dihydrochloride as an apoptosis-promoting anticancer agent. Mol Cancer Ther. 2006;5(9):2234-2240.ArticlePubMedPDF
REFERENCES
Figure 1
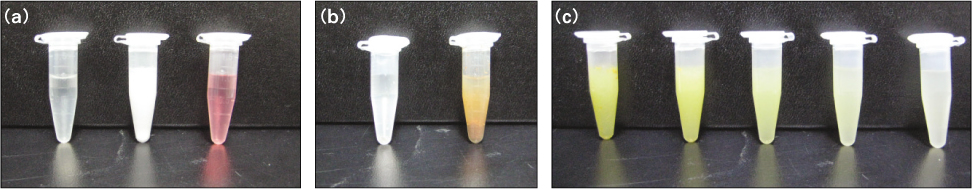
Analysis of color change and precipitate of 10 tested solutions: Microtube number #1-3(a): negative controls. No precipitation was observed. Microtube number #4-5(b): positive controls. Brown precipitate was formed in NaOCl/CHX mixture (b, right), but no reaction precipitate was formed in the ALX/NaOCl mixtures (microtube number #6-10).

Figure 3
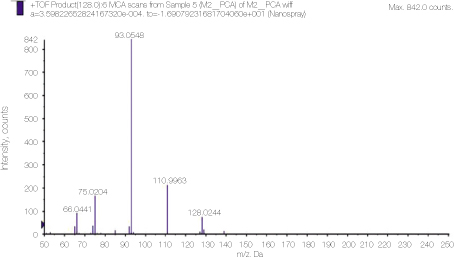
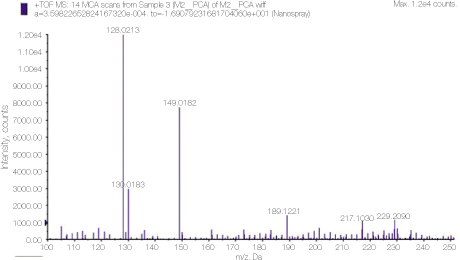
Detailed analysis of the peak 128 area in PCA spectrum shown figure 1. 110.93 and 93.0548 peaks show dissociation of NH3 or chlorine ion from PCA.

Figure 6
Mass spectrum of mixed solutions of NaOCl and ALX. The specific 128 peak of PCA is not observed.

Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Chemical Interaction of Alexidine and Sodium Hypochlorite
Hyeon-Sik Kim, Qiang Zhu, Seung-Ho Baek, Il-Young Jung, Won-Jun Son, Seok-Woo Chang, Woocheol Lee, Yu Gu, Yoon Lee, Sung-Tae Hong, Kwang-Shik Bae, Ji-Woong Kim, Kun Cho, Kee-Yeon Kum
Journal of Endodontics.2012; 38(1): 112. CrossRef
Analysis of para-chloroaniline after chemical interaction between alexidine and sodium hypochlorite using mass spectrometry: A preliminary study
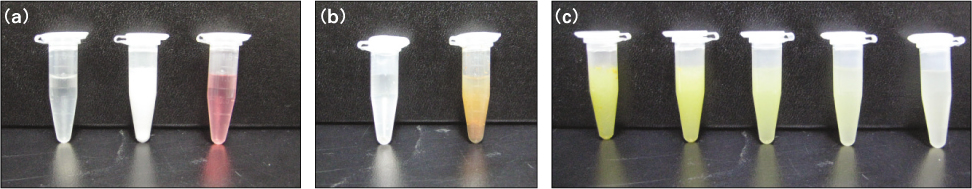
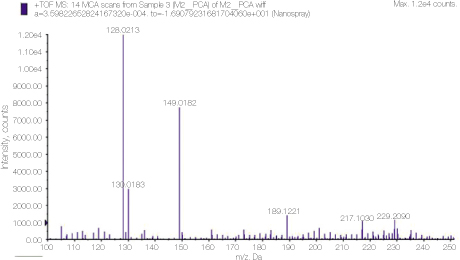
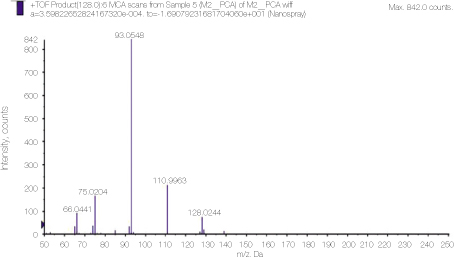
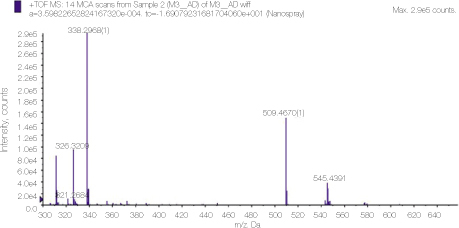
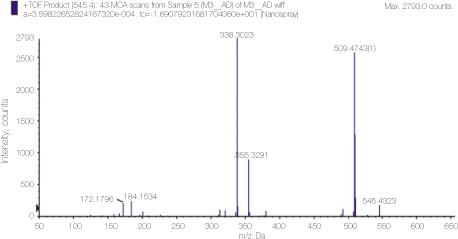
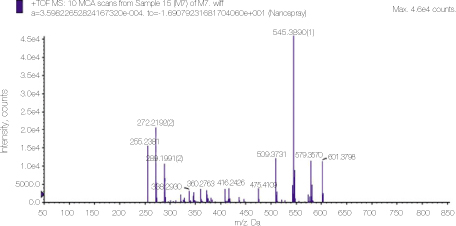
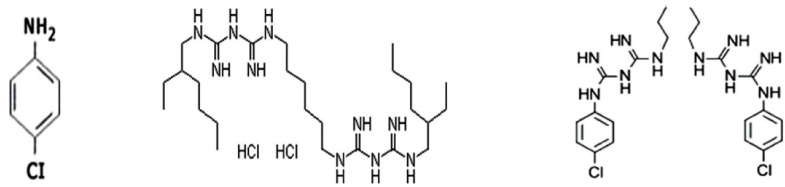
Figure 1
Analysis of color change and precipitate of 10 tested solutions: Microtube number #1-3(a): negative controls. No precipitation was observed. Microtube number #4-5(b): positive controls. Brown precipitate was formed in NaOCl/CHX mixture (b, right), but no reaction precipitate was formed in the ALX/NaOCl mixtures (microtube number #6-10).
Figure 2
Mass spectrometry clearly showed the peak 128, which is the molecular weight of PCA.
Figure 3
Detailed analysis of the peak 128 area in PCA spectrum shown figure 1. 110.93 and 93.0548 peaks show dissociation of NH3 or chlorine ion from PCA.
Figure 4
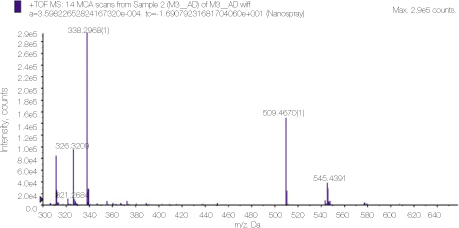
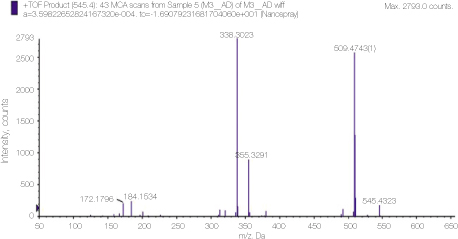
Mass spectrum showing the molecular peaks of alexidine and its derivatives.
Figure 5
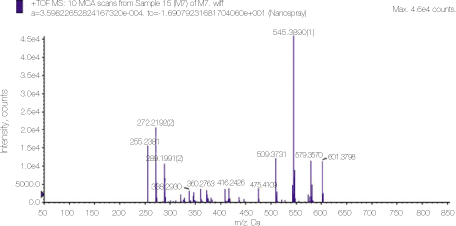
Mass spectrum of alexidine showing detailed analysis of the peak 545 area in figure 3.
Figure 6
Mass spectrum of mixed solutions of NaOCl and ALX. The specific 128 peak of PCA is not observed.
Figure 7
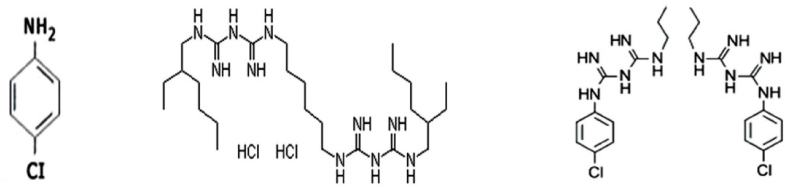
Molecular formula of PCA (a), ALX (b), and CHX (c). CHX contains phenol ring, ammonia and chlorine which are components of PCA. However, ALX does not consist of phenol ring.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Analysis of para-chloroaniline after chemical interaction between alexidine and sodium hypochlorite using mass spectrometry: A preliminary study
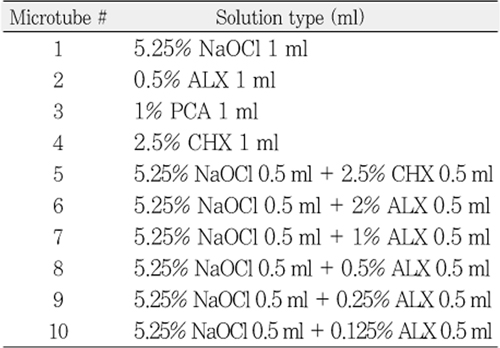
Composition and preparation of 6 test solutions for mass spectroscopy
Table 1
Composition and preparation of 6 test solutions for mass spectroscopy

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

