Articles
- Page Path
- HOME > Restor Dent Endod > Volume 27(5); 2002 > Article
-
Original Article
Sonicated extract of
Treponema denticola impairs the lymphocyte proliferation - Woo-Cheol Lee, Bruce J. Shenker*
-
2002;27(5):-478.
DOI: https://doi.org/10.5395/JKACD.2002.27.5.473
Published online: September 30, 2002
Department of Conservative Dentistry, Seoul National University, Korea.
*Department of Pathology, University of Pennsylvania, USA.
Copyright © 2002 Korean Academy of Conservative Dentistry
- 837 Views
- 0 Download
I. Introduction
Spirochetes are motile, Gram-negative, spiral organisms characterized by flexible cell walls and internal flagella. Members of these species have been implicated as pathogens in several human infections including syphilis, yaws and Lyme disease and this species has also been observed in oral diseases such as periodontal disease and periapical infections. Listgarten1) reported that spirochetes are conspicuous inhabitants of subgingival plaque in patients with gingivitis and periodontitis. Although these microorganisms are known to be important periodontal pathogens, recent studies suggest that this may be involved in pulpal and periapical infections as well. For example, Thilo et al.2) reported the root canal flora of decayed teeth contained as much as 6% of spirochetes. Likewise, Nair3) reported that spirochetes form a significant component of the flora of periapical specimens. Recently, using PCR, Siqueira4) reported that Treponema denticola accounted >50% of the flora in infected root canals and in acute alveolar abscesses5). Thus, spirochetes or their products may contribute to the inflammatory process and pathogenesis of these periradicular disorders.
In this regard, Shenker and colleague6) demonstrated that soluble sonic extracts of several strains of T. denticola inhibit human peripheral blood lymphocyte (HPBL) proliferative responses to both mitogens and antigens in vitro with no effect on cell viability. They have also observed that these effects are due to a protein compound of two polypeptides of 50 and 56 kD. It is interesting to note that these investigations recently reported that another oral pathogen, Fusobacterium nucleatum, also produces an immunomodulatory protein that is compound of two peptides of similar size as SIP7). Therefore, we propose that SIP may act to inhibit lymphocyte function by interfering with cell cycle progression.
The objective of this study was to determine if the T. denticola immunosuppressive protein (SIP) does indeed cause cell cycle arrest in activated T cells.
II. Materials and Methods
Spirochete strains were obtained from our own reservoir of Treponema denticola LL2513. Stock cultures of spirochetes were stored in -70℃ freezer. For the growth and the maintenance procedure, resuspend of spirochetes stock and transfer 0.5ml into each of 2 tubes of 20 ml of TYGVS (Trypticase, yeast extract, glucose, volatile fatty acids, serum) spirochete medium containing veal infusion broth (Difco Laboratories, Detroit, MI) supplemented with 30g of yeast extract (BBL), 30g of trypticase peptone (BBL) and 1.5g of ammonium sulfate then mixed with 3g of L-cysteine hydrochloride, 3g of glucose (Fisher) and 0.9ml of volatile fatty acid solution, and incubate in the anaerobic chamber for 3 to 4 days. After check the purity under the scope and then transfer 1ml of culture into two 20ml tubes of semisolid TYGVS broth (prereduced) and incubated in an anaerobic chamber for 3 to 4 days at 37℃. These cultures were ready to inoculate flask containing 500 ml TYGVS broth, which were then incubated for an additional 3 to 4 days. Centrifuged the culture of spirochetes in Sorvall RC5C (Sorvall Instruments, DuPont) at 8000 rpm for 30 min at 4℃ to harvest procedure. After decanted supernatant, resuspened pellets in PBS containing PMSF. Transfer each 30 ml suspension into a 40 ml centrifuge tube, spin again at 12,000 rpm for 20 min at 4℃. Resuspended each pellet with 50 mM Tris buffer (pH 8.0) and stored in the freezer before the sonication. Thawed the sample in tepid water and transferred to 50 ml conical tube with adding of 1/2 volume of glass beads. The equivalent of 1 liter of washed spirochetes was sonicated with macrotip (Branson sonicator model 350, Branson sonic Power Co. Danbury, CT) for a total of 7 minute with 30-sec pulses until the top layer gets clear. After setting of the beads, cellular debris and remaining bacterial cells were removed by centrifugation at 12,000×g for 20 min and the membrane fraction was sedimented by ultracentrifugation at 85,000×g for 60 min at 4℃; the supernatant was dialyzed against PBS (phosphate-buffered saline), divided into aliquots, and stored at -20℃. The protein that remained in suspension after the high-speed centrifugation was designated the cytoplasmic fraction and contained both cytoplasmic and periplasmic proteins. We named it as SIP and this protein was employed in most of the studies described in this paper.
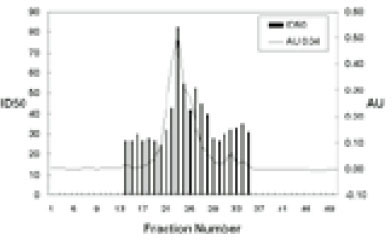
To ascertain if SIP has immunoreactivity, we conducted preliminary study. SIP was first fractioned by ammonium sulfate precipitation and fractions were monitored. As a result of bioactivity test, all activity precipitated between 60 and 80% (NH4)2SO4. Before the crude sample was applied to an ion-exchange column (Hi/Q; Pharmacia, Uppsala, Sweden), we performed dialysis against 10 mM tris buffer, pH 7.0, containing 10 mM NaCl and 1 mM EDTA. Take all active fractions above 10% gradient and run ion-exchange chromatography again by Mono Q5 (Pharmacia, Uppsala, Sweden) and chromatofocusing. The column was then extensively washed and eluted with a linear NaCl gradient (10 to 600mM). Fractions were collected and monitored for SIP activity expressed in ID50 units per milliliter, which represent the volume of the sample required to reduce [3H] thymidine incorporation to 50% of control values. Active fractions were pooled and concentrated for further fraction by gel filtration chromatography on a Superose 12 column (Pharmacia, Uppsala, Sweden) and then further fractionated by electrophoresis in 10% acrylamide gels under non-denaturing conditions.
Human peripheral blood mononuclear cells (HPBMC) were prepared according to the protocol used in Department of Pathology. HPBMC were isolated from 100 to 200 ml of heparinized venous blood obtained from healthy donors. The blood was diluted with an equal volume of HBSS (Hanks balanced salt solution) and the HPBMC were then isolated by buoyant density centrifugation on Ficoll-Hypaque (Pharmacia). The HPBMC were washed twice with HBSS and diluted to 10×106 to 20×106 viable cells per ml culture medium consisting of RPMI 1640. Viable-cell counts were performed by assessing tryptan blue dye exclusion. An HPBMC suspension (0.1ml) containing 2×105 cells was placed into each well of flat-bottomed microculture plates. Each culture received 0.1ml of medium or 0.1ml of various concentration of SIP diluted in medium. Right after the cells were incubated for 30 min at 37℃, the cell culture received an optimal mitogenic dose of phytohemagglutinin (PHA) (1µl/ml; Murex Diagnostics; Atlanta, GA) except control. The cells were incubated for 72h, and DNA synthesis was assessed by the incorporation of [3H] thymidine.
Cell cycle analysis was performed on HPBMC by a modification of the method of Schmid et al8). Briefly, 1ml culture (2×106 cells) was incubated for 24 to 72hr in the presence of medium (control), PHA, or PHA and SIP. Cells were harvested at 24-hr intervals, washed and then resuspended in 1ml of 80% ice cold ethanol for 2 hr at -20℃. After wash the cells, DNA was then stained by incubating cells with 0.5ml of PI staining for 30 min. Samples were analyzed on a Becton-Dickinson FACStarPLUS flow cytometer.
III. Results
Shenker and colleague6) previously reported that crude sonic extracts of Treponema denticola strain LL2513 contains a protein capable of inhibiting human T-lymphocyte activation by both antigens and mitogens. Using a combination of ion exchange, chromatofocusing and gel filtration chromatography, we purified this immunomodulatory protein (Fig. 1). Purified SIP consists of two polypeptides of 50 and 56 kDa and retains biological activity. Human lymphocytes exposed to various amounts of purified SIP (0.25 to 0.5µg) exhibit a dose-dependent reduction in their ability to proliferate in response to PHA (Fig. 2). In the presence of 0.25µg/ml SIP was observed 42.5 % inhibited and this increased to 75.1% in the presence of 0.5µg/ml SIP. Based upon these results, we calculated an ID50 of 0.5µg/ml for SIP; this concentration was used for most of the following experiments.
In addition to 3H-TdR incorporation, we also employed propidium iodide and flow cytometry to assess cell cycle progression in SIP-treated cells. As shown in Fig. 3 A, >97% of lymphocytes incubated in medium alone were found in the G0/G1 phase of the cell cycle. In contrast, treatment with PHA lead to lymphocyte proliferation with cells in the G0/G1 phase (58%), S phase (34.6%), and G2/M phase (7.4%). Cells pre-exposed to SIP were inhibited entering S, and G2/M phases; 87% of the cells were in the G0/G1 phase, 8% S phase and 4% G2/M phase.
The preceding experiments suggest that even in the presence of SIP, lymphocytes are indeed activated by PHA, however, the cells are not able to progress beyond the G1 phase of the cell cycle.
IV. Discussion
Pathogenesis of bacterial infection is contributed by virulence of microorganism. Several factors are involved in microbial virulence, such as promoting bacterial colonization, invading host tissue, and resisting or escaping host defense mechanism.
These factors render microorganisms to survive from the lethal action of host defense system. It is well known that several infectious diseases are associated with impairment of immunologic responsiveness23). In fact, several microorganisms such as Actinobacillus actinomycetemcomitans9,24), Fusobacterium nucleatum7,10), spirochetes6), Vibrio cholera11), group A streptococcus12,13), Pseudomonas aeruginosa14), Plasmodium berghei15), rubella, influenza, polio and parvovirus16,17,18,19,20) are proved to produce immunosuppressive factors. These immunomodulatory agents may act differently by intefering with either the induction or the expression of immune reaction. Sometimes these factors can activate T cells, sometimes can suppress T cells, or have direct effect on both precusor (stem cell) and mature effector cells. For example, sonic extracts of Fusobacterium nucleatum have been shown to evoke a concentration-dependent stimulatory or suppressive effect on T-cell proliferation in the presence of accessory cell from the dental pulp10). In addition, Actinobacillus actinomycetemcomitans produces heat labile protein which appears to suppress lymphocyte activation by blocking DNA, RNA, and protein synthesis9). Collectively, these studies demonstrate the importance of bacteria and/or bacterial products as exogenous immunoregulatory agents that could have profound effects on the course of infection.
Although it is not clear how this microorganism contributes to the disease process, several investigators have proposed that impaired host defenses may play a pivotal role. In this regard, there have been reports of abnormalities in chemotactic and phagocytic functions of peripheral blood PMN and monocytes in patients with certain oral diseases such as juvenile periodontitis20,21,22). Therefore, it is not unreasonable to propose that at least one aspect of susceptibility to certain disease may be due to the immunesuppression caused by or complicated by bacterial products. Bacterial products that may suppress immunologic responsiveness include toxins, enzymes, cell wall components (including lipopolysaccharide), and metabolic products. Microbial products represent an important source of immunoregulatory agents; in particular, products from several microorganisms are immunosuppressive. These immunosuppressive products can demonstrate that they alter the immune defense system via different mechanisms.
We demonstrated in this study that purified sonicated extracts of spirochetes were capable of inhibiting mitogen-induced human T-cell proliferation in a dose-dependent fashion (Fig. 2). Cell cycle analysis indicates that these cells remained in the G0/G1 phase of the cell cycle. It should be noted that the methods employed for the cell cycle analysis did not enable us to discriminate between the G0 and G1 phases. Thus we may need to further investigate by employing mutiparametric cell-cycle analysis. The multiparametric RNA/DNA or protein/DNA synthesis analysis is the method of choice to detect the unbalanced cell growth in relation to cell position in the cell cycle. Growth unbalance often occurs as a result of cell arrest in the cell cycle.
In conclusion, our data demonstrate that the spirochete inhibiting protein (SIP) disrupt the ability of T cells to properly transit the cell cycle. This disturbance would in turn adversely affect the development of normal immunologic defense mechanisms. Our results do not provide direct evidence that immunosuppression occurs in vivo. Nevertheless, it is reasonable to propose that if this organism acts in vivo as it does in vitro, inhibition of the immune response could result in the enhanced pathogenicity of Spirochete itself or that of other opportunistic organisms.
- 1. Listgarten MA. In: Genco RJ, Mergenhangen SE, editors. Colonization of subgingival areas by motile rods and spirochetes: clinical implication. Host-Parasite Interaction in Periodontal Diseases. 1982;Washington D.C: American Society of Microbiology; 112.
- 2. Thilo B, Baehni P, Holz J, Baume LJ. Distribution des bacteries dans les parties coronaire et apicale de dents a pulpe necrosee. SSO Schweiz Monatsschr Zahnheilkd. 1983;93: 335-350.PubMed
- 3. Nair PNR. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod. 1987;13: 29-39.ArticlePubMed
- 4. Siqueira JF, Rocas IN, Favieri A, Santos KRN. Detection of Treponema denticola in endodontics infections by 16S rRNA gene detected polymerase chain reaction. Oral Microbiol Immunol. 2000;15: 335-337.PubMed
- 5. Siqueira JF, Rocas IN, Oliveira JCM, Santos KRN. Detection of putative oral pathogens in acute periradicular abscesses by 16S rDNA-directed polymerase chain reaction. J Endod. 2001;27: 164-167.ArticlePubMed
- 6. Shenker BJ, Listgarten MA, Taichman NS. Suppression of human lymphocyte responses by oral spirochetes : A monocyte-dependent phenomenon. J Immunol. 1984;132: 2039-2045.ArticlePubMedPDF
- 7. Shenker BJ, Datar S. Fusobacterium nucleatum inhibits human T-cells by arresting cells in the mid G1 phase of the cell cycle. Infect Immun. 1995;63: 4830-4836.ArticlePubMedPMCPDF
- 8. Schumid I, Uittenbogaart CH, Giorgi JV. A gentle fixation and permeablization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry. 1991;12: 279-285.ArticlePubMed
- 9. Shenker BJ, Mcarthur WP, Tsai CC. Immune suppression induced by Actinobacillus actinomycetemcomitans. I. Effects on human peripheral blood lymphocyte responses to mitogens and antigens. J Immunol. 1982;128: 148-154.ArticlePubMedPDF
- 10. Yoshida H, Sundqvist G, Bergenholtz G. Effect of sonicated material from Fusobacterium nucleatum on the functional capacity od accessory cells derived from dental pulp. Oral Microbiol Immunol. 1995;10: 208-212.PubMed
- 11. Holmgren J, Lindholm L, Lonnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. I. binding properties and interference with lectin-induced cellular stimulation. J Exp Med. 1974;139: 801-819.PubMedPMC
- 12. Malakian AH, Schwab JH. Immunosuppressant from group A streptococci. Science. 1968;159: 880-881.ArticlePubMed
- 13. Hanna EE, Watson DW. Host-parasite relationships among group A streptococci. IV. Suppression of antibody response by streptococcal pyrogenic exotoxin. J Bacteriol. 1968;90: 14-21.ArticlePubMedPMCPDF
- 14. Floersheim GL, Hopff WH, Gasser M, Bucher K. Impairment of cell mediated immune response by Pseudomonas aeruginosa. Clin Exp Immunol. 1971;9: 241-247.PubMedPMC
- 15. Khansari N, Segre M, Segre D. Immunosuppression in murine malaria : a soluble immunosuppressive factor derived from Plasmodium berghei-infected blood. J Immunol. 1981;127: 1889-1893.ArticlePubMedPDF
- 16. Kauffman CA, Phair JP, Linneman CC, Schiff GM. Cell-mediated immunity in humans during viral infection. I. Effect of rubella on dermal hypersensitivity, PHA response and T lymphocytes numbers. Infect Immun. 1974;10: 212-215.ArticlePubMedPMCPDF
- 17. Olson GB, Dent PB, Rawls WE. Abnormalities of in vitro lymphocyte responses during rubella virus infections. J Exp Med. 1968;128: 47-68.ArticlePubMedPMCPDF
- 18. Kantzler GB, Lauteria SF, Cusumano CL, Lee JD, Ganguly R, Waldman RH. Immunosuppression during influenza virus infection. Infect Immun. 1974;10: 996-1002.ArticlePubMedPMCPDF
- 19. Engers HD, Louis JA, Zubler RH, Hirt B. Inhibition of Tx cell-mediated functions by MVM(i), a parvovirus closely related to minute virus of mice. J Immunol. 1981;127: 2280-2285.PubMed
- 20. Cianciola LJ, Genco RJ, Peters MR, McKenna J, VanOss CJ. Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature. 1977;265: 445-447.ArticlePubMedPDF
- 21. Clark RA, Page RC, Wilde G. Defective neutrophil chemotaxis in juvenile periodontitis. Infect Immun. 1977;18: 694-700.ArticlePubMedPMCPDF
- 22. Van Dyke TE, Horoszewicz HH, Cianciola LJ, Genco RJ. Neutrophil chemotaxis dysfunction in human periodontitis. Infect Immun. 1980;27: 124-132.ArticlePubMedPMCPDF
- 23. Schwab JH. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975;39: 121-143.ArticlePubMedPMCPDF
- 24. Shenker BJ, Tsai CC, Taichman NS. Suppression of lymphocyte responses by Actinobacillus actinomycetemcomitans. J Periodontal Res. 1982;17: 462.ArticlePubMed
REFERENCES
Fig. 2
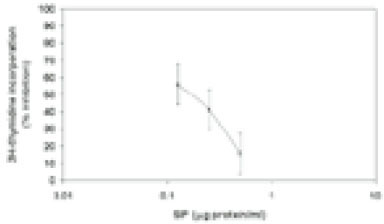
Effects of SIP on lymphocyte proliferation. HPBMC were incubated with various amounts SIP for 30 min and PHA (1.0µg/ml) was added. The cells were then incubated for 72 hr. 3H-TdR was added for the last 4 hr. Data was presented as a percent inhibition of 3H-TdR incorporation. Results represent the mean ± SD of 5 experiments

Fig. 3
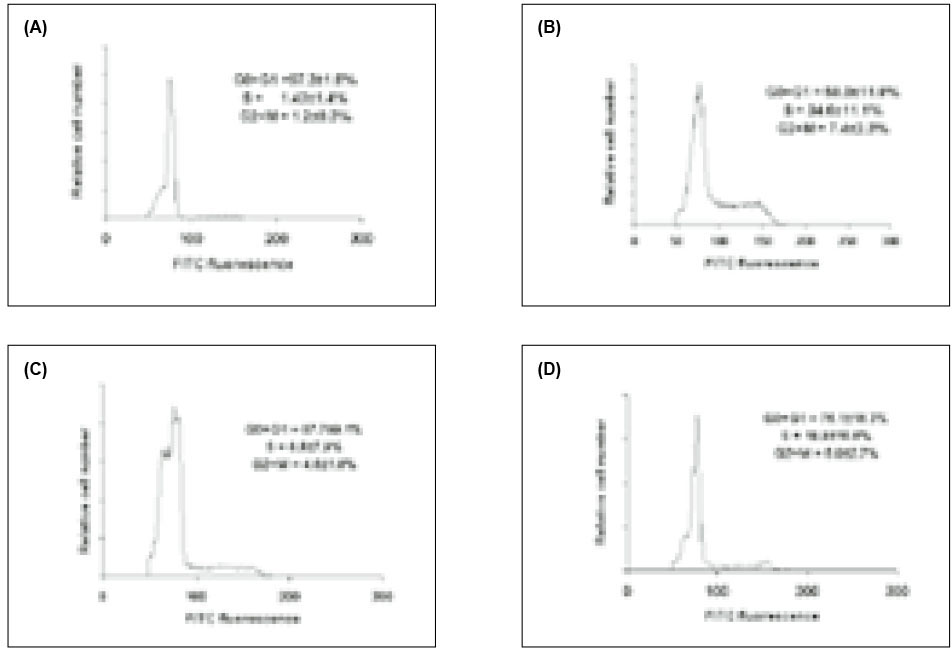
Cell cycle analysis using single PI staining. HPBMC were incubated with medium (A), PHA (B), PHA and 0.5µg of SIP per ml (C), 0.25µg of SIP per ml (D) for 72 h, and the cells were stained as described in materials and methods. Results are the mean of replicate cultures and are representative of three experiments.

Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

Sonicated extract of Treponema denticola impairs the lymphocyte proliferation
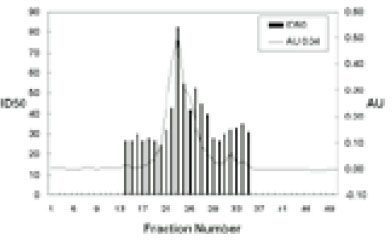
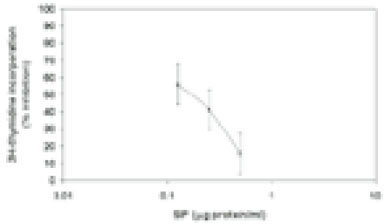
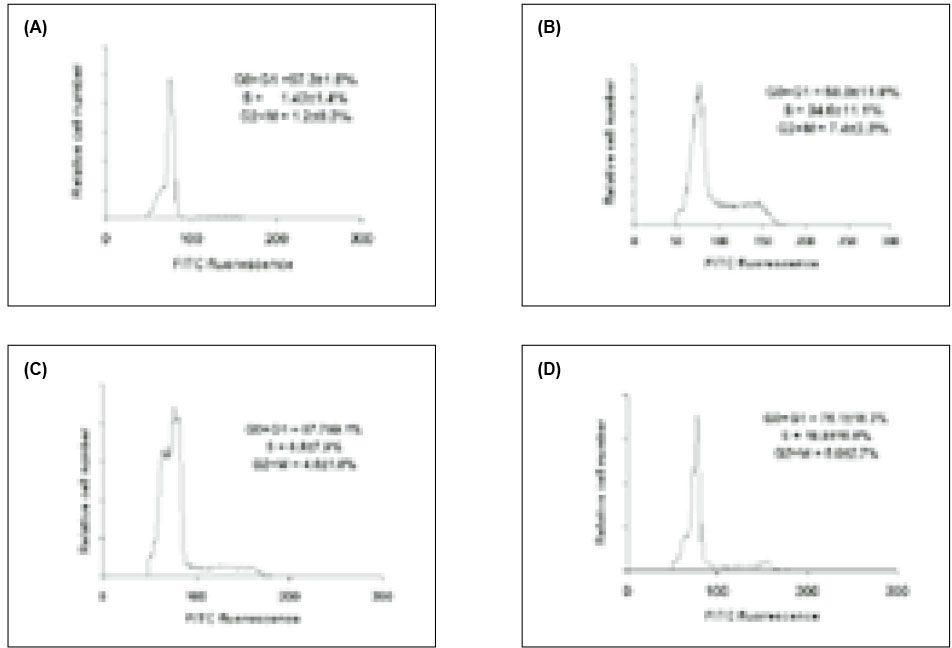
Fig. 1
Chromatography of soluble extracts of Treponema denticola LL2513.
Fig. 2
Effects of SIP on lymphocyte proliferation. HPBMC were incubated with various amounts SIP for 30 min and PHA (1.0µg/ml) was added. The cells were then incubated for 72 hr. 3H-TdR was added for the last 4 hr. Data was presented as a percent inhibition of 3H-TdR incorporation. Results represent the mean ± SD of 5 experiments
Fig. 3
Cell cycle analysis using single PI staining. HPBMC were incubated with medium (A), PHA (B), PHA and 0.5µg of SIP per ml (C), 0.25µg of SIP per ml (D) for 72 h, and the cells were stained as described in materials and methods. Results are the mean of replicate cultures and are representative of three experiments.
Fig. 1
Fig. 2
Fig. 3
Sonicated extract of Treponema denticola impairs the lymphocyte proliferation

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

