Articles
- Page Path
- HOME > Restor Dent Endod > Volume 33(5); 2008 > Article
- Original Article The remineralizing features of pH 5.5 solutions of different degree of saturations on artificially demineralized enamel
- Young-Jun Kwak, Eui-seoug Kim, Sung-Ho Park, Hyung Kyu Gong, Yoon Lee, Chan-Young Lee
-
2008;33(5):-492.
DOI: https://doi.org/10.5395/JKACD.2008.33.5.481
Published online: September 30, 2008
Department of Conservative Dentistry, Yonsei University, Seoul, Korea.
- Corresponding Author: Chan-Young Lee. Department of Conservative Dentistry, College of Dentistry, Yonsei University, 134, Shinchon-dong, Seodaemun-gu, Seoul, 120-752, Korea. Tel: 82-2-2228-8700, chanyoungl@yuhs.ac
• Received: August 12, 2008 • Revised: August 22, 2008 • Accepted: September 2, 2008
Copyright © 2008 The Korean Academy of Conservative Dentistry
- 1,286 Views
- 2 Download
- 1 Crossref
Abstract
-
The purpose of this study is to observe and compare the remineralization tendencies of artificially demineralized enamel by remineralization solutions of different degree of saturations at pH 5.5, using a polarizing microscope and computer programs (Photoshop, Image pro plus, Scion Image, Excel).For this study, 36 sound permanent teeth with no signs of demineralization, cracks, or dental restorations were used. The specimens were immersed in lactic acid demineralization solution for 3 days in order to produce dental caries artificially that consist of surface and subsurface lesions. Each of 9 or 10 specimens was immersed in pH 5.5 lactic acid buffered remineralization solution of three different degrees of saturation (0.25, 0.30, 0.35) for 12 days. After the demineralization and remineralization, images were taken by a polarizing microscope (× 100). The results were obtained by observing images of the specimens, and using computer programs, the density of caries lesions were determined.In conclusion, in the group with the lowest degree of saturation, remineralization occurred thoroughly from the surface to the subsurface lesion, whereas in the groups with greater degree of saturation showed no significant change in the subsurface lesion, although there was corresponding increase in the remineralization width on the surface zones.
- 1. Ingram GS, Silverstone LM. A chemical and histological study of artificial caries in human dental enamel in vitro. Caries Res. 1981;15: 393-398.ArticlePubMed
- 2. Shellis RP. Relationship between human enamel structure and the formation of caries-like lesions. Arch Oral Biol. 1984;29: 975-981.PubMed
- 3. Silverstone LM. Observation on the dark zone in early enamel caries and artificial like lesions. Caries Res. 1967;1: 260-274.PubMed
- 4. Brudevold F, McCann HG. Enamel solubility tests and their significance in regard to dental caries. Ann N Y Acad Sci. 1968;153: 20-51.
- 5. Brown WE, Patel PR, Chow LC. Formation of CaHPO4·2H2O from enamel mineral and its relationship to caries formation. J Dent Res. 1975;54: 475-481.ArticlePubMedPDF
- 6. Christoffersen J, Arends J. Progress of artificial lesion progression in enamel. Caries Res. 1982;16: 433-439.ArticlePubMed
- 7. Featherstone JDB, Duncan JF, Cutress TW. Surface layer phenomenon in vitro early caries-like lesions in human tooth enamel. Arch Oral Biol. 1978;23: 397-404.PubMed
- 8. Moreno EC, Zahradnik RT. Chemistry of enamel subsurface demineralization in vitro. J Dent Res. 1974;53: 226-235.ArticlePubMedPDF
- 9. Englander HR, Carter WMJ. The formation of lactic acid in dental plaque(III). J Dent Res. 1956;35: 792-799.ArticlePubMedPDF
- 10. Margolis HC, Moreno EC. Kinetic and thermodynamic aspect of enamel demineralization. Caries Res. 1985;19: 22-35.ArticlePubMed
- 11. Margolis HC, Moreno EC. Kinetics of hydroxyapatite dissolution. J Dent Res. 1990;Abstr. #1546.
- 12. Margolis HC, Moreno EC, Murphy BJ. Development of caries like lesions in partially saturated lactate buffers. Caries Res. 1985;19: 36-45.ArticlePubMed
- 13. Gray JA. Kinetics of enamel dissolution during formation of incipient caries-like lesions. Arch Oral Biol. 1966 04;11: 397-422.ArticlePubMed
- 14. Theuns HM, Van Dijk JWE, Groneveld A. Effect of time and degree of saturation of buffer solutions on artificial carious lesion formation in human tooth enamel. Caries Res. 1983;17: 503-512.ArticlePubMed
- 15. Kapur KK, Fischer EF, Manly RS. Influence of type of acid, pH, molarity, saturation in rate of enamel penetration. J Dent Res. 1962;41: 760-770.ArticlePubMedPDF
- 16. Lee CY. Artificial Caries Formation in Acid Buffering Solution. Yonsei Dent J. 1992;7: 34-41.
- 17. Silverstone LM, Wefer JS, Zimmerman BF, Clark BH, Featherstone MJ. Remineralization phenomena. Caries Res. 1977;11: 59-84.ArticlePubMed
- 18. ten Cate JM, Duijsters PPE. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16: 201-210.ArticlePubMed
- 19. ten Cate JM, Arends J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1981;15: 60-69.ArticlePubMed
- 20. Cate JM, Arends J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977;11: 277-286.ArticlePubMed
- 21. Han WS, Kum KY, Lee CY. The Influence of Fluoride on Remineralization of Artificial Dental Caries. J Korean Acad Conserv Dent. 1996;21: 161-173.
- 22. Feagin F, Patel PR, Koulourides T, Pigman W. Study of the effect Ca, P, F and hydrogen ion concentrations on the remineralization of partially demineralized human and bovine enamel surfaces. Arch Oral Biol. 1971;16: 535-548.PubMed
- 23. Featherstone JDB, Rodger BF. Effect of acetic, lactic and other organic acids on the formation of artificial caries lesion. Caries Res. 1981;15: 377-385.ArticlePubMed
- 24. Nikiforuk MJ. Environmental hypersensitivity living in a hostile world. Can Nurse. 1985;81: 42-46.
- 25. Margolis HC, Moreno EC, Murphy BJ. Effect of low levels of fluoride in solution on enamel demineralization. J Dent Res. 1986;65: 23-29.ArticlePubMedPDF
- 26. Lammers PC, Borggreven JM, Driessens FC. Influence of fluoride and pH on in vitro remneralization of bovine enamel. Caries Res. 1992;26: 8-13.ArticlePubMed
- 27. Park JW, Hur B, Lee CY. The Effects of the Degree of Saturation of Acidulated Buffer Solutions in Enamel and Dentin Remineralization and AFM Observation of Hydroxyapatite Crystals. J Korean Acad Conserv Dent. 2000;25: 459-473.
- 28. Kim MK, Kum KY, Lee CY. The Influence of pH on Remineralization of Artificial Dental Caries. J Korean Acad Conserv Dent. 1997;22: 193-208.
- 29. Kim SC, Lee CY. The Effect of the pH of Remineralized Buffer Solutions on Dentin Remineralizxation. J Korean Acad Conserv Dent. 2007;32: 151-161.Article
- 30. Margolis HC, Zhang YP, Lee CY, Kent RL Jr, Moreno EC. Kinetics of enamel demineralization in vitro. J Dent Res. 1999;78: 1326-1335.ArticlePubMedPDF
- 31. ten Cate JM. In vitro studies on the effects of F on de- and remineralization. J Dent Res. 1990;69: 614-619.ArticlePubMedPDF
- 32. White DJ. The use of synthetic polymer gels for artificial carious lesion preparation. Caries Res. 1987;21: 228-242.ArticlePubMed
- 33. Featherstone JDB. Comparison of artificial caries like lesions by quantitative microradiography and microhardness profile. Caries Res. 1983;17: 385-391.ArticlePubMed
- 34. Arends J, Jongebloed W, Ogaard B, Rolla G. SEM and microradiographic investigation of initial enamel caries. Scand J Dent Res. 1987;95: 193-201.ArticlePubMed
- 35. Strang R, MacDonald I, Creanor SL, Stephen KW. Mineral content variations in demineralized enamel (abstract). Caries Res. 1986;20: 185.
- 36. Buskes JA, Christoffersen J, Arends J. Lesion formation and lesion remineralization in enamel under constant composition condition. Caries Res. 1985;19: 490-496.ArticlePubMed
- 37. Nancollas GH, Purdie N. The kinetics of crystal growth. Q Rev. 1964;18: 1-20.Article
- 38. White DJ, Chen WC, Nancollas GH. Kinetic and physical aspects of enamel remineralization. Caries Res. 1988;22: 11-19.ArticlePubMed
- 39. Moreno EC, Zahradnik RT. Demineralization and remineralization of dental enamel. J Dent Res. 1979;58: 896-902.ArticlePubMedPDF
- 40. Exterkate RAM, Damen JJM, ten Cate JM. A single-section model for enamel de- and remineralization studies. 1. The effects of different Ca/P ratios in remineralization solutions. J Dent Res. 1993;72: 1599-1603.ArticlePubMedPDF
- 41. ten Cate JM, Arends J. Remineralization of artificial enamel lesions in vitro. III. A study of the deposition mechanism. Caries Res. 1980;14: 351-358.ArticlePubMed
- 42. Park SH, Lee CY, Lee JS. The Effect of Acid Concentration and pH of Lactate Buffer Solution on the Progress of Artificial Caries Lesion in Human Tooth Enamel. J Korean Acad Conserv Dent. 1993;18: 277-290.
- 43. Kum KY, Lee CY. The Effect of Four Kinds of Acid and Concentration on the Formation of Artificial Carious Lesion in Human Tooth Enamel. J Korean Acad Conserv Dent. 1996;21: 470-488.
REFERENCES
Figure 8
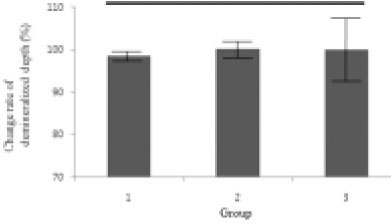
Change rate of demineralized depth (demineralized depth after remineralization / demineralized depth before remineralization) × 100 (%).

Figure 9
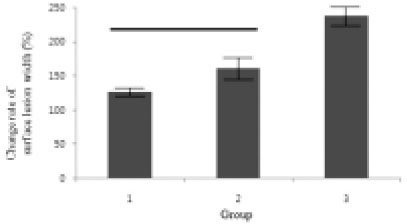
Change rate of surface lesion width (surface lesion width after remineralization / surface lesion width before remineralization) × 100 (%).

Figure 13
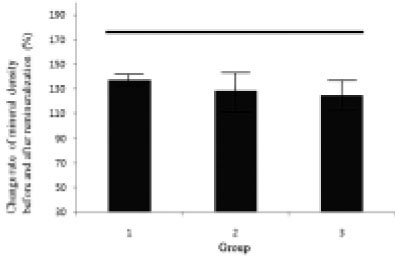
Change rate of mineral density before and after remineralization ((demineralized area before remineralization - demineralized area after remineralization / demineralized area before remineralization) × 100 + 100 (%))

Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

- Effect of fluoride concentration in pH 4.3 and pH 7.0 supersaturated solutions on the crystal growth of hydroxyapatite
Haneol Shin, Sung-Ho Park, Jeong-Won Park, Chan-Young Lee
Restorative Dentistry & Endodontics.2012; 37(1): 16. CrossRef
The remineralizing features of pH 5.5 solutions of different degree of saturations on artificially demineralized enamel
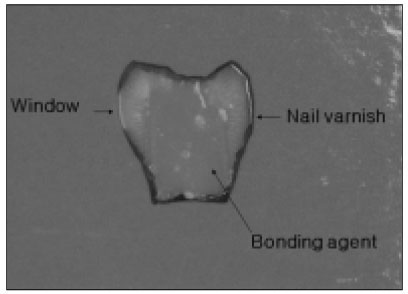
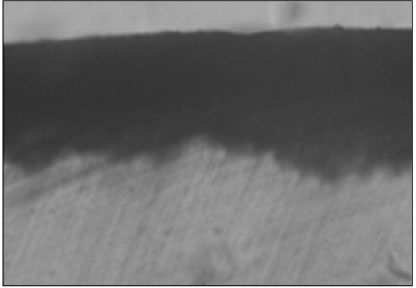
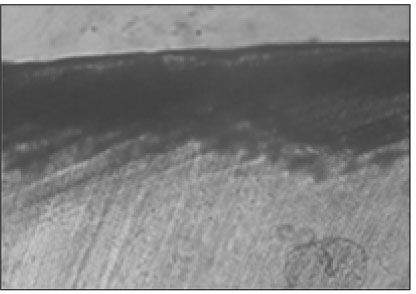
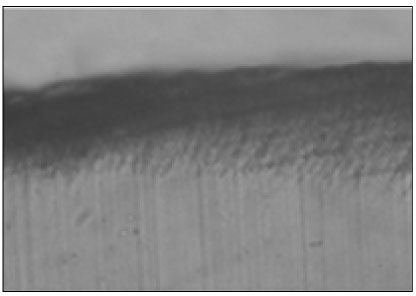


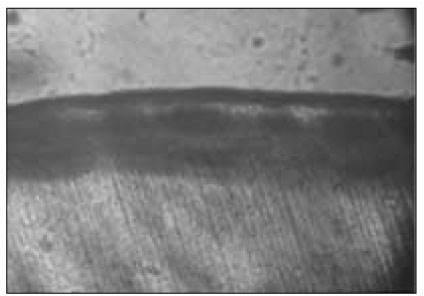
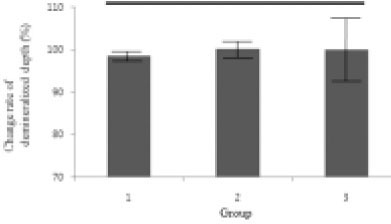
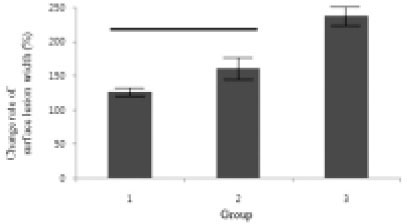
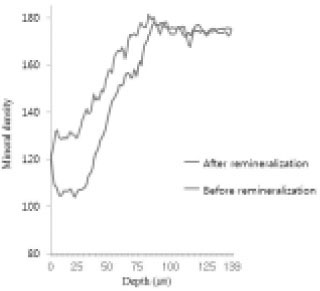
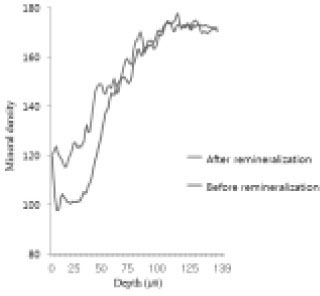
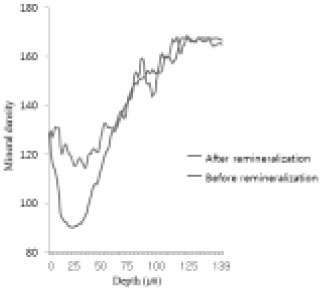
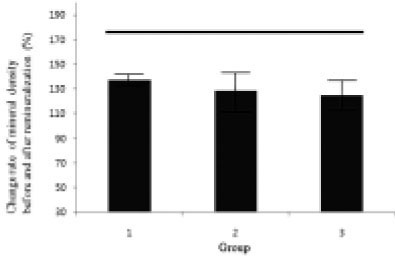
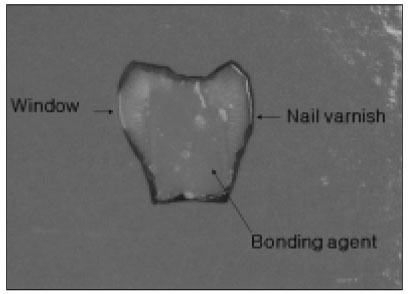
Figure 1
Specimen used in the experiment.
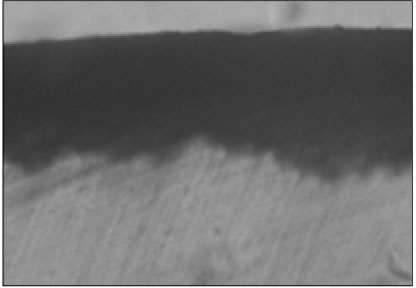
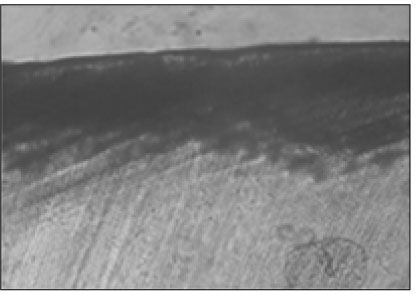
Figure 2
Polarizing microscopic observation of demineralized enamel (Group 1, × 100).
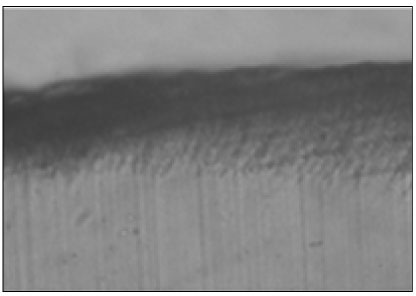
Figure 3
Polarizing microscopic observation of remineralized enamel (Group 1, × 100).
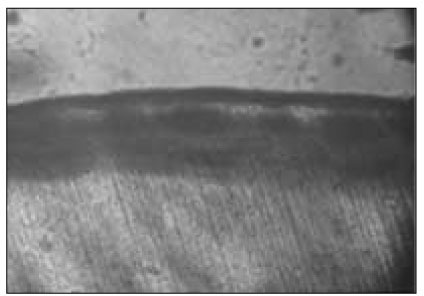
Figure 4
Polarizing microscopic observation of demineralized enamel (Group 2, × 100).
Figure 5
Polarizing microscopic observation of remineralized enamel (Group 2, × 100).
Figure 6
Polarizing microscopic observation of demineralized enamel (Group 3, × 100).
Figure 7
Polarizing microscopic observation of remineralized enamel (Group 3, × 100).
Figure 8
Change rate of demineralized depth (demineralized depth after remineralization / demineralized depth before remineralization) × 100 (%).
Figure 9
Change rate of surface lesion width (surface lesion width after remineralization / surface lesion width before remineralization) × 100 (%).
Figure 10
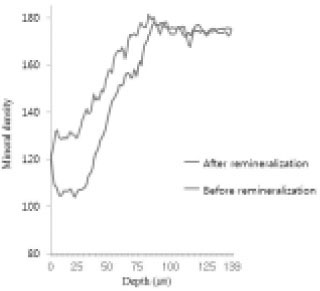
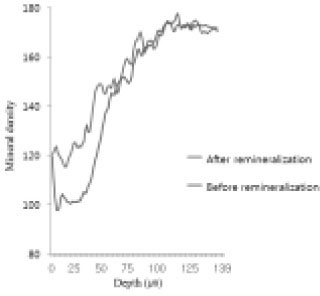
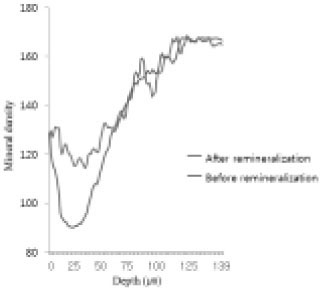
Comparison of density in enamel area before and after remineralization (Group 1).
Figure 11
Comparison of density in enamel area before and after remineralization (Group 2).
Figure 12
Comparison of density in enamel area before and after remineralization (Group 3).
Figure 13
Change rate of mineral density before and after remineralization ((demineralized area before remineralization - demineralized area after remineralization / demineralized area before remineralization) × 100 + 100 (%))
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 12
Figure 13
The remineralizing features of pH 5.5 solutions of different degree of saturations on artificially demineralized enamel
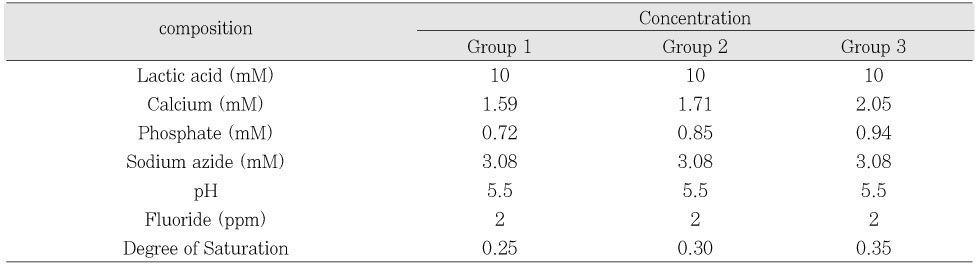
Initial composition of demineralization solution
Initial composition of remineralization solution
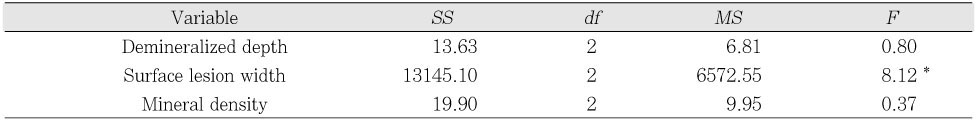
One-way ANOVA on the remineralizing features of pH 5.5 solutions of different degree of saturations on artificially demineralized enamel
*p < .05
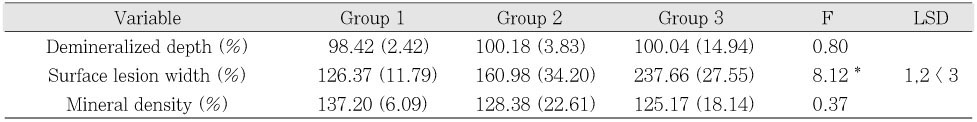
Quantitative value change (%) and the post-hoc result of enamel during de- and remineralization
Standard Deviation (SD) is in the parentheses.
*p < .05
Table 1
Initial composition of demineralization solution
Table 2
Initial composition of remineralization solution
Table 3
One-way ANOVA on the remineralizing features of pH 5.5 solutions of different degree of saturations on artificially demineralized enamel
*
Table 4
Quantitative value change (%) and the post-hoc result of enamel during de- and remineralization
Standard Deviation (SD) is in the parentheses. *

 KACD
KACD



 ePub Link
ePub Link Cite
Cite

