Articles
- Page Path
- HOME > Restor Dent Endod > Volume 31(6); 2006 > Article
- Original Article Aging effect on the microtensile bond strength of self-etching adhesives
- JS Park1, JS Kim1,2, MS Kim1, HH Son1,2, HC Kwon1,2, BH Cho1,2
-
2006;31(6):-426.
DOI: https://doi.org/10.5395/JKACD.2006.31.6.415
Published online: November 30, 2006
1Department of Conservative Dentistry, College of Dentistry, Seoul National University, Korea.
2Dental Research Institute, Korea.
- Corresponding author: Byeong-Hoon Cho. Department of Conservative Dentistry, College of Dentistry, Seoul National University, 28 Yeongeon-dong, Chongro-gu Seoul, Korea. 110-749. Tel: 82-2-2072-3514, Fax: 82-2-764-3514, chobh@snu.ac.kr
Copyright © 2006 Korean Academy of Conservative Dentistry
- 1,096 Views
- 0 Download
- 5 Crossref
Abstract
- In this study, the changes in the degree of conversion (DC) and the microtensile bond strength (MTBS) of self-etching adhesives to dentin was investigated according to the time after curing. The MTBS of Single Bond (SB, 3M ESPE, USA), Clearfil SE Bond (SE, Kuraray, Japan), Xeno-III (XIII, Dentsply, Germany), and Adper Prompt (AP, 3M ESPE, USA) were measured at 48h, at 1 week and after thermocycling for 5,000 cycles between 5℃ and 55℃. The DC of the adhesives were measured immediately, at 48h and at 7 days after curing using a Fourier Transform Infra-red Spectrometer. The fractured surfaces were also evaluated with scanning electron microscope. The MTBS and DC were significantly increased with time and there was an interaction between the variables of time and material (MTBS, 2-way ANOVA, p = 0.018; DC, Repeated Measures ANOVA, p < 0.001). The low DC was suggested as a cause of the low MTBS of self-etching adhesives, XIII and AP, but the increase in the MTBS of SE and AP after 48h could not be related with the changes in the DC. The microscopic maturation of the adhesive layer might be considered as the cause of increasing bond strength.
- 1. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16: 265-273.ArticlePubMed
- 2. Tantbirojn D, Cheng YS, Versluis A, Hodges JS, Douglas WH. Nominal shear or fracture mechanics in the assessment of composite-dentin adhesion? J Dent Res. 2000;79: 41-48.ArticlePubMedPDF
- 3. Dunn WJ, Soderholm KM. Comparison of shear and flexural bond strength tests versus failure modes of dentin bonding systems. Am J Dent. 2001;14: 297-303.PubMed
- 4. Wakefield CW, Draughn RA, Sneed WD, Davis TN. Shear bond strengths of six bonding systems using the pushout method of in vitro testing. Oper Dent. 1998;23: 69-76.PubMed
- 5. Wilder AD Jr, Swift EJ Jr, May KN Jr, Waddell SL. Bond strengths of conventional and simplified bonding systems. Am J Dent. 1998;11: 114-117.PubMed
- 6. Inoue S, Vargas MA, Abe Y, Yoshida Y, Lambrechts P, Vanherle G, Sano H, Van Meerbeek B. Microtensile bond strength of eleven contemporary adhesives to dentin. J Adhes Dent. 2001;3: 237-245.PubMed
- 7. Frankenberger R, Perdigao J, Rosa BT, Lopes M. "Nobottle" vs "multi-bottle" dentin adhesives - a microtensile bond strength and morphological study. Dent Mater. 2001;17: 373-380.ArticlePubMed
- 8. Tay FR, Gwinnett JA, Wei SH. The overwet phenomenon in two-component acetone-based primers containing aryl amine and carboxylic acid monomers. Dent Mater. 1997;13: 118-127.PubMed
- 9. Pashley DH, Ciucchi B, Sano H, Horner JA. Permeability of dentin to adhesive agents. Quintessence Int. 1993;24: 618-631.PubMed
- 10. Miyazaki S, Iwasaki K, Onose H, Moore BK. Enamel and dentin bond strengths of single application bonding systems. Am J Dent. 2001;14: 361-366.PubMed
- 11. Inoue S, Vargas MA, Abe Y, Yoshida Y, Lambrechts P, Vanherle G, Sano H, Van Meerbeek B. Microtensile bond strength of eleven contemporary adhesives to enamel. Am J Dent. 2003;16: 329-334.PubMed
- 12. Miyazaki M, Hirohata N, Takagaki K, Onose H, Moore BK. Influence of self-etching primer drying time on enamel bond strength of resin composites. J Dent. 1999;27: 203-207.ArticlePubMed
- 13. Sanares AM, Itthagarun A, King NM, Tay FR, Pashley DH. Adverse surface interactions between one-bottle light-cured adhesives and chemical-cured composites. Dent Mater. 2001;17: 542-556.ArticlePubMed
- 14. Suh BI, Feng L, Pashley DH, Tay FR. Factors contributing to the incompatibility between simplified-step adhesives and chemically cured or dual-cured composites. Part III. Effect of acidic resin monomers. J Adhes Dent. 2003;5: 267-282.PubMed
- 15. Sano H, Yoshikawa T, Pereira PN, Kanemur N, Morigami M, Tagami J, Pashley DH. Long-term durability of dentin bonds made with a self-etching primer, in vivo. J Dent Res. 1999;78: 906-911.ArticlePubMedPDF
- 16. Hashimoto M, Ohno H, Kaga M, Endo K, Sano H, Oguchi H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. J Dent Res. 2000;79: 1385-1391.ArticlePubMedPDF
- 17. Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. Degradation patterns of different adhesives and bonding procedures. J Biomed Mater Res B Appl Biomater. 2003;66: 324-330.ArticlePubMed
- 18. Asmussen E, Peutzfeldt A. Short- and Long-term bonding efficacy of a self-etching, one-step adhesive. J Adhes Dent. 2003;5: 41-45.PubMed
- 19. Tay FR, Pashley DH, Suh BI, Carvalho RM, Itthagarun A. Single-step adhesives are permeable membranes. J Dent. 2002;30: 371-382.ArticlePubMed
- 20. Meiers JC, Young D. Two-year composite/dentin bond stability. Am J Dent. 2001;14: 141-144.PubMed
- 21. Chersoni S, Suppa P, Grandini S, Goracci C, Monticelli F, Yiu C, Huang C, Prati C, Breschi L, Ferrari M, Pashley DH, Tay FR. In vivo and in vitro permeability of one-step self-etch adhesives. J Dent Res. 2004;83: 459-464.ArticlePubMedPDF
- 22. Ferracane JL, Greener EH. Fourier transform infrared analysis of degree of polymerization in unfilled resins--methods comparison. J Dent Res. 1984;63: 1093-1095.ArticlePubMedPDF
- 23. Imazato S, McCabe JF, Tarumi H, Ehara A, Ebisu S. Degree of conversion of composites measured by DTA and FTIR. Dent Mater. 2001;17: 178-183.ArticlePubMed
- 24. Yoshida K, greener EH. Effects of two amine reducing agents on the degree of conversion and physical properties of an unfilled light-cured resin. Dent Mater. 1993;9: 246-251.ArticlePubMed
- 25. Yoshiyama M, Carvalho R, Sano H, Horner J, Brewer PD, Pashley DH. Interfacial morphology and strength of bonds made to superficial versus deep dentin. Am J Dent. 1995;8: 297-302.PubMed
- 26. Pioch T, Stotz S, Buff E, Duschner H, Staehle HJ. Influence of different etching times on hybrid layer formation and tensile bond strength. Am J Dent. 1998;11: 202-206.PubMed
- 27. Perdigao J, May KN Jr, Wilder AD Jr, Lopes M. The effect of depth of dentin demineralization on bond strengths and morphology of the hybrid layer. Oper Dent. 2000;25: 186-194.PubMed
- 28. Cho BH, Dickens SH. Effects of the acetone content of single solution dentin bonding agents on the adhesive layer thickness and the microtensile bond strength. Dent Mater. 2004;20: 107-115.ArticlePubMed
- 29. Perdigao J, Lopes M, Geraldeli S, Lopes GC, Garcia-Godoy F. Effect of a sodium hypochlorite gel on dentin bonding. Dent Mater. 2000;16: 311-323.ArticlePubMed
- 30. Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20: 18-25.PubMed
- 31. Burrow MF, Takakura H, Nakajima M, Inai N, Tagami J, Takatsu T. The influence of age and depth of dentin on bonding. Dent Mater. 1994;10: 241-246.ArticlePubMed
- 32. Van Meerbeek B, Willems G, Celis JP, Roos JR, Braem M, Lambrechts P, Vanherle G. Assessment by nano-indentation of the hardness and elasticity of the resin-dentin bonding area. J Dent Res. 1993;72: 1434-1442.ArticlePubMedPDF
- 33. Lin C, Douglas WH. Failure mechanisms at the hyman dentin-resin interface: a fracture mechanics approach. J Biomech. 1994;27: 1037-1047.PubMed
- 34. Sano H, Takatsu T, Ciucchi B, Russell CM, Pashley DH. Tensile properties of resin-infiltrated demineralized human dentin. J Dent Res. 1995;74: 1093-1102.ArticlePubMedPDF
- 35. Armstrong SR, Keller JC, Boyer DB. Mode of failure in the dentin-adhesive resin-resin composite bonded joint as determined by strength-based (µTBS) and fracture-based (CNSB) mechanical testing. Dent Mater. 2001;17: 201-210.ArticlePubMed
- 36. Kim JS, Choi YH, Cho BH, Son HH, Lee IB, Um CM, Kim CK. The effect of light-cure time of adhesive resin on the thickness of the oxygen inhibited layer and the micro-tensile bond strength to dentine. J Biomed Mater Res B Appl Biomater. 2006;78(1):115-123.PubMed
- 37. Dickens SH, Cho BH. Interpretation of bond failure through conversion and residual solvent measurements and Weibull analyses of flexural and microtensile bond strengths of bonding agents. Dent Mater. 2005;21(4):354-364.ArticlePubMed
- 38. Carvalho RM, Chersoni S, Frankenberger R, Pashley DH, Prati C, Tay FR. A challenge to the conventional wisdom that simultaneous etching and resin infiltration always occurs in self-etch adhesives. Biomaterials. 2005;26: 1035-1042.ArticlePubMed
- 39. Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? Review. J Can Dent Assoc. 2003;69: 726-731.PubMed
- 40. Hashimoto M, Ohno H, Sano H, Tay FR, Kaga M, Kudou Y, Oguchi H, Araki Y, Kubota M. Micromorphological changes in resin-dentin bonds after 1 year of water storage. J Biomed Mater Res. 2002;63: 306-311.ArticlePubMed
- 41. Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent. 1999;27: 89-99.ArticlePubMed
- 42. Tay FR, Carvalho R, Sano H, Pashley DH. Effect of smear layers on the bonding of a self-etching primer to dentin. J Adhes Dent. 2000;2: 99-116.PubMed
- 43. Jung MK, Cho BH, Son HH, Um CM, Han YC, Choung SJ. Effect of additional coating of bonding resin on the microtensile bond strength of self-etching adhesives to dentin. J Korean Acad Conserv Dent. 2006;31: 103-112.Article
- 44. Uno S, Finger WJ. Function of the hybrid zone as a stress-absorbing layer in resin-dentin bonding. Quintessence Int. 1995;26: 733-738.PubMed
- 45. Choi KK, Condon JR, Ferracane JL. The effects of adhesive thickness on polymerization contraction stress of composite. J Dent Res. 2000;79: 812-817.ArticlePubMedPDF
- 46. Lambrechts P, Vanherle G. Microtensile bond strengths of one- and two-step self-etch adhesives to bur-cut enamel and dentin. Am J Dent. 2003;16: 414-420.PubMed
REFERENCES
Abbreviations:
BHT: 2,6-di-tert-butyl-p-cresol,
CQ: camphorquinone (2,3-bornanedione),
MDP: 10-methacryloxy methacrylate,
HEMA: 2-hydroxylethyl methacrylate,
Pyro-EMA-SK: tetra-methacryl-ethyl-pyrophosphate
PEM-F: Penta-methacryl-oxy-ethyl-cyclo-phosphazen-monofluoride,
UDMA: urethane dimethacrylate,
Bis-GMA: 2,2 bis [4-(2hydroxy3-methacryloyloxy propoxy) phenyl] propane
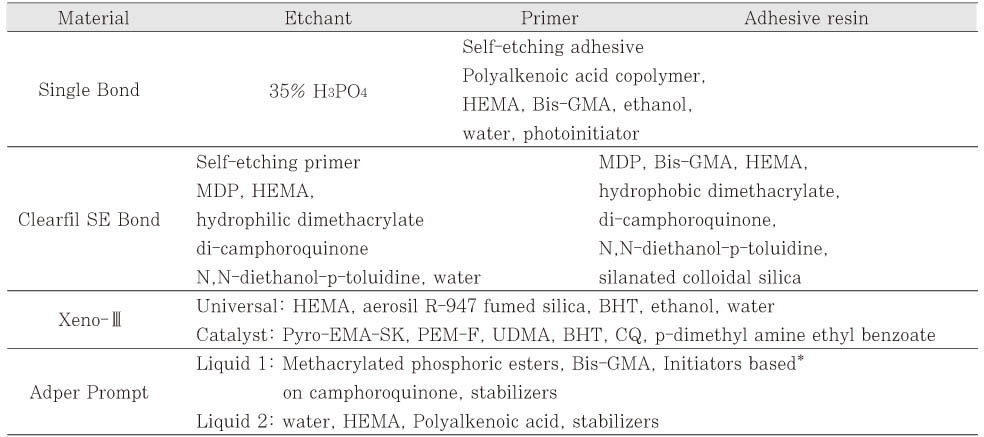
*The time at which the microtensile bond strength was measured using a universal testing machine.
**The specimens were thermally cycled 5000 times between 5℃ and 55℃ with 24 seconds of dwell time and 6 seconds of waiting time.
***The same superscript letters mean that there are no significant differences between groups, according to the adhesives (small letters) or the aging method (capital letters).
¶The results of Two-Way ANOVA suggested that the interaction effect of "adhesive * time" be expected in the measurements of the microtensile bond strength.
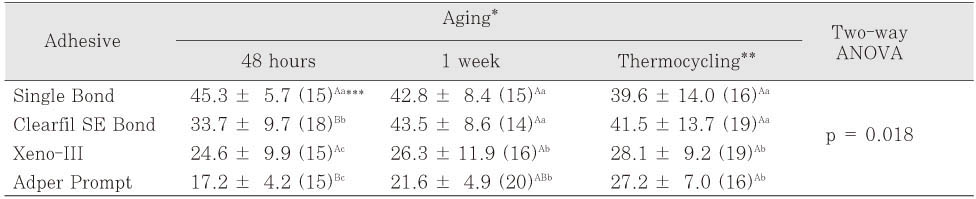
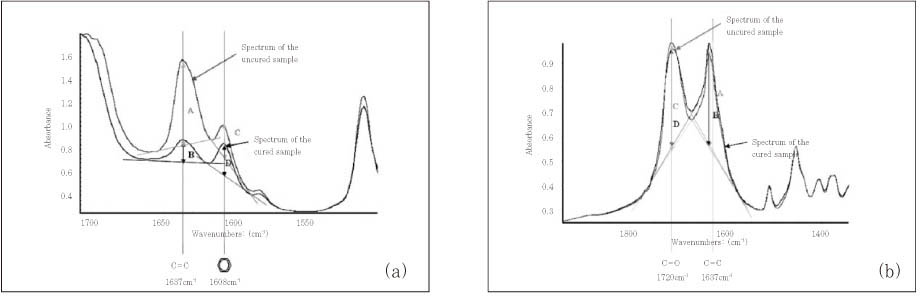
*The spectrum of the uncured adhesive was obtained before cure and those of the cured adhesives were obtained immediately after cure and at 48 hours and 1 week after cure.
**The same superscript letters mean that there are no significant differences between groups, according to the adhesives (small letters) or the aging period (capital letters).
§The internal references used for measuring degree of conversion were the absorbance peak at 1608 cm-1 of aromatic -C=C double bond for Single Bond, Clearfil SE Bond and Adper Prompt and that at 1720 cm-1 of carbonyl -C=O double bond for Xeno III, according to their base monomers.
¶The results of Repeated Measures ANOVA suggested that the interaction effect of "time * adhesive" be expected in the measurements of the degree of conversion.
Tables & Figures
REFERENCES
Citations

- Effect of Plasma Deposition Using Low-Power/Non-thermal Atmospheric Pressure Plasma on Promoting Adhesion of Composite Resin to Enamel
Geum-Jun Han, Jae-Hoon Kim, Sung-No Chung, Bae-Hyeock Chun, Chang-Keun Kim, Byeong-Hoon Cho
Plasma Chemistry and Plasma Processing.2014; 34(4): 933. CrossRef - The effect of priming etched dentin with solvent on the microtensile bond strength of hydrophobic dentin adhesive
Eun-Sook Park, Ji-Hyun Bae, Jong-Soon Kim, Jae-Hoon Kim, In-Bog Lee, Chang-Keun Kim, Ho-Hyun Son, Byeong-Hoon Cho
Journal of Korean Academy of Conservative Dentistry.2009; 34(1): 42. CrossRef - Effect of curing methods of resin cements on bond strength and adhesive interface of post
Mun-Hong Kim, Hae-Jung Kim, Young-Gon Cho
Journal of Korean Academy of Conservative Dentistry.2009; 34(2): 103. CrossRef - Difference in bond strength according to filling techniques and cavity walls in box-type occlusal composite resin restoration
Eun-Joo Ko, Dong-Hoon Shin
Journal of Korean Academy of Conservative Dentistry.2009; 34(4): 350. CrossRef - The effect of various bonding systems on the microtensile bond strength of immediate and delayed dentin sealing
Jin-hee Ha, Hyeon-Cheol Kim, Bock Hur, Jeong-Kil Park
Journal of Korean Academy of Conservative Dentistry.2008; 33(6): 526. CrossRef
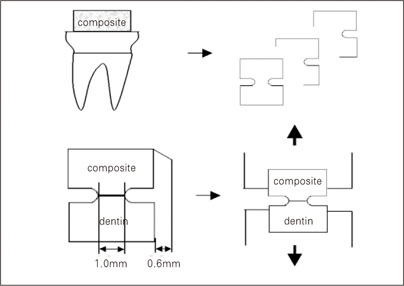
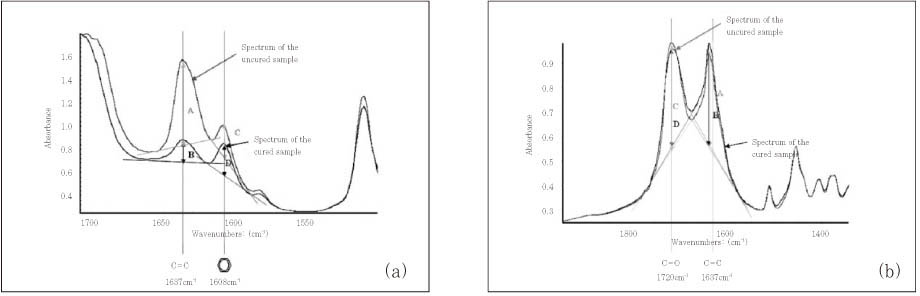
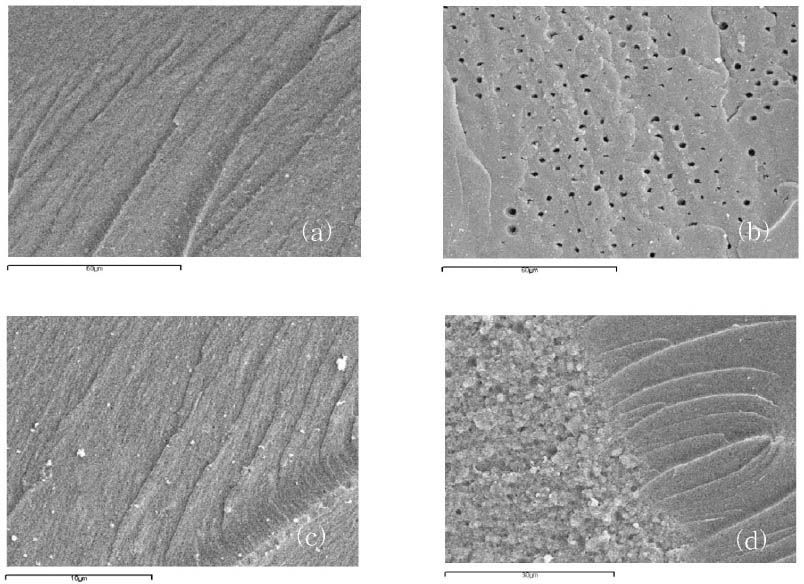
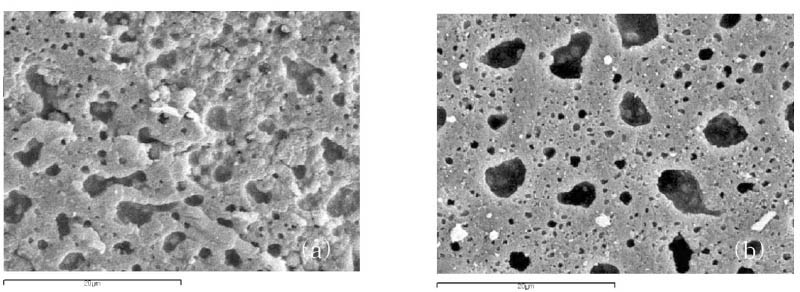
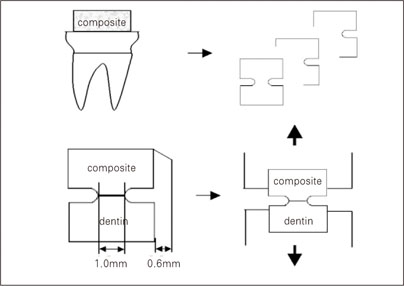
Figure 1
Figure 2
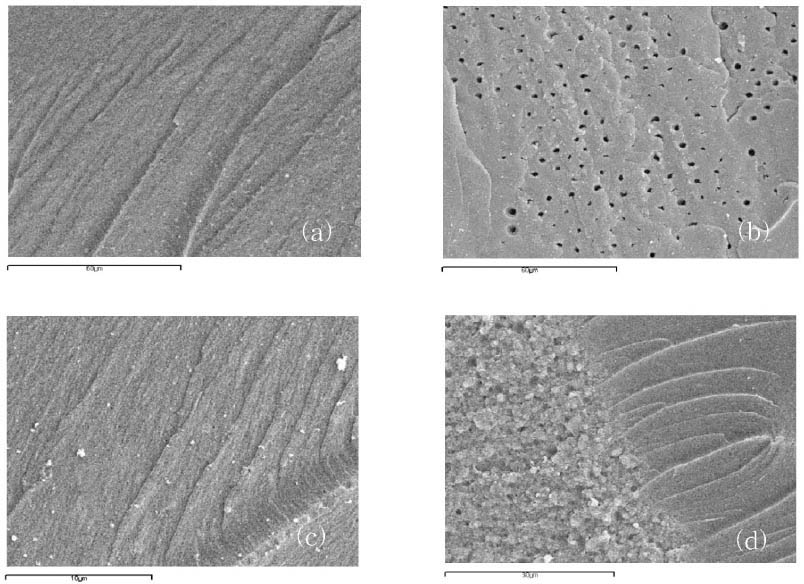
Figure 3
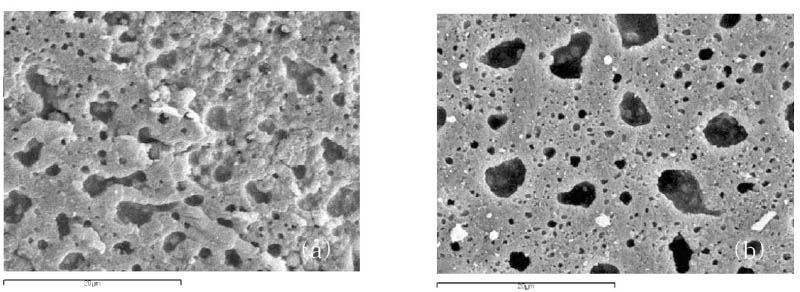
Figure 4
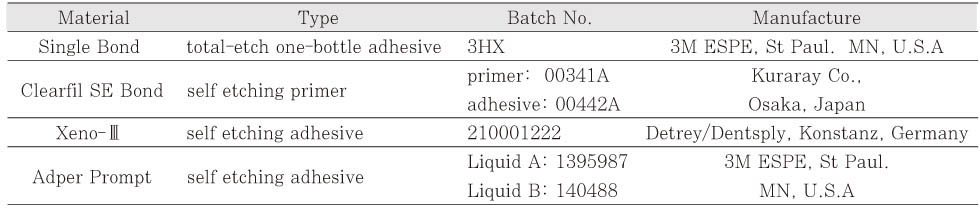
Dental adhesives used in this study
Compositions of dental adhesives used in this study
Abbreviations:
BHT: 2,6-di-tert-butyl-p-cresol,
CQ: camphorquinone (2,3-bornanedione),
MDP: 10-methacryloxy methacrylate,
HEMA: 2-hydroxylethyl methacrylate,
Pyro-EMA-SK: tetra-methacryl-ethyl-pyrophosphate
PEM-F: Penta-methacryl-oxy-ethyl-cyclo-phosphazen-monofluoride,
UDMA: urethane dimethacrylate,
Bis-GMA: 2,2 bis [4-(2hydroxy3-methacryloyloxy propoxy) phenyl] propane
Microtensile bond strength of dentin adhesives to superficial occlusal dentin at 48 hours and 1 week and after aging with thermocycling (Unit: MPa, mean ± standard deviation, The numbers in parentheses are those of the specimens tested)
*The time at which the microtensile bond strength was measured using a universal testing machine.
**The specimens were thermally cycled 5000 times between 5℃ and 55℃ with 24 seconds of dwell time and 6 seconds of waiting time.
***The same superscript letters mean that there are no significant differences between groups, according to the adhesives (small letters) or the aging method (capital letters).
¶The results of Two-Way ANOVA suggested that the interaction effect of "adhesive * time" be expected in the measurements of the microtensile bond strength.
The degree of conversion of four adhesives determined by mid-IR spectroscopy (Unit: %, mean ± standard deviation)
*The spectrum of the uncured adhesive was obtained before cure and those of the cured adhesives were obtained immediately after cure and at 48 hours and 1 week after cure.
**The same superscript letters mean that there are no significant differences between groups, according to the adhesives (small letters) or the aging period (capital letters).
§The internal references used for measuring degree of conversion were the absorbance peak at 1608 cm-1 of aromatic -C=C double bond for Single Bond, Clearfil SE Bond and Adper Prompt and that at 1720 cm-1 of carbonyl -C=O double bond for Xeno III, according to their base monomers.
¶The results of Repeated Measures ANOVA suggested that the interaction effect of "time * adhesive" be expected in the measurements of the degree of conversion.
Abbreviations: BHT: 2,6-di-tert-butyl-p-cresol, CQ: camphorquinone (2,3-bornanedione), MDP: 10-methacryloxy methacrylate, HEMA: 2-hydroxylethyl methacrylate, Pyro-EMA-SK: tetra-methacryl-ethyl-pyrophosphate PEM-F: Penta-methacryl-oxy-ethyl-cyclo-phosphazen-monofluoride, UDMA: urethane dimethacrylate, Bis-GMA: 2,2 bis [4-(2hydroxy3-methacryloyloxy propoxy) phenyl] propane
*The time at which the microtensile bond strength was measured using a universal testing machine. **The specimens were thermally cycled 5000 times between 5℃ and 55℃ with 24 seconds of dwell time and 6 seconds of waiting time. ***The same superscript letters mean that there are no significant differences between groups, according to the adhesives (small letters) or the aging method (capital letters). ¶The results of Two-Way ANOVA suggested that the interaction effect of "adhesive * time" be expected in the measurements of the microtensile bond strength.
*The spectrum of the uncured adhesive was obtained before cure and those of the cured adhesives were obtained immediately after cure and at 48 hours and 1 week after cure. **The same superscript letters mean that there are no significant differences between groups, according to the adhesives (small letters) or the aging period (capital letters). §The internal references used for measuring degree of conversion were the absorbance peak at 1608 cm-1 of aromatic -C=C double bond for Single Bond, Clearfil SE Bond and Adper Prompt and that at 1720 cm-1 of carbonyl -C=O double bond for Xeno III, according to their base monomers. ¶The results of Repeated Measures ANOVA suggested that the interaction effect of "time * adhesive" be expected in the measurements of the degree of conversion.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

