Abstract
-
The purpose of this study was to regenerate human dental pulp tissues similar to native pulp tissues. Using the mixture of type I collagen solution, primary cells collected from the different tissues (pulp, gingiva, and skin) and NIH 3T3 (1 × 105 cells/ml/well) were cultured at 12-well plate at 37℃ for 14 days. Standardized photographs were taken with digital camera during 14 days and the diameter of the contracted collagen gel matrix was measured and statistically analyzed with student t-test. As one of the pulp tissue engineering, normal human dental pulp tissue and collagen gel matrix cultured with dental pulp cells for 14 days were fixed and stained with Hematoxyline & Eosin.
According to this study, the results were as follows:
1. The contraction of collagen gel matrix cultured with pulp cells for 14 days was significantly higher than other fibroblasts (gingiva, skin) (p < 0.05).
2. The diameter of collagen gel matrix cultured with pulp cells was reduced to 70.4% after 7 days, and 57.1% after 14 days.
3. The collagen gel without any cells did not contract, whereas the collagen gel cultured with gingiva and skin showed mild contraction after 14 days (88.1% and 87.6% respectively).
4. The contraction of the collagen gel cultured with NIH 3T3 cells after 14 days was higher than those cultured with gingival and skin fibroblasts, but it was not statistically significant (72.1%, p > 0.05).
5. The collagen gel matrix cultured with pulp cells for 14 days showed similar shape with native pulp tissue without blood vessels.
This approach may provide a means of engineering a variety of other oral tissue as well and these cell behaviors may provide information needed to establish pulp tissue engineering protocols.
-
Keywords: Pulp tissue engineering; Pulp cells; Fibroblast; Tissue regeneration; Type I collagen gel; Matrix contraction
I. Introduction
Tissue engineering is strongly emerging as a new entity in medical and dental research. It has been greatly expected that tissue engineering concepts will be improved and its technique will give contribution to human health care. The new challenges of this field need the current knowledge concerning gene expression research over surgical techniques, cell biology, biomaterial science, chemistry, etc, and recent three groups of tools for tissue engineering of mineralized oral structures were shortly reviewed
1).
In vivo mesenchymal cells are surrounded by a three-dimensional extracellular matrix. Mesenchymal cells can produce matrix components and organize these components into higher order structures. Conversely, the extracellular matrix can provide important signals regulating cellular function, but it is not stable.
Dental pulp is a loose connective tissue, with extensive vascular and nerve supply, containing predominantly fibers and ground substance that make up the extracellular matrix (ECM), and cellular component including mainly fibroblasts and undifferentiated mesenchymal cells. Fibroblasts synthesize, organize and maintain connective tissues during development and in response to injury and fibrotic disease. The diverse 3 dimensional culture model system with fibroblast was introduced and the researches on fibroblast biology in three-dimensional collagen matrices were reviewed
2). After the pulp fibroblasts were isolated from the pulp tissues and seeded into polyglycoic acid (PGA) fiber matrix with static method, photomicrographs and scanning electron microscopy of the cell-seeded matrix were taken and its DNA contents were evaluated
3).
The uniquely specialized pulp cells (odontoblasts) are lining against the dentinal wall and their processes extend into the dentinal tubules. The volume of the mature pulp tissue is very small, and it appears relatively less difficult to regenerate this small volume of soft tissue than larger organs or tissues. However, it is considered rather difficult to regenerate the entire pulp tissue due to the following reasons: 1) the unique anatomical location of pulp tissue-enclosed in dentin having limited blood supply from one end for the in-growth of new tissue elements; 2) the small size of the pulpal canal space rendering a technically sensitive procedure to implant the regenerated pulpal tissue in vitro into the canal; and 3) the unique microscopic anatomy of pulp tissue, e.g., the odontoblasts and their relationship with the nerve fibers and vessels. While trying to regenerate the exact pulp tissue to its pristine condition appears difficult to achieve, regenerating a pulp connective tissue with vascular structure and nerve fibers appears possible.
The goal of tissue engineering is to regenerate living tissue substitutes in order to replace or enhance lost tissue function or structure. To accomplish this, the cells out of tissue must be organized and behave as if they are part of the original tissue by interacting with the surrounding ECM, which acts as a supporting material created by the cells and as a scaffolding on which to reside
4). The ECM is composed of a variety of macromolecules which can be grouped into four major classes (collagens, proteoglycans, cell interactive glycoproteins, and elastic fibers), each of which is responsible for specific ECM characteristics. Type I collagen is the most abundant type of collagen isolated from many adult connective tissues (skin, bone, and tendon). Pulp fibroblasts can produce both type I and type III collagens, whereas the majority of collagen molecules produced by odontoblasts are type I
5).
Synthetic matrices fabricated from naturally-derived (e.g., collagen) or synthetic materials (e.g., polyglycolic acid or PGA) are often utilized as a delivery vehicle for these cells and to guide the process of tissue formation
6). It has been shown by Rutherford's group (1999) that the fibroblast cultured from human adult dental pulps seeded onto PGA formed new tissue similar to that of native pulp after implanted into mouse dermis
7). They also found that when pulp cells were seeded onto the three different synthetic matrices (PGA fibers, type I collagen hydrogel, or alginate), the growth of the cells was moderate in collagen gel
8). Type I collagen gel has been used to measure the contraction of the collagen gel matrix mixed with bovine aortic endothelial cell
9) or human lung fibroblast
10).
In this study, to characterize the growth behavior of pulp cells in collagen matrix, bovine type I collagen was mixed with primary cells collected from the different tissues (pulp, gingiva, and skin) and NIH 3T3 (1×105 cells/ml/well) and the contraction of the collagen gel matrix in the time course was estimated. The result of this investigation may help to establish pulp tissue engineering protocol in vitro and in vivo.
II. Materials and method
Sample collection and Cell culture
Pulp cells or gingival fibroblasts or skin fibroblasts were collected and grown following the protocol described by Park
11). Third molar extractions or gingival tissue resection from patients receiving treatment at Kyung-Hee School of Dentistry, Department of Oral Surgery or Periodontal Clinic, followed a protocol approved by the Institutional Review Board, and human skin tissues from the Department of Plastic surgery at Kyung Hee Medical Center, Seoul, Korea.
Immediately after extraction, teeth were stored in phosphate buffered saline (PBS) and transported to the laboratory. Teeth were split open and the maximal amount of pulp tissue extracted and all kinds of the tissues (dental pulp and gingival, and skin tissues) were divided into several small fragments approximately 2 × 2 mm in size each. After repeated washing with PBS, the fragments were placed in a 60 mm culture dish containing Dulbecco's Modified Eagle Medium (DMEM; Life Technologies/GIBCO BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS).
Cells outgrown from the gingival or skin explants showing fibroblast-like phenotype were named human gingival fibroblasts (HGF) or human skin fibroblasts (HSF). Human pulp cells (HPC) from pulpal explants were similar to HGF morphologically, but showed more variation in size. These cells were allowed to reach confluence and passed at 1 : 2 ratio; passages 3 - 8 were used for experiments.
Cell culture media were supplemented with 100 units/ml penicillin-G, 100 mg/ml streptomycin, and 0.25 mg/ml fungizone (Gemini Bio-Products, Inc., Woodland, CA). In this study, primary cells of fibroblast-like phenotype outgrown from human pulp tissue and gingival tissue, and skin tissue (passage 3 - 5) were used for experiment and NIH 3T3 (ATCC CRL-165) grown in the same culture medium for the primary cells were used as a comparison.
Collagen gel preparation and collagen gel contraction assay
Each of the primary cells from human dental pulp tissues and gingival tissues, and skin tissues, at the concentration of 1 × 10
5 cells/ml/well, were mixed with bovine type I collagen gel solution (Vitrogen 100, Collagen Corp, Palo Alto, CA) according to Elsdale and Bard
12) with some modifications [8 parts type I collagen (3.97 mg/ml), 1 part 0.1N NaOH and 1 part 10 × DMEM], The DMEM supplemented with 10% fetal bovine serum and with 100 units/ml penicillin-G, 100 mg/ml streptomycin, and 0.25 mg/ml fungizone (Gemini Bio-Products, Inc., Woodland, CA) were used as a liquid medium, and collagen solution was placed in a microcentrifuge tube kept on ice to inhibit gelation of the collagen mixture. This neutralized, isotonic collagen solution was used to minimize injury of the primary cells. The mixture of the primary cells and collagen gel were placed into 12-well plates (500 ml/well) and incubated at 37℃ for 30 min to allow gelation. Culture medium (500 ml/well) was then added into each well rendering cell/collagen mixture lifted from the bottom and suspended in the medium. The plates were incubated at 37℃ with 5% CO
2 and the medium changed every 2-3 days. Cell culture was monitored everyday for 14 days using an inverted microscope and standardized photographic pictures were taken after 1/2 day (12 hours), 1 day, 3 days, 5 days, 7 days, 14 days. The diameters of the collagen matrix after shrinkage of the gels were measured with Image-pro plus software (Media Cybernetics, Silver spring, Maryland) at each time. The experiment was repeated twice and analyzed statistically with SPSS (version 11.5).
Immediately after the teeth were extracted, some of them were moved to the clean bench in the laboratory. Teeth were split into two pieces, and then pulp tissue fragments (n = 2) were collected with cotton plier carefully. Those pulp tissue fragments were processed for the histological evaluation.
After the collagen gel contraction assay, the pulp tissue fragments and the pulp cell-seeded collagen gel matrix were fixed in 10% phosphate neutralized formalin for 2 - 3 days, dehydrated in graded ethanol solutions, and embedded in paraffin. Sections (8 µm in thickness), were cut with a microtome and stained with hematoxylin and eosin.
III. Results
Collagen gel contraction assay
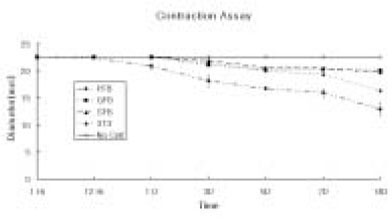
The contractive response of adult human pulp cells, gingival fibroblasts, skin fibroblasts, and NIH 3T3 cells mixed three-dimensionally in the type I collagen gel were evaluated during 14 days. The neutralized, isotonic bovine type I collagen gel solution was mixed with the primary cells, and the mixture of the primary cells and collagen gel were placed into 12-well plates (500 µl/well) and incubated at 37℃ with 5% CO
2 for 30 minutes to allow gelation. Culture medium (500 µl/well) was then added into each well rendering cell/collagen mixture lifted from the bottom and suspended in the medium. Unless the collagen gels were detached from the plate, they may not shrink. After additional 30 minutes of the incubation, the diameters of the collagen matrix after shrinkage of the gels were measured with Imagepro plus software. There was no contraction of collagen gel matrix without any cells during the entire experimental time (
Figure 1). Because the phenotype of the primary cells were round shape, which means they were not attached to the collagen gel three-dimensionally after the incubation at 37℃ for one hour, each of the collagen gel matrix were not contracted at all (
Figures 1 and
2).
12 hours and 1 day after the collagen gel matrix were incubated at 37℃, the diameter of the collagen gel were same with the collagen gel matrix after 1 hour incubation except pulp fibroblasts (22.4 mm, 12 hours and 20.85 mm, 1 day,
Figures 1,
3 and
4).
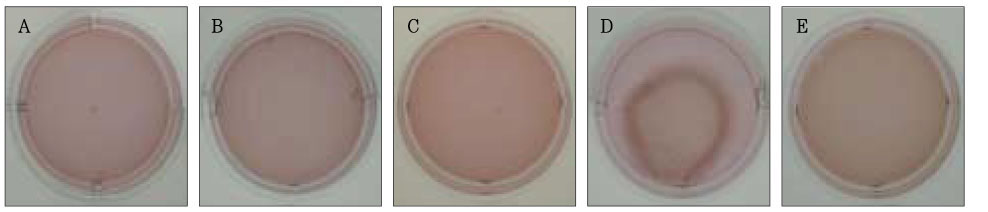
The collagen gel matrix seeded with gingival fibroblasts and skin fibroblasts and NIH 3T3 cells started to shrink after 3 days; nevertheless, the contraction was minimal (
Figure 5).
The contraction was dramatic in the collagen gel matrix seeded with the human pulp cells after 14 days (57.1%) compared with the contraction of the collagen gel seeded with other cells (gingival fibroblasts and skin fibroblasts,
Figures 1 and
6).
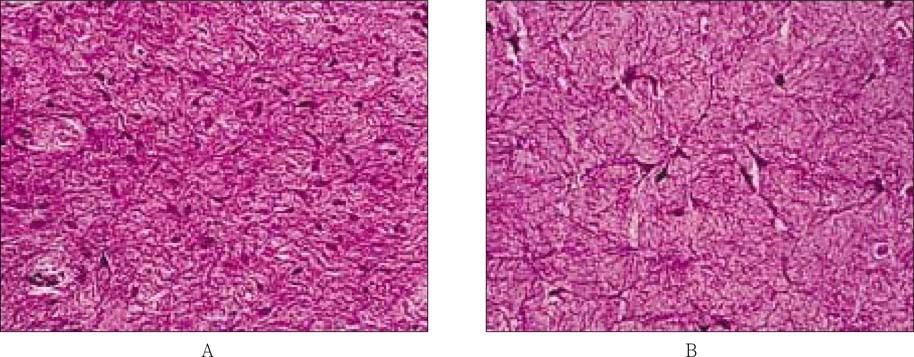
After the contraction of collagen gel matrix seeded with pulp fibroblast during 14 days, human natural adult pulp and contracted collagen gel matrix were processed, embedded, sectioned, and H&E stained for the histological finding. The stained samples were evaluated under the light microscope, and the microphotographs of the two samples were taken with × 400 magnifications.
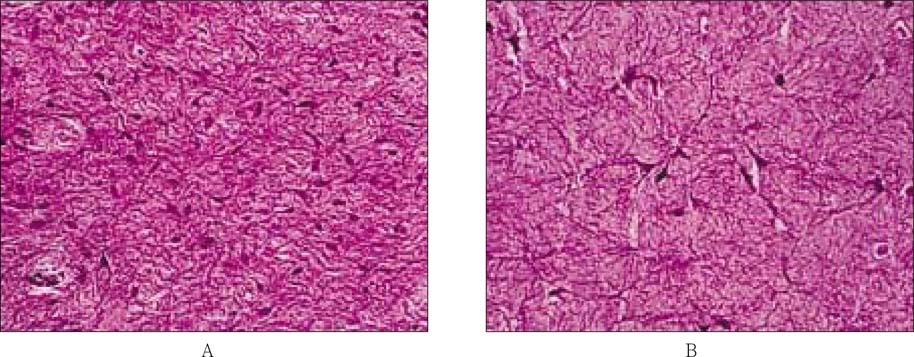
The normal adult pulp had lots of the spindle shape pulp cells, and small blood vessel, which were scattered within the collagen matrix (
Figure 7, A), whereas engineered pulp tissue had only pulp cells which was alive spindle shape without blood vessels (
Figure 7, B). Those two matrix look similar with each other.
IV. Discussion
This is the first investigation to compare the contraction of type I collagen in Vitro by culturing human pulp cells, and gingival fibroblasts, skin fibroblasts, and mouse cell line (NIH 3T3 cells) three-dimensionally. The use of type I collagen gels did not lead to the development of new tissues which resembled native pulp, while the cells adhered and proliferated in the collagen gel.
Collagen-Glycosaminoglycan matrix seeded with porcine dental pulp cells
in Vitro decreased in size to less than half of their original diameter by 28 days and the level of a-Smooth-muscle action (SMA) increased with passage number, so SMA containing pulp cells had the capability to contract a collagen-glycosaminoglycan analog of extracellular matrix
in vitro13).
Type I collagen has been widely utilized to engineer a variety of tissue type due to its ability to promote cell adhesion and allow cell-based remodeling. Type I collagen was the most abundant type of collagen isolated from many adult connective tissues (skin, bone, and tendon)
1), and out of the collagen molecules occurring in the pulp type I and III represent the bulk of the pulp tissue collagen. Because type I is the predominant type and may contribute to the establishment of the architecture of the pulp, in this study commercial bovine type I collagen was purchased and used as the scaffolds to grow the pulp fibroblast, gingival fibroblasts, skin fibroblasts, and NIH 3T3 cells three-dimensionally.
Human lung fibroblasts were cast into two different density of type I rat tail collagen (0.75 mg/ml and 2 mg/ml), the size of the lower density gel after 15 days was smaller than the higher density one
10). The contraction of type I collagen by bovine aortic endothelial (BAE) cells and human dermal fibroblast was compared in collagen gel contraction assay, the fibroblasts contracted rigid gels more effectively in comparison to BAE cells
9).
Human dental pulp cells contracted significantly comparing with NIH 3T3 cells in rat tail type I collagen, nevertheless the proliferation rate of the NIH 3T3 cells cultured in culture dishes and type I collagen gel increased more than human dental pulp cells
13). The basis of this reduced cellular proliferation rate on the collagen matrices was not identified but seemed to depend on fibrillar organization and required the native (triple helical) conformation of the collagen molecule
14). The more contraction of the collagen gels were made with the increased number of the pulp cells mixed with the same condition of collagen solution, nor contraction without any cells
13).
When skin fibroblasts are cultured on or in collagen gels, there was a significant reduction in their rate of proliferation
14). Skin fibroblast cultured in the collagen gel and culture dish with human serum had the more proliferation rate than bovine serum, and platelet-derived growth factor reduced mitotic rate observed with the cells on collagen gels
15).
When the porous absorbable polymeric scaffolds were used to seed the cells, the diverse seeding methods were introduced
16), and previous works were reported about using alginate hydrogels for bony regeneration
17) and tooth mold coated with collagen gel for complex tooth structure engineering
18).
The pulp tissue engineering may ultimately find clinical application as a novel approach to repair and/or regenerate dental pulp, and they may also provide a useful system to assess the biocompatibility of chemicals utilized in dental practice which come in contact with native pulp. It is possible that engineered dental pulp tissue will provide a model system in which reparative dentinogenesis can be studied. This work may also provide the first step to engineer an entire tooth, and these cell behaviors on the collagen matrix may provide information needed to establish pulp tissue engineering protocols.
V. Conclusion
The purpose of this study was to reveal if the bovine type I collagen can be used as scaffold to grow human dental pulp fibroblast, gingival fibroblast, skin fibroblast, and mouse skin fibroblasts (NIH 3T3 cells) and to characterize the behaviors of those cells on the collagen gel matrix. For the purpose, the contraction rate of the collagen matrix was measured for 14 days after those cells were mixed with the type I collagen solution three-dimensionally and the collagen gel plated with pulp fibroblast were evaluated histologically.
According to this study, the results were as follows:
The contraction of collagen gel matrix cultured with pulp cells for 14 days was significantly higher than other fibroblasts (gingiva, skin) (p < 0.05).
The diameter of collagen gel matrix cultured with pulp cells was reduced to 70.4% after 7 days, and 57.1% after 14 days.
The collagen gel without any cells did not contract, whereas the collagen gel cultured with gingiva and skin showed mild contraction after 14 days (88.1% and 87.6%).
The contraction of the collagen gel cultured with NIH 3T3 cells after 14 days was higher than those cultured with gingival and skin fibroblasts, but it was not statistically significant (72.1%, p > 0.05).
The collagen gel matrix cultured with pulp cells for 14 days showed similarity to native pulp tissue without blood vessels.
These data indicate that pulp cells contract the collagen fibers extensively, compared with other cells (gingiva, skin) and NIH 3T3 cells. The collagen gel matrix cultured with pulp cells showed similar histological appearance with native pulp tissue.
This approach may provide a means of engineering a variety of other oral tissue as well and these cell behaviors may provide information needed to establish pulp tissue engineering protocols.
-
This paper was supported by Wonkwang Health Science College in 2001.
REFERENCES
- 1. Dard M, Sewing A, Meyer J, Verrier S, Roessler S, Scharnweber D. Tools for tissue engineering of mineralized oral structures. Clin Oral Investig. 2000;4: 126-129.ArticlePubMedPDF
- 2. Mooney DJ, Powell C, Piana J, Rutherford B. Engineering dental pulp-like tissue in Vitro. Biotechnol Prog. 1996;12: 865-868.ArticlePubMed
- 3. Grinnell . Fibroblast biology in three-dimensional collagen matrix. Trends in Cell Biology. 2003;13: 264-269.ArticlePubMed
- 4. Mikos AG, Mcintire LV. Frontiers in tissue engineering. 1998;Pergamon. Houston. TX.
- 5. Hargreaves KM, Goodis HE. Seltzer and Bender's Dental Pulp. 2002;Chicago. IL: Quintessence Publishing Co. Inc.
- 6. Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nature Med. 1996;2: 824-826.ArticlePubMedPDF
- 7. Buurma B, Gu K, Rutherford R. Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur J Oral Sci. 1999;107: 282-289.ArticlePubMedPDF
- 8. Bohl KS, Shon J, Rutherford B, Mooney DJ. Role of synthetic extracellular matrix in development of engineered dental pulp. J Biomater Sci Polym Ed. 1998;9: 749-764.ArticlePubMed
- 9. Vernon RB, Sage EH. Contraction of fibrillar type I collagen by endothelial cells: A study in vitro. J Cell Biochem. 1996;60: 185-197.ArticlePubMed
- 10. Zhu YK, Umino T, Liu XD, Wang HJ, Romberger DJ, Spurzem JR, Rennard SI. Contraction of fibroblast-containing collagen gels: initial collagen concentration regulates the degree of contraction and cell survival. In Vitro Cell Dev Biol Anim. 2001;37: 10-16.ArticlePubMed
- 11. Park SH. Culturing the human dental pulp cells in the collagen matrix and on the ground tooth surface. J Korean Acad Conserv Dent. 2003;28: 419-424.Article
- 12. Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54: 626-637.ArticlePubMedPMCPDF
- 13. Brock DP, Marty-Raix R, Spector M. a-Smooth-muscle actin in and contraction of porcine dental pulp cells. J Dent Res. 2002;81: 203-208.ArticlePubMedPDF
- 14. Schor SL. Cell proliferation and migration on collagen substrata in vitro. J Cell Sci. 1980;41: 159-175.ArticlePubMedPDF
- 15. Rhudy RW, Mcpherson JM. Influence of the extracellular matrix on the proliferative response of human skin fibroblasts to serum and purified platelet-derived growth factor. J Cell Physiol. 1988;137: 185-191.ArticlePubMed
- 16. Burg KJL, Holder WD, Culberson CR, Beiler RJ, Greeene KG, Loebsack AB, Roland WD, Eiselt P, Mooney DJ, Halberstadt CR. Comparative study of seeding methods for three-dimensional polymeric scaffolds. J Biomed Mater Res. 2000;51: 642-649.ArticlePubMed
- 17. Alsberg E, Anderson KW, Alberiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80: 2025-2029.ArticlePubMedPDF
- 18. Young CS, Terada S, Vacanti JP, Honda M, Barrtlett JD, Yelick PC. Tissue engineering of complex tooth stuructures on biodegradable polymer scaffolds. J Dent Res. 2002;81: 695-700.ArticlePubMedPDF
Figure 1Collagen Gel Contraction Assay. Changes in the diameter of the different cell-seeded collagen gel matrix. 1 × 105 cells of the primary cultured gingival fibroblasts (GFB), skin fibroblast (SFB), NIH 3T3 cells (3T3) were mixed with type I collagen gel solution. Only collagen gel solution (500 µl/well) were plated as control, and the cell-seeded collagen gel solution (500 µl/well) were plated in the 12-well-plates. The values of the diameter represent the mean ± standard deviation calculated from two-samples at each point for 14 days.
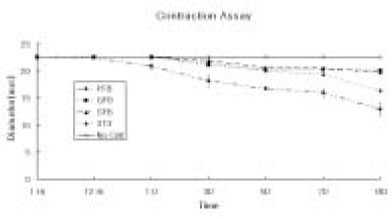
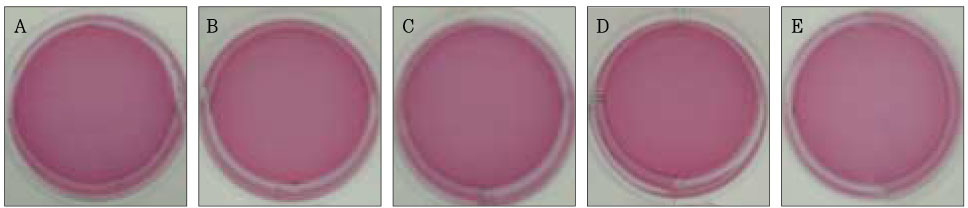
Figure 2Photographs of collagen gel matrix 1 hour after the collagen gel solution without cells (A), 1 × 105 cells of the primary cultured gingival fibroblasts (B), skin fibroblasts (C), pulp cells (D), and NIH 3T3 cells (E) were plated in the 12-well plates.
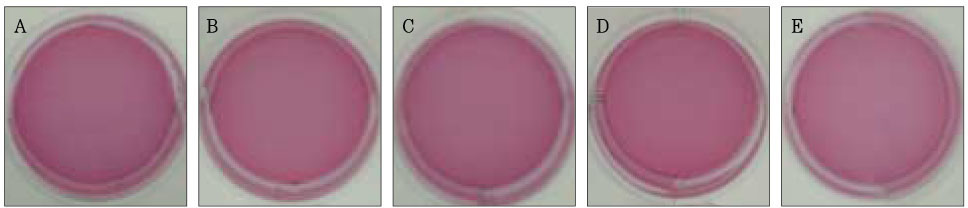
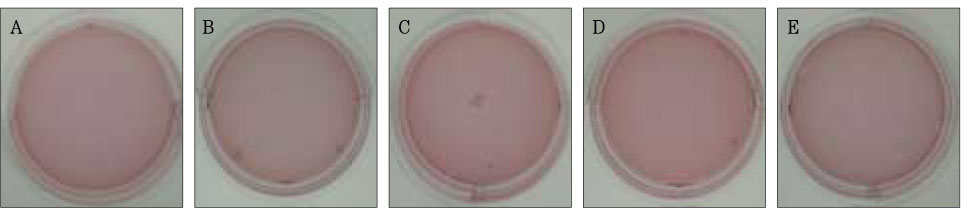
Figure 3Photographs of collagen gel matrix 1 day after the collagen gel solution without cells (A), 1 × 105 cells of the primary cultured gingival fibroblasts (B), skin fibroblasts (C), pulp cells (D), and NIH 3T3 cells (E) were plated in the 12-well plates.
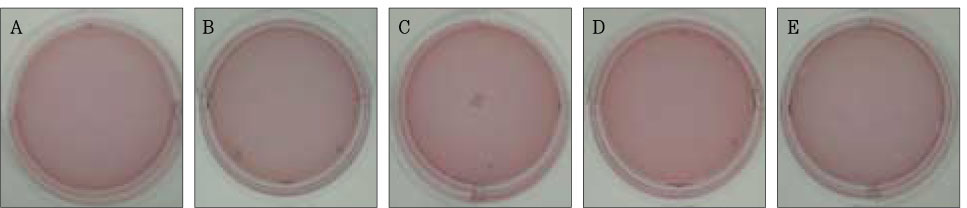
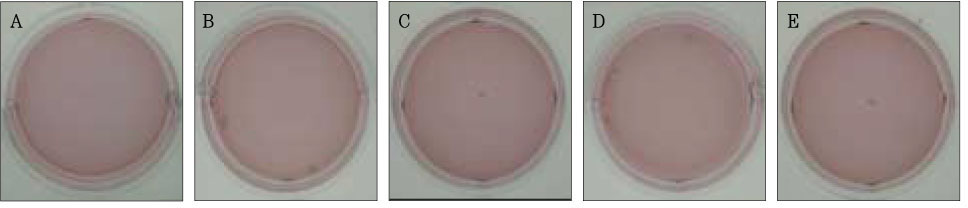
Figure 4Photographs of collagen gel matrix 3 days after the collagen gel solution without cells (A), 1 × 105 cells of the primary cultured gingival fibroblasts (B), skin fibroblasts (C), pulp cells (D), and NIH 3T3 cells (E) were plated in the 12-well plates.
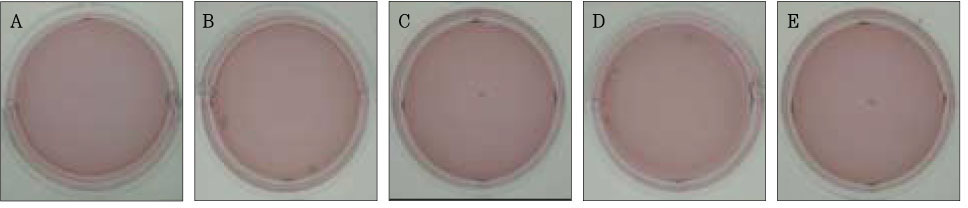
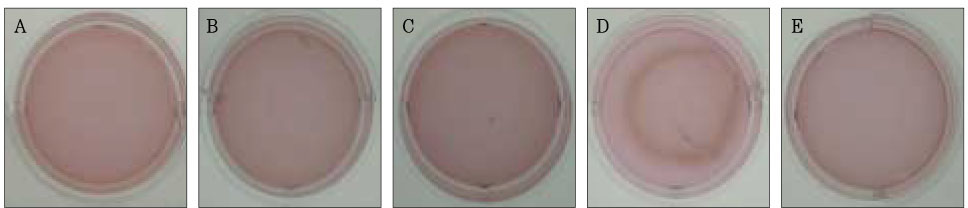
Figure 5Photographs of collagen gel matrix 5 days after the collagen gel solution without cells (A), 1 × 105 cells of the primary cultured gingival fibroblasts (B), skin fibroblasts (C), pulp cells (D), and NIH 3T3 cells (E) were plated in the 12-well plates.
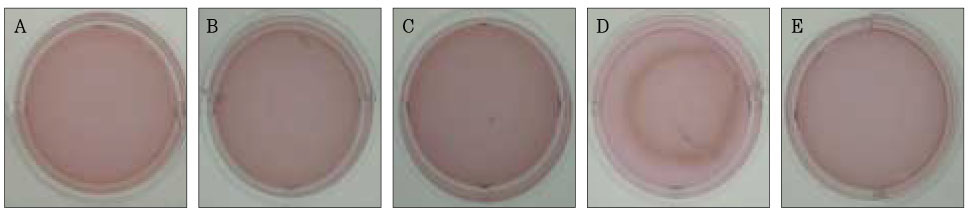
Figure 6Photographs of collagen gel matrix 7 days after the collagen gel solution without cells (A), 1 × 105 cells of the primary cultured gingival fibroblasts (B), skin fibroblasts (C), pulp cells (D), and NIH 3T3 cells (E) were plated in the 12-well plates.
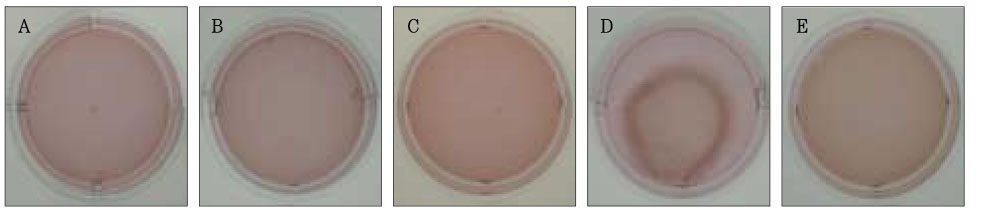
Figure 7Microphotographs of histological sections of the normal adult pulp (A) and engineered pulp tissue (B) stained with hematoxylin and eosin. The original magnifications of the photographs were × 400.







 KACD
KACD


 ePub Link
ePub Link Cite
Cite

