Articles
- Page Path
- HOME > Restor Dent Endod > Volume 27(2); 2002 > Article
- Original Article Antimicrobial activity of eight root canal sealers before and after setting
- Denny Y Fang*, WooCheol Lee*, Chern H Lai**
-
2002;27(2):-211.
DOI: https://doi.org/10.5395/JKACD.2002.27.2.207
Published online: March 31, 2002
*Department of Endodontics, University of Pennsylvania, School of Dental Medicine, USA.
**Department of Periodontics & Microbiological Testing Laboratory, University of Pennsylvania, School of Dental Medicine, USA.
Copyright © 2002 Korean Academy of Conservative Dentistry
- 965 Views
- 1 Download
I. Introduction
The goal of endodontic treatment is to eliminate or to lower the bacterial concentration gradient from the root canal and to prevent reinfection1,2). In addition to cleaning and shaping procedure, root canal filling material may play a critical role in destroying bacteria which remained in the root canal system. Killing bacteria by root canal filling procedure is either done by the hermetic obturating seal or by the direct bactericidal properties of the obturating materials. Several endodontic sealers have been found to possess antibacterial properties depending on their chemical components, such as calcium hydroxide, eugenol, and fluoride3,4,5). Different types of sealers have been introduced in the market and several studies have been done to evaluate antimicrobial effect of those sealers6). However, data were limited to the initial antimicrobial activity only because the highest microbial inhibition is thought to occur immediately after the sealer has been mixed and to decrease as it becomes hard. Therefore, it is important to compare the antibacterial activities of endodontic sealers before and after setting.
The purpose of this study is to compare the antimicrobial potential among the eight commercially available sealers by using the agar diffusion test. The bacterial species tested against were Enterococcus faecalis and Staphylococcus aureus. This study also compared the freshly mixed and one-week set to evaluate if the antimicrobial effect sustained once the sealers have completely set.
II. Materials and Methods
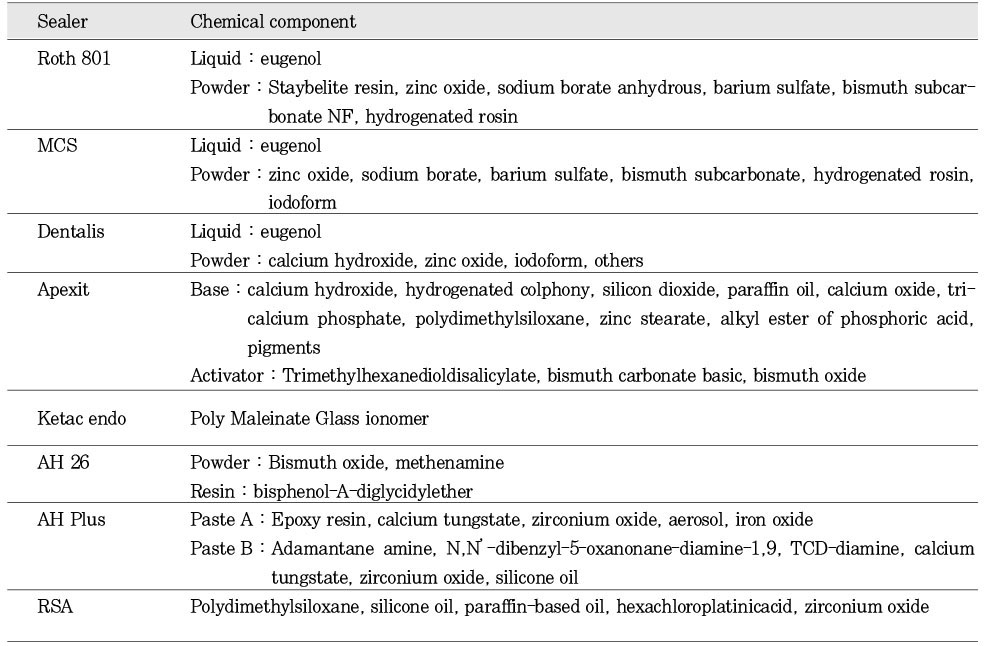
The sealers used in this study were : Roth 801 (Roth Int., Chicago, IL), MCS (Lone Star Tech., Westport, CT), Dentalis (DiaDent Group Int., B.C., Canada), Apexit (Vivadent Ets., Schaan, Liechtenstein), Ketac Endo (ESPE, Norristown, PA), AH 26 (Dentsply De Trey, Konstanz, Germany), AH Plus (Dentsply De Trey, Konstanz, Germany), and RSA (Roeko, Langenau, Germany). The chemical components of each sealer were listed in Table 1. Each of the eight sealers was mixed according to its manufacture's instruction. Each sealer was divided into two groups; dry and wet group. The dry group was the sealer-coated paper disk (Becton Dickinson, Cockeysville, MD), which was set and stored in a sterile petri dish for one week. The wet group was the paper disk coated with the freshly mixed sealer prior to the agar diffusion experiment.
An agar diffusion test was used to evaluate the bacterial inhibition of each sealer. Two facultative anaerobes, Enterococcus faecalis (ATCC 29212) and Staphylococcus aureus (ATCC 29213) were tested. All the straines were obtained from the Department of Periodontics & Microbiological Testing Laboratory, University of Pennsylvania, School of Dental Medicine. 200µl of bacterial suspension (McFarland Standard 0.5=150 ×106 cells) were spread on Brucellar blood agar plates. Both groups of sealer incorporated paper disks, dry and wet, were placed in the center of the plates. As a control, a disk saturated with normal saline solution was placed on the plate for each series of experiment. After 35℃ incubation in an anaerobic chamber containing a mixture of gas with 80% N2, 10% CO2 and 10% H2 for 48 hours, the agar plates were examined for bacterial growth inhibition. The diameter of the inhibition zone formed beyond the 6mm paper disk was measured by millimeters. Greater diameter of zone of inhibition was interpreted to indicate greater antimicrobial activity of the involved sealers. For consistency of the result, each sealer was retested for three times.
The Fisher's PLSD analysis was used to detect any significant difference between two different sealers and the conditions (between dry and wet) of each sealer.
III. Results
The saline saturated disk did not exhibit zones of inhibition when tested against either E. faecalis or S. aureus.
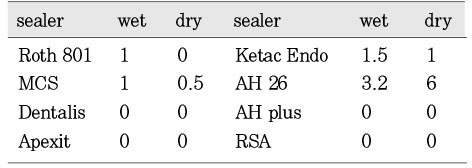
Fig. 2 showed the mean inhibition diameter of the wet and dry group sealers against Enterococcus faecalis. The Fisher's PLSD analysis found both one-week set (dry) and freshly mixed (wet) AH26 have significantly higher antimicrobial activity than the dry and wet Roth 801, dry MCS, dry and wet Dentalis, dry and wet Apexit, dry Ketac Endo, dry and wet AH Plus, and the dry and wet RSA (p<0.05). Only the dry AH26 has significantly greater antimicrobial activity than the wet AH 26, the wet Ketac Endo and the wet MCS. Fig. 1 demonstrated the inhibition zone produced by dry AH26 The dry Roth 801, dry and wet Dentalis, dry and wet Apexit, dry and wet AH Plus, and dry and wet RSA have no antimicrobial activity against E. faecalis.
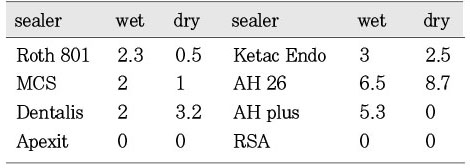
Fig. 3 showed the mean inhibition diameter of the wet and dry group sealers against Staphylococcus aureus. The Fisher's PLSD analysis found the dry AH26 has significantly better antimicrobial activity than the dry and wet Roth 801, dry and wet Apexit, dry and wet AH Plus, and the dry and wet RSA (p<0.05). And the wet AH26 is significantly better than the dry AH Plus. The dry and wet Apexit, dry AH Plus, and dry and wet RSA have no antimicrobial activity against S. aureus.
IV. Discussion
This study demonstrated eight different sealers in their antimicrobial capability by using the agar diffusion test. The bacterial strains tested against were E. faecalis and S. Aureus. These bacteria were selected because they were highly resistant to calcium hydroxide treatment8,9). They both can survive in a harsh environment. Especially, E. faecalis is able to survive longer than seven days without any nutrient10). E. faecalis is also a common single isolate from the canals of the refractory cases9). When tested against E. faecalis, with the release of formaldehyde as an antimicrobial agent7,11), AH26 has significantly stronger bacterialcidal effect than Roth 801, MCS, Dentalis, Apexit, Ketac Endo, AH Plus, and RSA. The sealers, Dentalis, Apexit, AH Plus and RSA, do not provide any antimicrobial effect against E. faecalis. There is no significant difference between the freshly mixed sealer and the one-week set sealer in terms of its sustaining antimicrobial effect. Except in the group of AH26, the oneweek set sealer has significantly higher bacterialcidal effect than the freshly mixed. We assume that antimicrobial component in the AH26 may release in higher concentration once the sealer has completely set. This is a similar phenomenon to Al-Khatib's5) experiment of agar diffusion test against Bacteroids endodontalis on 7-days and 35-days.
When tested against the weaker bacteria strain S. aureus as a comparison to the E. faecalis group, most of the sealers perform equally well in their antimicrobial activity except Apexit and RSA sealer groups. The RSA sealer does not provide any antimicrobial capability because none of its chemical components has bacterialcidal effect. The chemical component of the RSA sealer is very similar to the silicone based Endo-Fill sealer which Görduysus12) also found no antimicrobial capability. The fact that Apexit did not show any microbial inhibition corroborates those observed by Duarte et al.13) who found that Apexit produced least alkaline pH and calcium ion compared with other two calsium hydroxide-based sealers such as sealapex and sealer 26. Optimal pH for growth of S. aureus is between 7 and 7.5 with a 4.2 to 9.3 range. The result in this study confirmed that alkaline pH produced by Apexit did not exceed this value, thus allowing this microorganism to grow7). Glass ionomer cement possess strong antibacterial properties14,15), it is mainly due to a fluoride and other ingradients. Ketac Endo is a newly developed glass ionomer-based sealer. According to the recent studies16,17,18), this sealer was superior in its ease of manipulation, radiopacity, and setting time compared to Grossman's sealer as well as its adaptation to the canal walls. The data of the current study indicated that Ketac Endo did possess antibacterial activity against both tested microorganisms. Our data also consist with Siqueira's findings6) that ZOE-based sealers demonstrate a strong bacterial inhibition, which seems related to a high concentration of eugenol.
The Agar diffusion test used to evaluate antibacterial activity of sealers is the most commonly used technique6,15). This method is relatively insensitive and data are highly dependent on molecular size and the diffusion constant of the antimicrobial component, inoculum size, incubation time, and degree of material-agar contact19). Moreover, since the antimicrobial substance must diffuse through the aqueous agar medium, only water soluble agents can be tested. Therefore, Agar diffusion technique has some type of limitation and the interpretation of data obtained by this test should be reevaluated.
AH 26 and AH Plus are basically same material. The difference between them lies in the presence of silicone and aerosol in the fomula as well as the elimination of formaldehyde release from the latter material. Even though this study proved the superiority of antimicrobial acitivity of the AH26 sealer over the others, its cytotoxicity effect from the release of the formaldehyde cannot be neglected20). Careful usage and manipulation of this sealer should be considered. It is recommended that maintain the sealer inside the canal system and contact with the periapical tissue as little as possible.
- 1. Kakehashi S, Stanley HR, Fizgerald RJ. The effects of surgical exposure of dental pulps in germfree and converntional rats. Oral surg. 1965;20: 340-349.PubMed
- 2. Sundqvist G. Bacteriological studies of necrotic dental pulps. Umea University Odontol Dissertation No. 7. 1976;Sweden: Umea.
- 3. Pumarola J, Berastegui E, Brau E, Canalda C, Anta MTJ. Antimicrobial activity of seven root canal sealers. Oral Surg. 1992;74: 216-220.ArticlePubMed
- 4. Orstravik D. Antibacterial properties of root canal sealers, cements and pastes. Int Endod J. 1981;14: 125-133.ArticlePubMed
- 5. Al-Khatib ZZ, Baum RH, Morse DR, Tesilsoy C, Bhambhani S, Furst ML. The Antimicrobial effect of various endodontic sealers. Oral surg. 1990;70: 784-790.ArticlePubMed
- 6. Siqueira JF, Goncalves RB. Antibacterial activities of root canal sealers against selected anaerobic bacteria. J Endod. 1996;22: 79-80.Article
- 7. Kaplan AE, Picca M, Gonzalez MI, Macchi RL, Molgatini SL. Antimicrobial effect of six endodontic sealers: an in vitro evaluation. Endod Dent Traumatol. 1999;15: 42-45.ArticlePubMed
- 8. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30: 297-306.ArticlePubMed
- 9. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85: 86-93.ArticlePubMed
- 10. Stevens RH, Grossman LI. Evaluation of the antimicrobial potential of calcium hydroxide as an intracanal medicament. J Endod. 1983;9: 372-374.ArticlePubMed
- 11. Spangberg LSW, Barbosa SV, Lavigne GD. AH26 releases formaldehyde. J Endod. 1993;19: 596-598.ArticlePubMed
- 12. Görduysus Ö. An evaluation of antimicrobial efficiency of endo-fill root canal sealant and filling material. J Endod. 1999;25: 652.ArticlePubMed
- 13. Duarte MAH, Demarchi AC, Giaxa MH, Kuga MC, Fraga S, Duarte de Souza LC. Evaluation of pH and calcium ion release of three root canal sealers. J Endod. 2000;26: 389-390.ArticlePubMed
- 14. Svanberg M, Mjor IA, Orstavik D. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J Dent Res. 1990;69: 861-864.PubMed
- 15. Loyola-Rodriguez JP, Garcia-Godoy F, Lindquist R. Growth inhibition of glass ionomer cements on mutans streptococci. Pediatr Dent. 1994;16: 346-349.PubMed
- 16. Ray H, Seltzer S. A new glass ionomer root canal sealer. J Endod. 1991;17: 598-603.ArticlePubMed
- 17. Friedman S, Lost C, Zarrabian M, Trope M. Evaluation of success and failure after endodontic therapy using a glass ionomer cement sealer. J Endod. 1995;21: 384-390.ArticlePubMed
- 18. Shalhav M, Fuss Z, Weiss EI. In vitro antibacterial activity of a glass ionomer endodontic sealer. J Endod. 1997;23: 616-619.ArticlePubMed
- 19. Tobias RS. Antibacterial properties of dental restorative materials: a review. Int Endod J. 1988;21: 155-160.ArticlePubMed
- 20. Cohen BI, Pagnillo MK, Musikant BL, Deutsch AS. Formaldehyde evaluation from endodontic materials. Oral Health. 1998;88: 37-39.
REFERENCES
Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

Antimicrobial activity of eight root canal sealers before and after setting
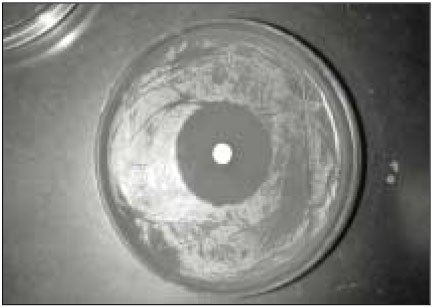
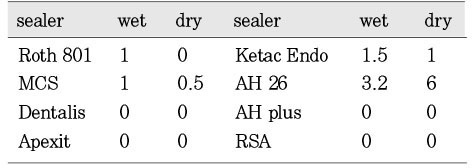
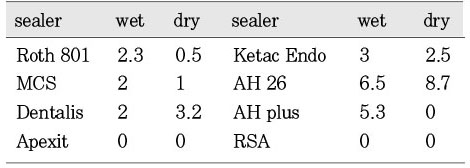
Fig. 1
Inhibition zone produced by dry AH26 against Enterococcus faecalis.
Fig. 2
Mean inhibition diameter (mm) of the wet and dry group sealers against the Enterococcus faecalis.
Fig. 3
Mean inhibition diameter (mm) of the wet and dry group sealers against the Staphylococcus aureus.
Fig. 1
Fig. 2
Fig. 3
Antimicrobial activity of eight root canal sealers before and after setting
Chemical component of each sealer used in this study
Table 1
Chemical component of each sealer used in this study

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

