Abstract
-
Objectives
The present study investigated the prevalence of mechanical allodynia (MA) in healthy teeth adjacent and contralateral to endodontically diseased teeth.
-
Materials and Methods
This cross-sectional study included 114 patients with symptomatic irreversible pulpitis and apical periodontitis in permanent mandibular first molars who possessed healthy teeth adjacent and contralateral to the endodontically diseased tooth. The mechanical sensitivity of the teeth was determined by percussion testing. The presence or absence of pain on percussion in the teeth adjacent and contralateral to the endodontically diseased tooth and the tooth distal to the contralateral symmetrical tooth was recorded according to coding criteria. The prevalence of MA was computed as a percentage, and binary logistic regression analysis was done. The Fisher exact test and Mann-Whitney U test were used for binary and ordinal data.
-
Results
Age and sex did not influence the prevalence of MA. An increased prevalence of MA was found in patients with higher levels of spontaneous pain (p < 0.001). The prevalence of allodynia was 57% in teeth adjacent to endodontically diseased teeth and 10.5% in teeth contralateral to endodontically diseased teeth. In addition, on the ipsilateral side, there were more painful sensations distal to the diseased tooth than mesially.
-
Conclusions
Despite being disease-free, teeth adjacent and contralateral to endodontically diseased teeth exhibited pain on percussion. There was a direct association between the severity of the patient’s pain and the presence of MA.
-
Keywords: Central sensitization; Mechanical allodynia; Molar; Pain; Periapical periodontitis; Pulpitis
INTRODUCTION
Odontogenic pain is a common type of orofacial pain that affects millions of people worldwide. The stimulation of pulpal or periradicular nociceptors can be the cause of odontogenic pain. The occurrence of pain in response to a mechanical stimulus that normally does not provoke pain is an indicator of mechanical allodynia (MA), which involves a decreased neuron excitability threshold and is demonstrated by sensitivity to percussion, biting, or pressure [
1,
2].
Pain experienced by the patient before, during, and after root canal treatment is an important aspect of clinical endodontics. Unfortunately, there is a poor correlation between endodontic pain and the pathological processes taking place in the pulp and periapical tissues. Therefore, a study of how pain correlates with diverse clinical presentations can provide insight into determining the extent of pulpal and periapical inflammation and infection [
3].
In addition to a detailed history of pain during the patient’s initial appointment, surrogate tests such as thermal testing, as well as percussion and palpation tests, are crucial for endodontic diagnosis. The most common cause of a painful reaction to mechanical sensory tests is inflammation or infection of the periapical tissues. Nonetheless, it is also plausible that, even when the periapical tissues are free of pathology, percussion sensitivity could detect peripheral and central sensitization (CS)-generated MA, which is caused by inflammatory and hypersensitive pulpal neuronal afferents [
1,
4]. A deeper understanding of what each type of mechanical sensory test conveys, along with the way it relates to overall pain, can aid in our comprehension of mechanical testing. The diagnosis of pulpal and periapical diseases and pain perception are influenced by an intricate and dynamic interplay. It has been proven that healthy teeth have mechanical thresholds like their normal contralateral counterparts [
5]. Conversely, teeth with symptomatic irreversible pulpitis (SIP) have considerably lower mechanical pain thresholds than teeth on the contralateral side [
4]. When compared to healthy individuals, patients with SIP also showed lowered mechanical pain thresholds in teeth adjacent and contralateral to the endodontically diseased teeth [
6]. This was the first evidence that CS induced by pulpal inflammation resulted in discernible changes in the pain thresholds of teeth adjacent and contralateral to teeth with the endodontic disease.
CS refers to the increased function of neurons in nociceptive pathways due to increased membrane excitability and synaptic efficacy as well as lowered inhibition. This is a manifestation of the somatosensory nervous system’s remarkable plasticity in response to activity, inflammation, and neural injury [
7,
8]. The increased activation of pulpal nociceptors has been shown to induce changes in wide dynamic range second-order neurons in the trigeminal complex of the medullary dorsal horn. Since CS can continue despite the elimination of its primary etiology, it frequently underpins persistent pain states [
9,
10]. In addition, it has been reported to cause contralateral alterations in the trigeminal system [
11]. This unexplored pain state, which plays a crucial role in the elevated state of nociception that can affect neighboring and contralateral teeth, is probably present in most patients needing endodontic treatment [
12,
13]. Therefore, differentiating between odontogenic and non-odontogenic pain is crucial for determining a correct diagnosis and adequate treatment plan. Recognizing and quantifying CS predictors could help the clinician achieve this objective.
To date, few scientific investigations have investigated this issue [
1,
4,
6]. Therefore, this study aimed to assess MA in healthy teeth adjacent and contralateral to endodontically diseased teeth, and to identify potential predictors of MA such as age, sex, and the severity of preoperative pain.
MATERIALS AND METHODS
This cross-sectional study conformed to the principles of the Declaration of Helsinki and was designed according to Strengthening the Reporting of Observational Studies in Epidemiology guidelines [
14].
Approval was granted by the Institutional Ethical Committee of Bharati Vidyapeeth Dental College and Hospital, Navi Mumbai (protocol No: IEC336072022, version 001; August 9, 2023). Patients were screened for enrollment at the outpatient department of Conservative Dentistry and Endodontics, Bharati Vidyapeeth Dental College and Hospital, Navi Mumbai from August 2023 to February 2024.
Sample size calculation
The sample size (
n = 348) was estimated using data for MA reported by Kayaoglu
et al. [
1] and calculated using a 2-sided single-proportion test for the prevalence of MA in healthy teeth adjacent to endodontically diseased teeth. A sample size of 114 was found to be adequately powered to detect a prevalence of MA between 39.4% and 65.7% in healthy teeth adjacent (ipsilateral) to endodontically diseased teeth. The confidence level was 95% with an alpha of 0.05 in the 2-sided test.
The null hypothesis was that the prevalence of MA in ipsilateral healthy teeth adjacent to endodontically diseased teeth would be 52.5%. The alternate hypothesis was that the prevalence of MA in ipsilateral healthy teeth adjacent to endodontically diseased teeth would not be between 39.4% and 65.7% (a 25% margin), which meant that the prevalence of MA would be either < 39.4% or > 65.7%.
Inclusion and exclusion criteria
We included patients aged > 18 years and < 60 years of either sex, with deep carious lesions with SIP and symptomatic apical periodontitis (SAP) in the mandibular permanent first molars with healthy teeth proximal and distal to the diseased tooth, a healthy tooth contralateral to the diseased tooth, and a healthy tooth distal to the contralateral symmetrical tooth. We excluded patients who were unable to communicate and give informed consent; had a history of significant medical problem(s) (American Society of Anesthesiologists physical status classification III or greater); teeth with periapical radiolucency other than slight widening of the periodontal ligament space (periapical index > 3) [
15,
16]; persistent use of antidepressants, narcotics, or sedatives; or analgesic intake within the past 24 hours. Once eligibility was confirmed, patients were briefed about the treatment protocol before obtaining informed consent.
Patients were provided with a thorough explanation of the study’s aims and methods. The cases were preoperatively evaluated using the cold test (Roeko Endo-Frost; Coltene-Whaledent, Langenau, Germany) and preoperative radiographs (Carestream CS 5200; Carestream Dental LLC, Atlanta, GA, USA). Two radiographs were taken on the ipsilateral and contralateral sides of the diseased tooth to check the coronal, periodontal, and periradicular status. Percussion tests were performed vertically on the occlusal tooth surface using the blunt end of an examination probe. The presence, absence, and intensity of pain identified during percussion tests in the teeth adjacent and contralateral to the endodontically diseased mandibular first permanent molars and teeth distal to the contralateral mandibular first molar were recorded using a visual analogue scale (VAS) and a code criterion (
Table 1) [
4]. The VAS consisted of a line divided into 10 mm segments, which the patients marked to grade their pain severity considering the following: no pain (0 mm), mild pain (1–3 mm), moderate pain (4–6 mm), and severe pain (7–10 mm). For the code criteria, each patient was assigned 1 or more grades. Grade Y1 denoted pain on percussion of adjacent teeth (ipsilateral). Grades Y2, Y3, and Y4 were separate categories for pain on percussion of the contralateral side. Subcodes 1 and 0 denoted the presence or absence of pain on percussion in adjacent or contralateral teeth (
Table 1).
Table 1Coding criteria
|
Code |
Explanation |
|
Y1 |
|
|
1 |
At least 1 of the adjacent teeth presents moderate to severe pain on percussion. |
|
0 |
Otherwise |
|
Y2 |
|
|
1 |
Contralateral symmetrical tooth presents moderate to severe pain on percussion. |
|
0 |
Otherwise |
|
Y3 |
|
|
1 |
Tooth distal to the contralateral symmetrical tooth presents moderate to severe pain on percussion |
|
0 |
Otherwise |
|
Y4 |
|
|
1 |
Contralateral symmetrical tooth and tooth distal to the contralateral symmetrical tooth present moderate to severe pain on percussion. |
|
0 |
Otherwise |
Data analysis
All data were entered onto a spreadsheet using Microsoft Office Excel 2021 (Office 365) and examined for errors and inconsistencies. Data analysis was performed using Windows-based MedCalc statistical software version 20.014 (MedCalc Software bvba, Ostend, Belgium;
http://www.medcalc.rorg; 2021).
Measurement and ranking data for pain scores were presented as means with standard deviation (SD), standard error (SE), and 95% confidence intervals (CIs). Binary (presence or absence of pain) data and nominal data were presented as numbers with proportions (%).
Statistical analysis
The prevalence of MA was calculated in all patients, based on findings of MA in healthy teeth adjacent and contralateral to endodontically involved teeth. The denominator was the total number of patients with endodontically diseased teeth. The prevalence of MA in ipsilateral and contralateral teeth was computed as a percentage. For binary data, the Fisher exact test was utilized, and the Mann-Whitney U test was used for ordinal data. For continuous data, the t-test was used for comparisons. Binary logistic regression analysis was used with the presence of MA as a dependent variable and other factors (age, sex, preoperative pain) as independent variables. All analyses were carried out utilizing 2-sided tests with an alpha value of 0.05 (corresponding to a 95% confidence level).
RESULTS
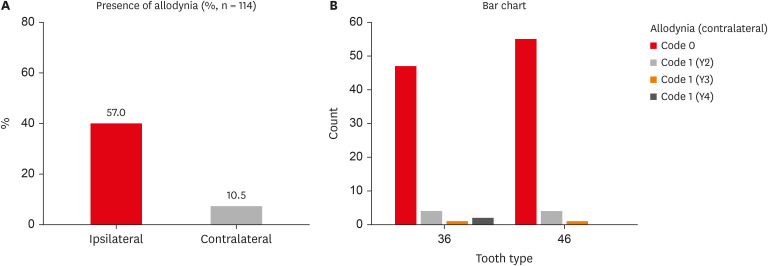
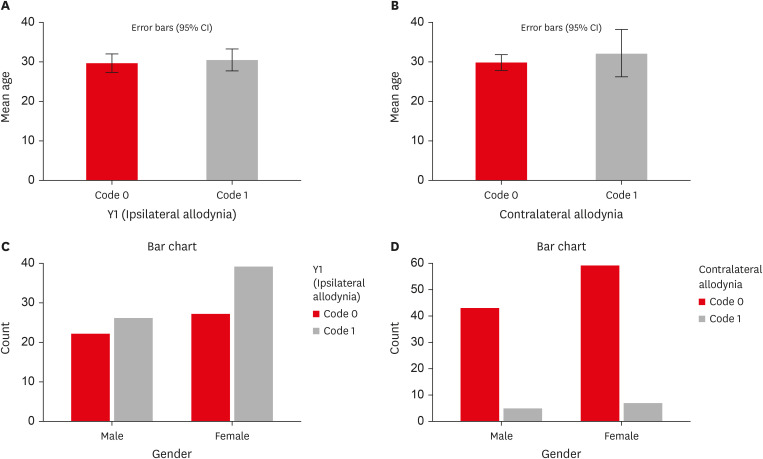
Of the 114 patients included in the study, 66 were women and 48 were men, with a mean age of 29 years. MA was detected in 57% (65/114) of teeth adjacent to the endodontically diseased tooth (
Figure 1A), and 10.5% (12/114) was detected in teeth contralateral to the endodontically diseased tooth (
Table 2). Furthermore, teeth distal to the endodontically diseased tooth were more painful than teeth located mesially. Y1-Code 1 was present in 65 of 114 teeth (57%), Y2-Code 1 in 8 of 114 teeth (7%), Y3-Code 1 in 2 of 114 teeth (1.75%), and Y4-Code 1 in 2 of 114 teeth (1.75%). Laterality did not have any significant influence on the prevalence of MA (
Table 3,
Figure 1B). Age and sex showed no statistically significant associations with the presence of MA (
p > 0.05) (
Tables 4 and
5,
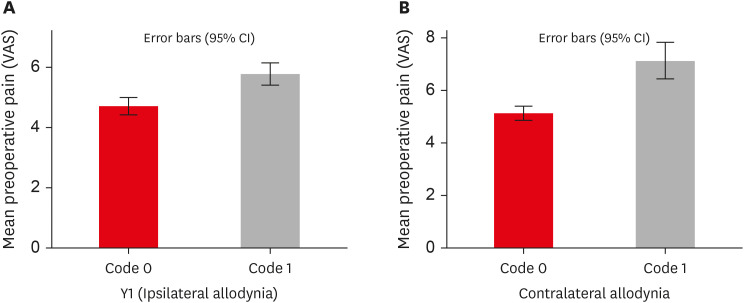
Figure 2). MA was more prevalent in women than in men on the ipsilateral (women: 60%, men: 40%) and contralateral sides (women: 58.3%, men: 41.7%), but the difference was not statistically significant. Patients with higher levels of spontaneous pain were associated with an increased prevalence of MA (
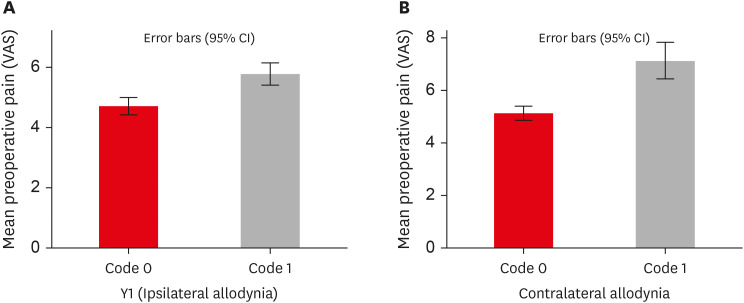
p < 0.001) (
Table 4,
Figure 3).
Figure 1
Prevalence of ipsilateral and contralateral MA. (A) Graph representing the percentage of ipsilateral and contralateral MA. (B) Graph representing contralateral MA in individual codes (Y2, Y3, Y4). Refer to Table 1 for code criteria (Y2, Y3, Y4 and code 0, code 1).
MA, mechanical allodynia.
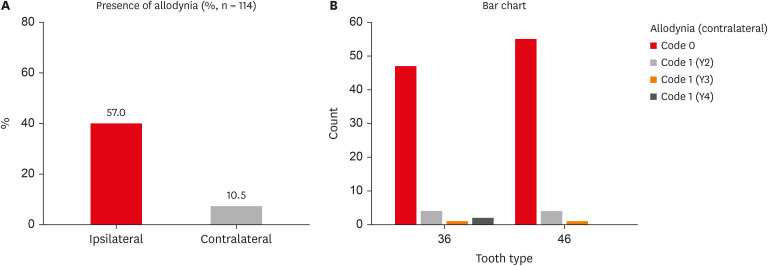
Table 2 Percentage and number of patients with and without ipsilateral and contralateral mechanical allodynia (MA)
|
Variables |
MA present |
MA absent |
|
No. |
% |
No. |
% |
|
Ipsilateral MA |
65 |
57.0% |
49 |
43.0% |
|
Contralateral MA |
12 |
10.5% |
102 |
89.5% |
Table 3 Contralateral mechanical allodynia (MA) in individual codes (Y2, Y3, Y4)
|
Variables |
Y2/Y3/Y4 (contralateral MA) |
Total |
|
Code 0 |
Y2 (Code 1) |
Y3 (Code 1) |
Y4 (Code 1) |
|
Tooth |
|
|
|
|
|
|
36 |
47 (46.1%) |
4 (50.0%) |
1 (50.0%) |
2 (100.0%) |
54 (47.4%) |
|
46 |
55 (53.9%) |
4 (50.0%) |
1 (50.0%) |
0 (0.0%) |
60 (52.6%) |
|
Total |
102 |
8 |
2 |
2 |
114 |
Table 4 Influence of age and preoperative pain level on the prevalence of ipsilateral (Y1) and contralateral mechanical allodynia (MA) (Y2, Y3, Y4)
|
Variables |
Code 0 |
Code 1 |
p
|
|
No. |
Mean ± SD |
Min–Max |
No. |
Mean ± SD |
Min–Max |
|
Ipsilateral MA (Y1) |
|
|
|
|
|
|
|
|
Age (yr) |
49 |
29.67 ± 8.28 |
18–50 |
65 |
30.48 ± 11.27 |
18–60 |
0.675 |
|
Preoperative pain score (VAS) |
49 |
4.69 ± 1.02 |
3–7 |
65 |
5.77 ± 1.51 |
2–9 |
< 0.001 |
|
Contralateral MA (Y2, Y3, Y4) |
|
|
|
|
|
|
|
|
Age (yr) |
102 |
29.89 ± 10.14 |
18–60 |
12 |
32.17 ± 9.49 |
18–50 |
0.461 |
|
Preoperative pain score (VAS) |
102 |
5.10 ± 1.31 |
2–9 |
12 |
7.08 ± 1.08 |
5–9 |
< 0.001 |
Table 5 Influence of sex on the prevalence of ipsilateral (Y1) and contralateral mechanical allodynia (MA) (Y2, Y3, Y4)
|
Variables |
Code 0 |
Code 1 |
Total |
p
|
|
Ipsilateral MA (Y1) |
|
|
|
|
|
Sex |
|
|
|
0.600 |
|
|
Male |
22 (44.9%) |
26 (40.0%) |
48 (42.1%) |
|
|
Female |
27 (55.1%) |
39 (60.0%) |
66 (57.9%) |
|
Total |
49 |
65 |
114 |
|
|
Contralateral MA (Y2, Y3, Y4) |
|
|
|
|
|
Sex |
|
|
|
0.974 |
|
|
Male |
43 (42.2%) |
5 (41.7%) |
48 (42.1%) |
|
|
Female |
59 (57.8%) |
7 (58.3%) |
66 (57.9%) |
|
Total |
102 |
12 |
114 |
|
Figure 2
Influence of age and sex on the prevalence of ipsilateral and contralateral MA. (A) Graph representing the influence of age on the prevalence of ipsilateral MA. (B) Graph representing the influence of age on the prevalence of contralateral MA. (C) Graph representing the influence of sex on the prevalence of ipsilateral MA (Y1). (D) Graph representing the influence of sex on the prevalence of contralateral MA (Y2, Y3, Y4). Refer to Table 1 for code criteria (Y1, Y2, Y3, Y4 and code 0, code 1).
MA, mechanical allodynia; CI, confidence interval.
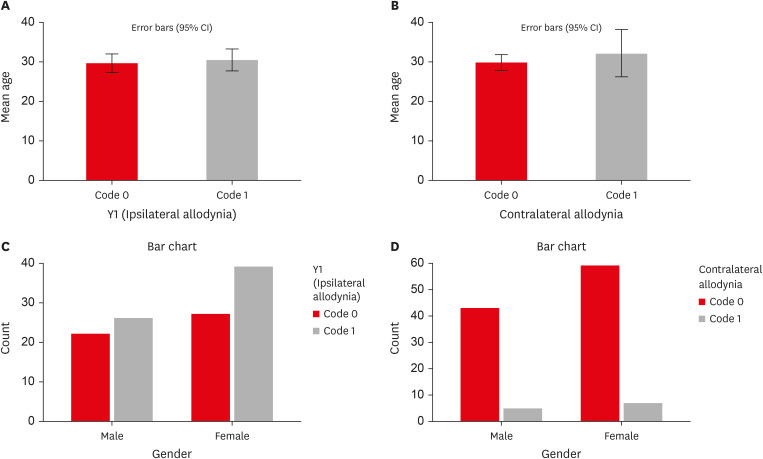
Figure 3
Influence of preoperative pain levels on prevalence of ipsilateral and contralateral MA. (A) Graph representing the association between preoperative pain and ipsilateral MA. (B) Graph representing the association between preoperative pain and contralateral MA. Refer to Table 1 for code criteria (code 0, code 1, Y1).
VAS, visual analogue scale; MA, mechanical allodynia; CI, confidence interval.
DISCUSSION
This study was unique in that it assessed MA not only in healthy teeth adjacent to endodontically diseased teeth, but also in healthy teeth contralateral to endodontically diseased teeth. The main conclusion of this study was that healthy teeth adjacent and contralateral to teeth with symptomatic irreversible pulpitis and apical periodontitis frequently exhibited MA. The prevalence of MA was 57% in healthy teeth adjacent to endodontically diseased teeth and 10.5% in teeth contralateral to endodontically diseased teeth. The prevalence of ipsilateral allodynia was higher than the prevalence of contralateral allodynia. Furthermore, teeth distal to endodontically diseased mandibular first molars were more painful than mesial teeth on the ipsilateral side. Age and sex had no significant influence on the prevalence of MA. The incidence of both ipsilateral and contralateral allodynia was strongly influenced by the intensity of preoperative pain.
Ipsilateral pain generated by high threshold nociceptive cells has been documented in rat teeth [
17]. In mice, the hind paw (a more distant region) exhibited MA following experimental dental pulp injury [
18]. The authors hypothesized that dental pulp injury is associated with substantial neuroplasticity, which may contribute to allodynia and persistent pain [
18]. These studies on rats furnish valuable scientific evidence, but they cannot be used to validate the findings of our study. In a human study, distinct regions of hyperalgesia and allodynia have been identified around intradermal capsaicin injection sites. The hyperalgesia propagated radially outward, covering extensive areas, with pain levels gradually decreasing with increased distance from the injection site [
19].
The ipsilateral pain correlations revealed in our study suggest that CS can coexist with peripheral sensitization. Under usual conditions, sensory experiences elicited by innocuous and noxious stimuli are distinct and discrete. The environment is hyperactive in CS, leading to the convergence of 2 sensory pathways, which then results in the loss of usual discrimination and expansion of the receptivity field of the dorsal horn neurons. Thus, synaptic efficiency increases and inhibitory regulation decreases and pain is elicited by stimuli that were previously subthreshold or non-painful [
7,
8]. This forms the basis for MA in the diseased tooth as well as healthy adjacent teeth.
Our observation of MA in contralateral healthy teeth agrees with the results of Khan
et al. [
6], who found decreased biting force in healthy teeth contralateral to teeth with irreversible pulpitis and acute apical periodontitis. Pain was also observed in teeth distal to the contralateral symmetrical tooth, which again is an indication of CS. Experiments have demonstrated that noxious conditioning (injury or injection of capsaicin) on one side of the body can result in allodynia in the untreated (pathology-free) contralateral side [
20]. Although the precise neurophysiology of this reaction needs further study, previous research has suggested that peripheral and central (spinal and supraspinal) mechanisms play a role. Certain peripheral receptors, such as transient receptor potential vanilloid TRPV1, PIEZO2, and purinoceptor P2X3, can be stimulated and upregulated to produce and sustain contralateral effects [
21,
22]. Contralateral effects at the spinal level may be mediated by the midline crossover of commissural interneurons that end in the contralateral dorsal horn or the overlapping central terminals of primary afferent neurons [
23]. At the spinal level, astrocytic and microglial cells also make a substantial contribution [
24]. In addition, involvement of the descending pain regulation system and activation of higher brain centers like periaqueductal gray matter and anterior cingulate cortex are examples of supraspinal processes [
25].
A study by Owatz
et al. [
4] found MA in 57.2% of patients with irreversible pulpitis and acute periradicular periodontitis. They postulated 3 mechanisms for the MA: 1) stimulation of pulpal mechanoreceptive nociceptive neurons, 2) diffusion of inflammatory cytokines or bacterial metabolites into the periradicular region, and 3) activation of periradicular mechanoreceptive nociceptive neurons and CS [
4]. Another study reported a 55% drop in the mechanical pain threshold in teeth diagnosed with SIP and in teeth with SAP [
12]. They also concluded that sex was a key predictor of MA, with women exhibiting much lower pain thresholds than men on both sides [
12]. In addition, teeth with previously initiated treatment showed a significant decrease (64%) in the mechanical pain threshold [
12]. These results imply that pulpitis or incomplete root canal therapy-related inflammation has a significant impact on periradicular nociceptors. This may have practical implications—namely, it may be best to finish root canal therapy as soon as feasible to shorten the duration of the primary afferent barrage, thus preventing the development of CS [
12]. Furthermore, evidence suggests that moderate to severe preoperative MA is a predictor of postoperative pain in patients undergoing root canal treatment [
2].
One intriguing finding of our study is that teeth distal to the diseased tooth (first molar) had higher percussion sensitivity than teeth mesial to the diseased tooth on the ipsilateral side. A possible explanation could be that biting force increases from the incisors to molars in patients with normal overjet and overbite [
26,
27]. MA, which is a result of central and peripheral sensitization, might be a protective body response, compelling a person to minimize bite force in the presence of an injured or diseased tooth [
1]. Typical nociceptive behavior, hyperalgesia, and allodynia may differ across the sexes, suggesting that sex hormones are involved in mediating these nociceptive distinctions. Although the influence of sex on MA in endodontic patients is unclear, a prior study reported a substantial sex difference in the prevalence of MA [
5]. The biological differences between men and women might explain the increased occurrence of MA in women [
28,
29]. Fluctuating female hormone levels may be linked with varying amounts of serotonin and noradrenaline, leading to greater pain predominance in women throughout the menstrual period and when receiving hormonal replacement therapy or using oral contraceptives [
30,
31]. Our study demonstrated no significant influence of sex on either ipsilateral or contralateral painful associations. This disparity in results could be attributed to differing measurement methods. More research is needed to understand how sex affects MA. Traditional methods for detecting MA include the percussion test, which is commonly performed with a mirror or probe handle, and the bite test on a hard object such as the Tooth Slooth [
1]. However, these tests are not quantitative and have intrinsic variability in force vectors and subjective outcomes.
A limitation of our study was that the assessment was conducted using qualitative clinical percussion testing. The utilization of a quantitative bite fork force transducer is another alternative, but it necessitates additional inclusion criteria, such as the existence of an antagonist tooth [
1]. A bite fork force sensor device is used to measure MA by determining occlusal force on the ipsilateral and contralateral sides [
32]. Usually, patients are unable to bite on the affected tooth with the same force as on the contralateral tooth. Furthermore, the design of a bite fork force sensor prevents examination of a tooth that has extensive decay [
1]. The bite fork force sensor is designed to be placed under 1 cusp at a time and features an acrylic cone that works as an occlusal guide, allowing it to be positioned in a consistent location each time [
5]. Since the extent of caries and cuspal involvement varied in the teeth included in our study, it was not practical to use a bite fork force sensor device. The accessibility, feasibility, and clinical relevance of routine clinical percussion testing made it advantageous [
4]. Furthermore, it required less of an armamentarium and was cheaper, faster, and convenient. A limitation of the routine percussion test was that it was not quantitative and used an undefined amount of force. Nevertheless, there was obvious variation in how patients responded to mechanical testing, given the intrinsic diversity of pain reporting. No examiner effect was detected, as all percussion tests were carried out by the same examiner. In the present study, we included only mandibular first molars to minimize the effect of tooth type on the prevalence of MA and because this tooth is commonly affected by dental caries and endodontic involvement.
Our study included a relatively small sample of 114 patients with SIP and SAP. Therefore, the prevalence of MA in the study cohort (patients presenting to the study site with endodontically diseased teeth) may not be representative of the entire population. Our results were site-specific and may not be extrapolated to the population in general. In addition, since the primary objective of this study was to investigate the prevalence of MA in ipsilateral teeth, the sample size was estimated based on available data for MA in ipsilateral teeth. The data for contralateral MA were independently reported.
Since all clinicians have encountered unexplained sensitivity to percussion in otherwise healthy teeth adjacent to a tooth with obvious pulpal and periapical pathology, our findings are of substantial clinical significance. MA is not limited to pulp and periapical pathologies, but has also been observed after third molar extractions and surgery [
33]. Thus, the occurrence of CS should be explored in greater depth to help clinicians across the various domains of dentistry comprehend and diagnose diverse conditions.
It is suggested that dentists use caution when interpreting the findings of percussion hypersensitivity testing. It is more likely to indicate a decreased pain threshold or increased pain sensitivity as a result of peripheral or central sensitization than to pinpoint the precise tooth with periapical inflammation or infection [
34,
35]. In addition, the presence of MA with moderate to severe preoperative pain should be taken into account while designing an effective postoperative pain management plan. Research has shown that both local anesthetics and a typical analgesic regimen (non-steroidal anti-inflammatory drugs) may not be enough to relieve MA [
6,
36]. This topic warrants further research to find an ultimate solution to MA.
CONCLUSIONS
Within the constraints of this investigation, it can be concluded that healthy teeth adjacent and contralateral to endodontically diseased mandibular first molars frequently exhibit MA. The prevalence of ipsilateral MA was comparatively higher than the prevalence of contralateral MA. In addition, teeth distal to endodontically diseased mandibular first molars were more painful than teeth located mesially on the ipsilateral side. Patients with higher levels of spontaneous pain were associated with an increased prevalence of MA. Thus, dentists need to be prepared to confront percussion-sensitive healthy teeth in patients with endodontically diseased and symptomatic teeth. The patient should also be educated about the condition and be instructed to expect gradual resolution upon treatment of the affected tooth.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Patankar VR, Jain AK, Rao RD.
Data curation: Patankar VR.
Formal analysis: Patankar VR, Jain AK, Rao RD, Rao PR.
Investigation: Patankar VR.
Methodology: Patankar VR.
Project administration: Patankar VR.
Resources: Patankar VR, Jain AK, Rao RD, Rao PR.
Software: Patankar VR, Jain AK, Rao RD, Rao PR.
Supervision: Jain AK, Rao RD, Rao PR.
Validation: Patankar VR, Jain AK, Rao RD.
Visualization: Patankar VR, Jain AK, Rao RD.
Writing - original draft: Patankar VR.
Writing - review & editing: Patankar VR, Jain AK, Rao RD, Rao PR.
REFERENCES
- 1. Kayaoglu G, Ekici M, Altunkaynak B. Mechanical allodynia in healthy teeth adjacent and contrala-teral to endodontically diseased teeth: a clinical study. J Endod 2020;46:611-618.ArticlePubMed
- 2. Jang YE, Kim Y, Kim BS. Influence of preoperative mechanical allodynia on predicting postoperative pain after root canal treatment: a prospective clinical study. J Endod 2021;47:770-778.e1.ArticlePubMed
- 3. Gutmann JL, Baumgartner JC, Gluskin AH, Hartwell GR, Walton RE. Identify and define all diagnostic terms for periapical/periradicular health and disease states. J Endod 2009;35:1658-1674.ArticlePubMed
- 4. Owatz CB, Khan AA, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. The incidence of mechanical allodynia in patients with irreversible pulpitis. J Endod 2007;33:552-556.ArticlePubMed
- 5. Khan AA, McCreary B, Owatz CB, Schindler WG, Schwartz SA, Keiser K, et al. The development of a diagnostic instrument for the measurement of mechanical allodynia. J Endod 2007;33:663-666.ArticlePubMed
- 6. Khan AA, Owatz CB, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. Measurement of mechanical allodynia and local anesthetic efficacy in patients with irreversible pulpitis and acute periradicular periodontitis. J Endod 2007;33:796-799.ArticlePubMed
- 7. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(Supplement):S2-SS15.ArticlePubMedPMC
- 8. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895-926.ArticlePubMedPMC
- 9. Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 2000;11:57-91.ArticlePubMedPDF
- 10. Chiang CY, Park SJ, Kwan CL, Hu JW, Sessle BJ. NMDA receptor mechanisms contribute to neuroplasticity induced in caudalis nociceptive neurons by tooth pulp stimulation. J Neurophysiol 1998;80:2621-2631.ArticlePubMed
- 11. Worsley MA, Allen CE, Billinton A, King AE, Boissonade FM. Chronic tooth pulp inflammation induces persistent expression of phosphorylated ERK (pERK) and phosphorylated p38 (pp38) in trigeminal subnucleus caudalis. Neuroscience 2014;269:318-330.ArticlePubMedPMC
- 12. Alelyani AA, Azar PS, Khan AA, Chrepa V, Diogenes A. Quantitative assessment of mechanical allodynia and central sensitization in endodontic patients. J Endod 2020;46:1841-1848.ArticlePubMed
- 13. Hashemipour MA, Borna R. Incidence and characteristics of acute referred orofacial pain caused by a posterior single tooth pulpitis in an Iranian population. Pain Pract 2014;14:151-157.ArticlePubMed
- 14. Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019;13(Supplement 1):S31-S34.ArticlePubMedPMC
- 15. Orstavik D, Kerekes K, Eriksen HM. The periapical index: a scoring system for radiographic assessment of apical periodontitis. Endod Dent Traumatol 1986;2:20-34.ArticlePubMed
- 16. Zanini M, Decerle N, Hennequin M, Cousson PY. Revisiting Orstavik’s PAI score to produce a reliable and reproducible assessment of the outcomes of endodontic treatments in routine practice. Eur J Dent Educ 2021;25:291-298.ArticlePubMedPDF
- 17. Matsuura S, Shimizu K, Shinoda M, Ohara K, Ogiso B, Honda K, et al. Mechanisms underlying ectopic persistent tooth-pulp pain following pulpal inflammation. PLoS One 2013;8:e52840.ArticlePubMedPMC
- 18. Lee C, Ramsey A, De Brito-Gariepy H, Michot B, Podborits E, Melnyk J, et al. Molecular, cellular and behavioral changes associated with pathological pain signaling occur after dental pulp injury. Mol Pain 2017;13:1744806917715173.ArticlePubMedPMCPDF
- 19. Torebjörk HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol 1992;448:765-780.ArticlePubMedPMC
- 20. Shenker NG, Haigh RC, Mapp PI, Harris N, Blake DR. Contralateral hyperalgesia and allodynia following intradermal capsaicin injection in man. Rheumatology (Oxford) 2008;47:1417-1421.ArticlePubMedPMC
- 21. Simonic-Kocijan S, Zhao X, Liu W, Wu Y, Uhac I, Wang K. TRPV1 channel-mediated bilateral allodynia induced by unilateral masseter muscle inflammation in rats. Mol Pain 2013;9:68.ArticlePubMedPMCPDF
- 22. Tariba Knežević P, Vukman R, Antonić R, Kovač Z, Uhač I, Simonić-Kocijan S. The role of P2X3 receptors in bilateral masseter muscle allodynia in rats. Croat Med J 2016;57:530-539.ArticlePubMedPMC
- 23. Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci 1999;22:122-127.ArticlePubMed
- 24. Schreiber KL, Beitz AJ, Wilcox GL. Activation of spinal microglia in a murine model of peripheral inflammation-induced, long-lasting contralateral allodynia. Neurosci Lett 2008;440:63-67.ArticlePubMedPMC
- 25. Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 2009;89:707-758.ArticlePubMed
- 26. Serra CM, Manns AE. Bite force measurements with hard and soft bite surfaces. J Oral Rehabil 2013;40:563-568.ArticlePubMedPDF
- 27. Ferrario VF, Sforza C, Serrao G, Dellavia C, Tartaglia GM. Single tooth bite forces in healthy young adults. J Oral Rehabil 2004;31:18-22.ArticlePubMed
- 28. Unruh AM. Gender variations in clinical pain experience. Pain 1996;65:123-167.ArticlePubMed
- 29. Fillingim RB, Maixner W. Gender differences in the responses to noxious stimuli. Pain Forum 1995;4:209-221.Article
- 30. Nagendrababu V, Gutmann JL. Factors associated with postobturation pain following single-visit nonsurgical root canal treatment: a systematic review. Quintessence Int 2017;48:193-208.PubMed
- 31. Machado R, Comparin D, Ignácio SA, da Silva Neto UX. Postoperative pain after endodontic treatment of necrotic teeth with large intentional foraminal enlargement. Restor Dent Endod 2021;46:e31.ArticlePubMedPMCPDF
- 32. Kishnani S, Saha SG, Bhardwaj A, Dubey S, Saha M, Kala S, et al. Unmasking the effect of analgesics on endodontic diagnosis using a novel bite force sensor device: a prospective, randomized clinical trial. J Clin Diagn Res 2016;10:ZC38-ZC42.ArticlePubMedPMC
- 33. Eliav E, Gracely RH. Sensory changes in the territory of the lingual and inferior alveolar nerves following lower third molar extraction. Pain 1998;77:191-199.ArticlePubMed
- 34. Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 2019;33:131-139.ArticlePubMedPMCPDF
- 35. Erdogan O, Malek M, Gibbs JL. Associations between pain severity, clinical findings, and endodontic disease: a cross-sectional study. J Endod 2021;47:1376-1382.ArticlePubMed
- 36. Read JK, McClanahan SB, Khan AA, Lunos S, Bowles WR. Effect of ibuprofen on masking endodontic diagnosis. J Endod 2014;40:1058-1062.ArticlePubMed
 , Ashish K Jain1
, Ashish K Jain1 , Rahul D Rao1
, Rahul D Rao1 , Prajakta R Rao2
, Prajakta R Rao2





 KACD
KACD
 ePub Link
ePub Link Cite
Cite

