Articles
- Page Path
- HOME > Restor Dent Endod > Volume 49(2); 2024 > Article
- Research Article Single-session associative protocol for dentin hypersensitivity management: a 1-year randomized, blinded clinical study
-
Thayna Carolina Zeni
 , Poliana Maria de Faveri Cardoso
, Poliana Maria de Faveri Cardoso , Rafael da Silva Vanolli
, Rafael da Silva Vanolli , Márcio José Mendonça
, Márcio José Mendonça , Julio Katuhide Ueda
, Julio Katuhide Ueda , Veridiana Camilotti
, Veridiana Camilotti
-
Restor Dent Endod 2024;49(2):e15.
DOI: https://doi.org/10.5395/rde.2024.49.e15
Published online: March 20, 2024
Department of Restorative Dentistry, School of Dentistry, Western State University of Paraná, Cascavel, PR, Brazil.
- Correspondence to Poliana Maria de Faveri Cardoso, DDS. Department of Restorative Dentistry, School of Dentistry, Western State University of Paraná, 2069 Universitaria St. Cascavel, PR 858191110, Brazil. polif1704@gmail.com
Copyright © 2024. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives This study aimed to establish a single-session associative protocol for non-restorative management of dentin hypersensitivity (DH).
-
Materials and Methods Twenty-four individuals with DH and a minimum sensitivity level of 4 on the visual analog scale (VAS) were selected. The study was conducted in a split-mouth design, with each participant (n = 20) having at least 1 affected tooth in all quadrants. The management protocols consisted of control group: universal adhesive, Neural Desensitizing Protocol group: 5% potassium nitrate, Mixed Desensitizing Protocol (PAM) group: 5% sodium fluoride and 5% potassium nitrate, Remineralizing Desensitizing Protocol (PDR) group: surface-partially reacted glass technology photopolymerizable varnish. Evaluations were performed immediately after application, at 1 week, 1 month, 2 months, and 12 months using the VAS sensitivity test.
-
Results The scores were subjected to statistical analysis using the Friedman test (p < 0.05), Durbin-Conover test (p < 0.05), and Wilcoxon test (p < 0.05). At the 12-month evaluation, all groups showed statistically significant differences compared to the initial assessment. For the evaluation after 12 months, there was a statistically significant difference between the PAM group, the control group, and the PDR group.
-
Conclusions It can be concluded that all groups were effective in controlling DH, but there were significant results in the control group and PDR group. The clinical relevance of this study is to demonstrate that the application of single-session desensitizing protocols can be effective in controlling DH for up to 12 months.
-
Trial Registration Brazilian Clinical Trials Registry Identifier: RBR-4r63d7s
INTRODUCTION
MATERIALS AND METHODS
Search method flowchart.
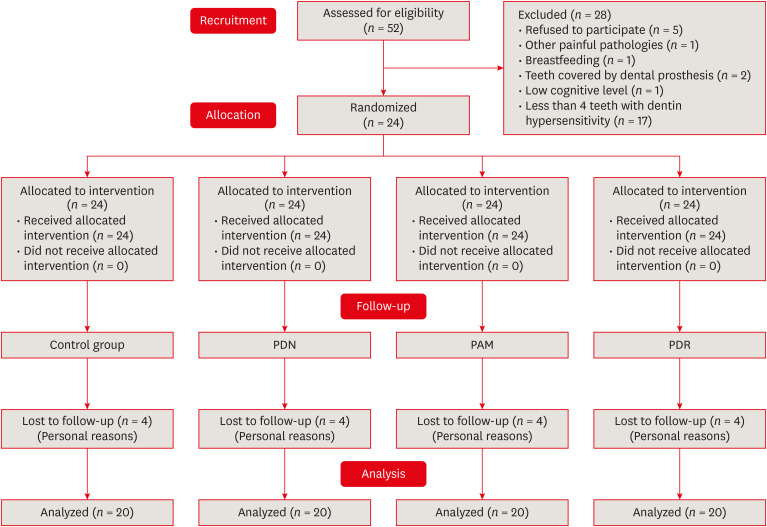
1. Interventions
Description of application and composition of products used according to each manufacturer
Description of application of management protocols
RESULTS
Patient data regarding sex, race, and age
| Variables | Values | |
|---|---|---|
| Sex | ||
| Female | 19 (79.17) | |
| Male | 5 (20.83) | |
| Race | ||
| Caucasian | 20 (83.33) | |
| Non-Caucasian | 4 (16.67) | |
| Age (yr) | ||
| 11–20 | 1 (4.17) | |
| 21–30 | 9 (37.50) | |
| 31–40 | 6 (25.00) | |
| 41–50 | 5 (20.83) | |
| 51–60 | 3 (12.50) | |
Median values and interquartile ranges of sensitivity level during the execution of the tooth whitening protocol using desensitizing agents control group, PDN group, PAM group, and PDR group at different assessment times
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Zeni TC, Cardoso PMdeF Vanolli RdaS.
Data curation: Vanolli RdaS, Mendonça MJ, Camilotti V.
Formal analysis: Cardoso PMdeF, Mendonça MJ.
Funding acquisition: Vanolli RdaS, Ueda JK Investigation.
Methodology: Mendonça MJ.
Project administration: Zeni TC.
Resources: Camilotti V.
Software: Cardoso PMdeF, Camilotti V.
Supervision: Vanolli RdaS, Validation.
Visualization: Zeni TC, Writing - original draft.
Writing - review & editing: Ueda JK, Cardoso PMdeF, Mendonça MJ Camilotti V.
- 1. Favaro Zeola L, Soares PV, Cunha-Cruz J. Prevalence of dentin hypersensitivity: systematic review and meta-analysis. J Dent 2019;81:1-6.ArticlePubMed
- 2. Addy M. Dentine hypersensitivity new perspectives on an old problem. Int Dent J 2002;52:367-375.Article
- 3. Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc 2003;69:221-226.PubMed
- 4. Davari A, Ataei E, Assarzadeh H. Dentin hypersensitivity: etiology, diagnosis and treatment; a literature review. J Dent (Shiraz) 2013;14:136-145.PubMedPMC
- 5. Ishihata H, Finger WJ, Kanehira M, Shimauchi H, Komatsu M. In vitro dentin permeability after application of Gluma® desensitizer as aqueous solution or aqueous fumed silica dispersion. J Appl Oral Sci 2011;19:147-153.ArticlePubMedPMC
- 6. García-Delaney C, Abad-Sánchez D, Arnabat-Domínguez J, Valmaseda-Castellón E, Gay-Escoda C. Evaluation of the effectiveness of the photobiomodulation in the treatment of dentin hypersensitivity after basic therapy. A randomized clinical trial. J Clin Exp Dent 2017;9:e694-e702.PubMedPMC
- 7. Aw TC, Lepe X, Johnson GH, Mancl L. Characteristics of noncarious cervical lesions: a clinical investigation. J Am Dent Assoc 2002;133:725-733.PubMed
- 8. Braennstroem M, Astroem A. A study on the mechanism of pain elicited from the dentin. J Dent Res 1964;43:619-625.ArticlePubMedPDF
- 9. Douglas-de-Oliveira DW, Vitor GP, Silveira JO, Martins CC, Costa FO, Cota LO. Effect of dentin hypersensitivity treatment on oral health related quality of life - a systematic review and meta-analysis. J Dent 2018;71:1-8.ArticlePubMed
- 10. Teixeira DNR, Zeola LF, Machado AC, Gomes RR, Souza PG, Mendes DC, et al. Relationship between noncarious cervical lesions, cervical dentin hypersensitivity, gingival recession, and associated risk factors: a cross-sectional study. J Dent 2018;76:93-97.ArticlePubMed
- 11. Liu XX, Tenenbaum HC, Wilder RS, Quock R, Hewlett ER, Ren YF. Pathogenesis, diagnosis and management of dentin hypersensitivity: an evidence-based overview for dental practitioners. BMC Oral Health 2020;20:220.ArticlePubMedPMCPDF
- 12. Shah D, Mital K. The role of trypsin:chymotrypsin in tissue repair. Adv Ther 2018;35:31-42.ArticlePubMedPDF
- 13. Joshi S, Gowda AS, Joshi C. Comparative evaluation of NovaMin desensitizer and Gluma desensitizer on dentinal tubule occlusion: a scanning electron microscopic study. J Periodontal Implant Sci 2013;43:269-275.ArticlePubMedPMCPDF
- 14. Ma S, Imazato S, Chen JH, Mayanagi G, Takahashi N, Ishimoto T, et al. Effects of a coating resin containing S-PRG filler to prevent demineralization of root surfaces. Dent Mater J 2012;31:909-915.ArticlePubMed
- 15. Ravishankar P, Viswanath V, Archana D, Keerthi V, Dhanapal S, Lavanya Priya KP. The effect of three desensitizing agents on dentin hypersensitivity: a randomized, split-mouth clinical trial. Indian J Dent Res 2018;29:51-55.ArticlePubMed
- 16. Rabiabasree R, Krishnakumar R, Prabhu AS, Naik NS, Shashibhushan KK, Janarthanan K. Inhibitory effect of a resin coat-containing prereacted glass fillers on the enamel demineralization of the primary teeth: an in vitro pilot study. J Indian Soc Pedod Prev Dent 2019;37:146-150.ArticlePubMed
- 17. Spinola MD, Moecke SE, Rossi NR, Nakatsuka T, Borges AB, Torres CRG. Efficacy of S-PRG filler containing varnishes on enamel demineralization prevention. Sci Rep 2020;10:18992.ArticlePubMedPMCPDF
- 18. Suzuki M, Yamada A, Saito K, Hino R, Sugawara Y, Ono M, et al. Application of a tooth-surface coating material containing pre-reacted glass-ionomer fillers for caries prevention. Pediatr Dent J 2015;25:72-78.Article
- 19. Miki S, Kitagawa H, Kitagawa R, Kiba W, Hayashi M, Imazato S. Antibacterial activity of resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent Mater 2016;32:1095-1102.ArticlePubMed
- 20. Kaga M, Kakuda S, Ida Y, Toshima H, Hashimoto M, Endo K, et al. Inhibition of enamel demineralization by buffering effect of S-PRG filler-containing dental sealant. Eur J Oral Sci 2014;122:78-83.PubMed
- 21. Iijima M, Ito S, Nakagaki S, Kohda N, Muguruma T, Saito T, et al. Effects of immersion in solution of an experimental toothpaste containing S-PRG filler on like-remineralizing ability of etched enamel. Dent Mater J 2014;33:430-436.ArticlePubMed
- 22. Rusnac ME, Gasparik C, Irimie AI, Grecu AG, Mesaroş AŞ, Dudea D. Giomers in dentistry - at the boundary between dental composites and glass-ionomers. Med Pharm Reports 2019;92:1-6.ArticlePubMedPMCPDF
- 23. Sarkis-Onofre R, Cenci MS, Moher D, Pereira-Cenci T. Research reporting guidelines in dentistry: a survey of editors. Braz Dent J 2017;28:3-8.ArticlePubMed
- 24. Camilotti V, Zilly J, Busato PM, Nassar CA, Nassar PO. Desensitizing treatments for dentin hypersensitivity: a randomized, split-mouth clinical trial. Braz Oral Res 2012;26:263-268.ArticlePubMed
- 25. Lopes AO, Aranha ACC. Comparative evaluation of the effects of Nd:YAG laser and a desensitizer agent on the treatment of dentin hypersensitivity: a clinical study. Photomed Laser Surg 2013;31:132-138.ArticlePubMedPMC
- 26. Moura GF, Zeola LF, Silva MB, Sousa SC, Guedes FR, Soares PV. Four-session protocol effectiveness in reducing cervical dentin hypersensitivity: a 24-week randomized clinical trial. Photobiomodul Photomed Laser Surg 2019;37:117-123.ArticlePubMed
- 27. Galvão AD, Zeola LF, Moura GF, Teixeira DNR, Gonzaga RCQ, da Silva GR, et al. A long-term evaluation of experimental potassium oxalate concentrations on dentin hypersensitivity reduction: a triple-blind randomized clinical trial. J Dent 2019;89:103180.ArticlePubMed
- 28. Narayanan R, Prabhuji MLV, Paramashivaiah R, Bhavikatti SK. Low-level laser therapy in combination with desensitising agent reduces dentin hypersensitivity in fluorotic and non-fluorotic teeth - a randomised, controlled, double-blind clinical trial. Oral Health Prev Dent 2019;17:547-556.PubMed
- 29. Lopes AO, de Paula Eduardo C, Aranha ACC. Evaluation of different treatment protocols for dentin hypersensitivity: an 18-month randomized clinical trial. Lasers Med Sci 2017;32:1023-1030.ArticlePubMedPDF
- 30. Younus MZ, Ahmed MA, Syed AUY, Baloch JM, Ali M, Sheikh A. Comparison between effectiveness of dentine desensitizer and one bottle self-etch adhesive on dentine hypersensitivity. Technol Health Care 2021;29:1153-1159.ArticlePubMed
- 31. Askari M, Yazdani R. Comparison of two desensitizing agents for decreasing dentin hypersensitivity following periodontal surgeries: a randomized clinical trial. Quintessence Int 2019;50:320-329.PubMed
- 32. Yoshida Y, Inoue S. Chemical analyses in dental adhesive technology. Jpn Dent Sci Rev 2012;48:141-152.Article
- 33. Kwon SR, Dawson DV, Schenck DM, Fiegel J, Wertz PW. Spectrophotometric evaluation of potassium nitrate penetration into the pulp cavity. Oper Dent 2015;40:614-621.ArticlePubMedPDF
- 34. Rösing CK, Fiorini T, Liberman DN, Cavagni J. Dentine hypersensitivity: analysis of self-care products. Braz Oral Res 2009;23(Supplement 1):56-63.ArticlePubMed
- 35. Godoy CE, Consani S, Guimarães AT, Laurindo BM, Mendonça MJ, Camilotti V. Effect of two desensitizing agents applied previous to in-office bleaching on the degree of whitening and dentin sensitivity: a randomized, controlled, double-blind clinical trial. Am J Dent 2021;34:70-74.PubMed
- 36. Grippo JO, Simring M, Coleman TA. Abfraction, abrasion, biocorrosion, and the enigma of noncarious cervical lesions: a 20-year perspective. J Esthet Restor Dent 2012;24:10-23.ArticlePubMed
- 37. Ali NY, Hassanein OE, Hamza HS, Baz MAE. Clinical efficacy of giomer versus sodium fluoride varnish for management of hypersensitivity: randomized control trail. Egypt Dent J 2021;67:905-915.Article
REFERENCES
Tables & Figures
REFERENCES
Citations

- In vivo and in situ evaluation of innovative approaches in dentin hypersensitivity treatment
Heba Abd El-Fattah Mohamed, Dina Ezzeldin Mohamed, Elhassan Hassanein, Heba El-din Salah El-din Hamza
BMC Oral Health.2025;[Epub] CrossRef - Publication trends and scientific profile of clinical trials on universal adhesives in dentistry: A metrics-based review
Aurélio de Oliveira Rocha, Lucas Menezes dos Anjos, Michael Willian Favoreto, Michely Cristina Goebel, Bruno Henriques, Alessandra Reis, Alessandro D. Loguercio, Mariane Cardoso
Journal of Dentistry.2025; 161: 105965. CrossRef - EVALUATION OF PUSH-OUT BOND STRENGTH OF GLASS FIBER POSTS USING DIFFERENT LUTING CEMENTS
Jannah Mohammed, Maha Agha
BULLETIN OF STOMATOLOGY AND MAXILLOFACIAL SURGERY.2025; : 274. CrossRef - EVALUATION OF PUSH-OUT BOND STRENGTH OF GLASS FIBER POSTS USING DIFFERENT LUTING CEMENTS
Jannah Mohammed, Jannah Mohammed
BULLETIN OF STOMATOLOGY AND MAXILLOFACIAL SURGERY.2025; : 274. CrossRef - CLINICAL AND BEHAVIORAL DETERMINANTS OF DENTIN SENSITIVITY AMONG DENTAL STUDENTS: AN INSTITUTIONAL CROSS-SECTIONAL STUDY
Giuseppe Eliseo ALLOCCA, Alexandrina MUNTEAN , Cristian Doru OLTEANU , Sorana Maria BUCUR
Medicine and Materials.2025; 5(2): 73. CrossRef - Desensitizing efficacy of a universal dentin adhesive containing mesoporous bioactive glass on dentin hypersensitivity: a randomized clinical trial with a split-mouth model
Hyun-Jung Kim, Soram Oh, Jiyoung Kwon, Kyoung-Kyu Choi, Ji-Hyun Jang, Duck-Su Kim
Scientific Reports.2024;[Epub] CrossRef
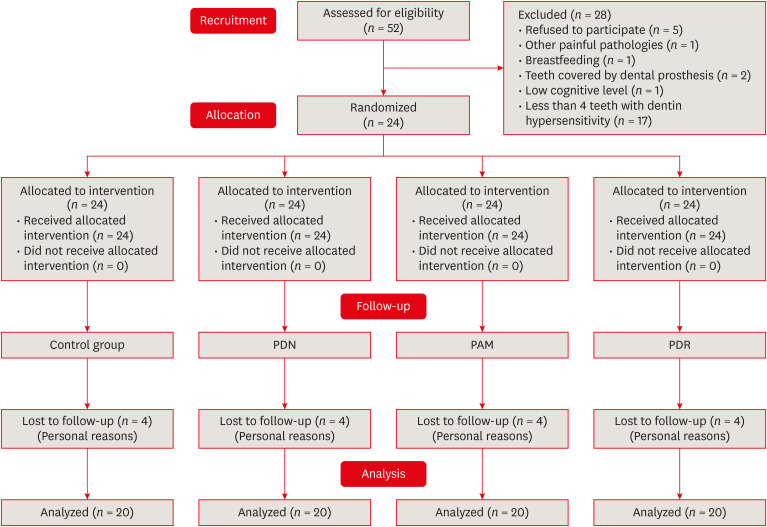
Figure 1
Description of application and composition of products used according to each manufacturer
| Product | Application | Composition |
|---|---|---|
| Enamelast (Ultradent, South Jordan, UT, USA) | Prophylaxis, relative isolation, thoroughly dry the area and apply the product to the sensitive region, wait for 1 min. Remove excess with cotton. | Five percent sodium fluoride in varnish suspension. |
| Desensibilize KF 2% (FGM, Joinville, SC, Brazil) | Prophylaxis, relative isolation, thoroughly dry the area and apply the product to the sensitive region and wait for 15 min. Remove excess with cotton and rinse abundantly with water. | Active ingredients: 5% potassium nitrate, 2% sodium fluoride. |
| Single Bond Universal (3M ESPE, St. Paul, MO, USA) | Prophylaxis, relative isolation, dry the region, apply Single Bond by rubbing for 20 sec, light air blasts for 5 sec, light-cure for 10 sec with Bluephase LED light-curing unit (Ivoclar Vivadent, Barueri, SP, Brazil) at an intensity of 1,200 mW/cm2. | HEMA, ethanol, water, initiators, silane, filler, dimethacrylate, MDP, Vitrebond Copolymer. |
| PRG Barrier Coat (Shofu, Kyoto, Japan) | Prophylaxis, relative isolation, dry the region. Add a drop of activator into the capsule with the base. Remove excess material from the brush on the edges of the capsule. The working time of the material is about 2 min. Apply a uniform layer on the surface, from the cervical to the incisal margin. Leave for 3 sec. In case of saliva contamination, remove with gauze and repeat the procedure. Light-cure for 10 sec with LED or other light-curing units. The formed film is approximately 15 μm thick without aesthetic compromise. | Base: fluoroaluminosilicate glass-based S-PRG filler, distilled water, methacrylic acid monomer, and other components. Active: phosphoric acid monomer, methacrylic acid monomer, Bis-MPEPP, carboxylic acid monomer, TEGDMA, polymerization initiator, and other components. |
LED, light-emitting diode; HEMA, 2-hydroxyethyl methacrylate; MDP, methacryloyloxydecyl dihydrogen phosphate; S-PRG, surface-partially reacted glass; Bis-MPEPP, bisphenol A polyethoxymethacrylate; TEGDMA, triethylene glycol dimethacrylate.
Description of application of management protocols
| Protocol | Step 1 | Step 2 |
|---|---|---|
| Control group | Application of adhesive system (Single Bond Universal, 3M ESPE, St. Paul, MO, USA) | N/A |
| PDN group | Application of neural desensitizing agent (Desensibilize KF 2, FGM, Joinville, SC, Brazil) | Application of adhesive system (Single Bond Universal) |
| PAM group | Application of neural desensitizing agent (Desensibilize KF 2%) | Application of obliterating agent (Enamelast, Ultradent, South Jordan, UT, USA) |
| PDR group | Application of neural desensitizing agent (Desensibilize KF 2%) | Application of remineralizing agent (PRG Barrier Coat, Shofu, Kyoto, Japan) |
PDN, Neural Desensitizing Protocol; PAM, Mixed Desensitizing Protocol; PDR, Remineralizing Desensitizing Protocol; PDN group, application of the neural desensitizing agent followed by the application of the adhesive; PAM group, application of the neural desensitizing agent followed by the application of the obliterating agent; PDR group, application of the neural desensitizing agent followed by the application of the remineralizing agent.
Patient data regarding sex, race, and age
| Variables | Values | |
|---|---|---|
| Sex | ||
| Female | 19 (79.17) | |
| Male | 5 (20.83) | |
| Race | ||
| Caucasian | 20 (83.33) | |
| Non-Caucasian | 4 (16.67) | |
| Age (yr) | ||
| 11–20 | 1 (4.17) | |
| 21–30 | 9 (37.50) | |
| 31–40 | 6 (25.00) | |
| 41–50 | 5 (20.83) | |
| 51–60 | 3 (12.50) | |
Values are presented as number (%).
Median values and interquartile ranges of sensitivity level during the execution of the tooth whitening protocol using desensitizing agents control group, PDN group, PAM group, and PDR group at different assessment times
| Time | Control group | PDN group | PAM group | PDR group |
|---|---|---|---|---|
| T1 | 7.00 ± 2.12Aab | 7.17 ± 2.12Aab | 8.00 ± 1.57Aa | 6.67 ± 3.00Ab |
| T2 | 3.54 ± 2.6Ba | 3.93 ± 4.00Ba | 3.80 ± 4.00Ba | 3.73 ± 2.57Ba |
| T3 | 3.83 ± 2.5Ba | 4.42 ± 3.7Ca | 4.00 ± 2.5Ba | 3.83 ± 2.5BCa |
| T4 | 3.00 ± 4.63Ba | 3.25 ± 3.25Da | 3.00 ± 3.75Ba | 3.00 ± 3.45Ca |
| T5 | 1.67 ± 2.2Ca | 2.50 ± 2.77BDab | 2.71 ± 3.77Cb | 1.67 ± 3.18Da |
PDN, Neural Desensitizing Protocol; PAM, Mixed Desensitizing Protocol; PDR, Remineralizing Desensitizing Protocol.
Different uppercase letters in the same column indicate significant differences (
LED, light-emitting diode; HEMA, 2-hydroxyethyl methacrylate; MDP, methacryloyloxydecyl dihydrogen phosphate; S-PRG, surface-partially reacted glass; Bis-MPEPP, bisphenol A polyethoxymethacrylate; TEGDMA, triethylene glycol dimethacrylate.
PDN, Neural Desensitizing Protocol; PAM, Mixed Desensitizing Protocol; PDR, Remineralizing Desensitizing Protocol; PDN group, application of the neural desensitizing agent followed by the application of the adhesive; PAM group, application of the neural desensitizing agent followed by the application of the obliterating agent; PDR group, application of the neural desensitizing agent followed by the application of the remineralizing agent.
Values are presented as number (%).
PDN, Neural Desensitizing Protocol; PAM, Mixed Desensitizing Protocol; PDR, Remineralizing Desensitizing Protocol.
Different uppercase letters in the same column indicate significant differences (

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

