Abstract
-
Objectives
This study evaluated the impact of different methods of irrigant agitation on smear layer removal in the apical third of curved mesial canals of 3 dimensionally (D) printed mandibular molars.
-
Materials and Methods
Sixty 3D-printed mandibular second molars were used, presenting a 70° curvature and a Vertucci type II configuration in the mesial root. A round cavity was cut 2 mm from the apex using a trephine of 2 mm in diameter, 60 bovine dentin disks were made, and a smear layer was formed. The dentin disks had the adaptation checked in the apical third of the teeth with wax. The dentin disks were evaluated in environmental scanning electron microscope before and after the following irrigant agitation methods: G1(PIK Ultrasonic Tip), G2 (Passive Ultrasonic Irrigation with Irrisonic– PUI), G3 (Easy Clean), G4 (HBW Ultrasonic Tip), G5 (Ultramint X Ultrasonic tip), and G6 (conventional irrigation-CI) (n = 10). All groups were irrigated with 2.5% sodium hypochlorite and 17% ethylenediaminetetraacetic acid.
-
Results
All dentin disks were 100% covered by the smear layer before treatment, and all groups significantly reduced the percentage of the smear layer after treatment. After the irrigation protocols, the Ultra-X group showed the lowest coverage percentage, statistically differing from the conventional, PIK, and HBW groups (p < 0.05). There was no significant difference among Ultramint X, PUI-Irrisonic, and Easy Clean (p > 0.05). None of the agitation methods could remove the smear layer altogether.
-
Conclusions
Ultramint X resulted in the most significant number of completely clean specimens.
-
Keywords: Irrigation methods; Scanning electron microscopy; Smear layer; 3D-printed
INTRODUCTION
Sufficient cleansing and disinfection, along with appropriate shaping of the root canal, constitute the primary goals of endodontic therapy. Throughout the process of preparing the root canal, remnants of dentin may collect in the branching structures, irregularities, and isthmus of the root canal. Furthermore, a smear layer and smear plugs develop on the surfaces of dentinal tubules [
1].
The smear layer (SL) can present a thickness ranging from 1 to 2 μm, and the smear plug has the capability to infiltrate dentinal tubules with a diameter of up to 40 μm. This penetration presents a challenge to the processes of root canal cleaning, the antimicrobial effectiveness of intracanal dressing, and the disinfection of dentin walls [
1,
2]. Effectively addressing the apical third of curved canals represents a significant hurdle in endodontic procedures [
3]. In the apical third of the root canal, the smear layer can hinder proper root canal filling, promoting the ingress of periapical fluid and, consequently, leading to treatment failure [
3]. Consequently, the comprehensive elimination of the smear layer is imperative to facilitate adequate root canal disinfection and filling [
4,
5,
6,
7].
Various irrigation solutions and methodologies have been suggested to enhance the efficacy of root canal cleaning and disinfection [
4]. Among these, sodium hypochlorite (NaOCl) and ethylenediaminetetraacetic acid (EDTA) stand out as the most frequently employed irrigation solutions. NaOCl exhibits proficient antimicrobial properties and effective tissue dissolution capacity. However, due to its lack of chelating capabilities, the inclusion of EDTA is indispensable for the successful removal of the smear layer [
4,
8].
Numerous studies have shown that the conventional irrigation technique falls short in achieving thorough cleaning of flat and isthmus areas [
4,
8]. Passive ultrasonic irrigation (PUI) is an alternative technique that enhances cleaning efficacy by dispersing the irrigant through sound transmission and cavitation. This method proves effective in reaching challenging areas, facilitating the removal of the smear layer, bacteria (both planktonic and biofilm), and organic tissue from the intricate anatomical details of the canal [
9,
10]. Nevertheless, its effectiveness is not absolute [
11].
Easy Clean, a product developed by Easy Dental Equipment in Belo Horizonte, MG, Brazil, constitutes an instrument crafted from a polymer known as acrylonitrile butadiene styrene. This rotating device features a cross-sectional design resembling an 'airplane wing,' measuring 0.25 mm in diameter at the tip, with a taper of 0.04 mm/mm. It is employed with either reciprocating or rotational movements [
11,
12]. Several studies have indicated that the utilization of Easy Clean yields cleaning results in isthmus and flat areas comparable to those achieved with passive ultrasonic irrigation (PUI) [
11,
12].
Ultramint X (MK Life Medica and Dental Products in Porto Alegre, Brazil), represents a cordless ultrasonic device designed to activate irrigants, sharing similarities with passive ultrasonic irrigation (PUI). Operating at a frequency of 45 kHz, this device, as per the manufacturer's claims, enhances cleaning efficacy within the root canal and in challenging anatomical regions [
13]. The device accommodates 3 types of tips—silver, blue, and gold—each featuring a 2% taper. These tips vary in size and flexibility, facilitating their use in canals of diverse shapes [
13].
The HBW ultrasonic ring is specifically engineered for the concurrent irrigation and instrumentation of root canals. Crafted from stainless steel 316, the ring is precisely cut to facilitate the effortless insertion of an endodontic instrument. According to the manufacturer, the ring serves various purposes, including exploration, creating conservative openings, extracting fractured instruments, and enhancing irrigation within the root canal [
14].
A recently developed experimental ultrasonic tip named PIK has been introduced by Medidenta based in Las Vegas, NV, USA. Constructed from poly(ether-ether-ether-ketone) polymer, this tip is designed to be used with a dedicated ultrasound insert featuring the I-Vac technology. The red-tipped PIK has a size of #25. There needs to be more studies assessing its cleaning efficacy compared to other irrigation methods.
Therefore, this study aimed to evaluate the effect of different agitation methods on the smear layer removal in dentin disks fitted in the apical portion of mesial curved canals. The null hypothesis tested is that all agitation methods are more effective than conventional irrigation.
MATERIALS AND METHODS
Sample calculation
The sample calculation was performed using the G* Power program v 3.1 for Mac by selecting the analysis of variance (ANOVA) test using data from a previous study on cement penetration [
9]. The effect size for the present study was 1.66, the alpha type error at 0.05, the beta power at 0.80, and the radius at 1. A total of 9 specimens per group were needed. To account for specimen loss, the number of specimens was increased by 10% per group. Thus, 10 teeth were used per group.
This study did not use human teeth and used fragments of bovine teeth and the project was approved by the local animal research ethics committee approved this study (001/2022).
Sixty circular dentin disks with 2 mm in diameter were obtained from the apical third of bovine roots with a trephine. The discs were standardized in 2 mm diameter and 2 millimeters thick, the dentinal surface was completely flat and fully covered with a smear layer. The disks were sanded with a water sandpaper of 320 grit to form a smear layer (Norton Saint Gobain, Guarulhos, SP, Brazil), the time used to form the smear layer was 30 seconds on each dentin disk. To confirm the smear layer formation, all specimens were evaluated by environmental scanning electron microscopy (Aspex Express; Fei Europe, Eindhoven, Netherlands) with the magnification of 500×, beam energy of 20 kV, filament drive of 62.5%, and emission current of 47.2 μA.
Sixty mandibular second molars were utilized in this study, fabricated through 3D printing technology (IM do Brasil, São Paulo, SP, Brazil). The teeth featured a Vertucci type II canal configuration with a mesial root curvature of 70°. The preparation of the mesial roots involved the use of Genius Pro Flex (Medidenta, Las Vegas, NV, USA). The working length was established using a size 15 K-file, which was inserted into the root canal and carefully observed until it reached the precise level of the apical foramen. Subsequently, 1 millimeter was subtracted from that measurement, resulting in a standardized working length of 21 mm for all teeth. The final preparation of the teeth was carried out up to a size 40 with a taper of 0.04. A 2 mm diameter diamond bur, operated with a high-speed handpiece, was employed to create a perforation in the distal face of the mesial root, situated 2 mm from the apical foramen. This perforation exposed the root canal. At this specific location, previously prepared dentin disks were positioned. These disks were meticulously adapted and fixed in place using wax to ensure secure fixation, preparing them for subsequent cleaning protocols (
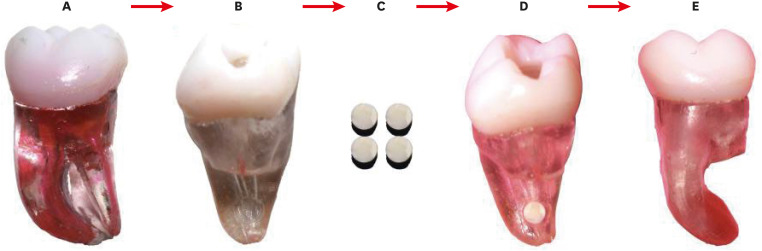
Figure 1).
Figure 1
The sequence of placement of the dentin disk into the mesial root of a 3D-printed molar. (A) 3D tooth with 70° curvature. (B) Medial root showing the hole on the distal side, 2 mm from the apical foramen. (C) Dentin discs with a diameter of 2 mm. (D) Dentin disc adaptation at the apical third level. (E) Sealed with wax at the apical third level.
3D, 3-dimensionally.
The 3D teeth with the dentin disks were randomly divided into the 6 following groups (n = 10) according to the final irrigation protocol:
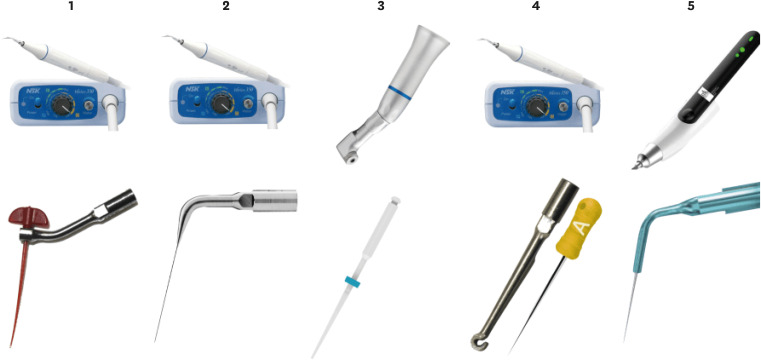
Group 1 (PIK): the canal was manually irrigated with a syringe with a 30-gauge needle with an apical outlet (MKLIFE, Porto Alegre/RS, Brazil). The needle was inserted 3 mm short of the working length and the canal was irrigated with 0.5 mL of 2.5% NaOCl (Bioquímica, São José do Rio Preto - SP, Brazil) with a flow rate of 0.5 mL/5 seconds. Activation was performed with the PIK placed in the insert and coupled to the NSK Varios 350 ultrasound device (NSK, Kanuma, Tochigi, Japan) at a power of 30% for 20 seconds, with the tip positioned 1 mm short of the working length. Two more activations were performed with the same volume and flow rate. Subsequently, the same protocol was used but with 17%% EDTA (Biodinamika Ibiporã - Paraná, Brazil). Finally, the same irrigation protocol was performed with 2.5% NaOCl, totaling 1.5 mL of NaOCl and 3 cycles of 20 seconds of activation. Three mL of sodium hypochlorite and 1.5 mm of EDTA were used (
Figure 2).
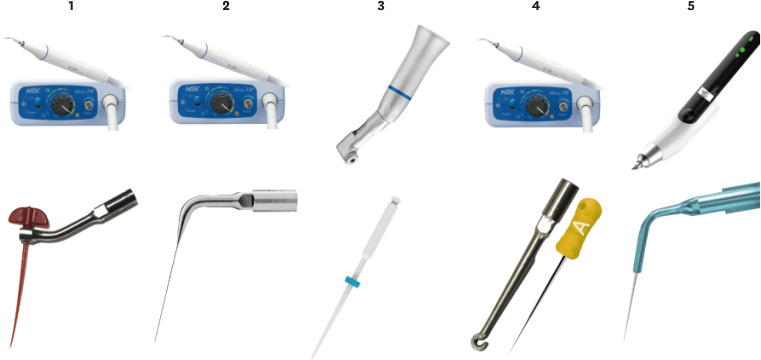
Figure 2
The image shows the different experimental groups.1: PIK adapted to an ultrasonic insert plus NSK ultrasonic. 2: Irrisonic E1 insert plus NSK ultrasonic. 3: Easyclean 25.04 with a pneumatic contra-angle. 4: HBW insert and digital spacing A plus NSK ultrasonic. 5: X Blue insert plus Ultra X ultrasonic.
PUI, passive ultrasonic irrigation.
Group 2 (PUI): The agitation was performed with Irrisonic ultrasound insert E1 (Helse Ultrasonic, Santa Rosa de Viterbo - SP, Brazil), which consists of a 20-gauge stainless steel insert with 1% taper coupled to an NSK Varios 350 ultrasound (NSK, Kanuma, Tochigi, Japan), with the pre-curved tip inserted until it reached 1 mm of the working length and the selected power was 20%. The irrigation protocol was similar to group 1. 3 mL of sodium hypochlorite and 1.5 mm of EDTA were used (
Figure 2).
Group 3 (EC): the agitation of the irrigant was performed with Easy Clean coupled to a contra-angle operated by a micromotor with approximately 12,000 revolutions per minute (KaVo Kerr Group, Charlotte, NC), used with a rotary motion. The Easy Clean tip was inserted into the canal until 1 mm short of the working length. The entire irrigation protocol was the same as in Group 1. Three mL of sodium hypochlorite and 1.5 mm of EDTA were used (
Figure 2).
Group 4 (HBW): The HBW ultrasound ring was used with a 20-gauge yellow digital spacer (Denstply-Sirona, Ballaigues, Switzerland) coupled to the NSK Varios 350 ultrasound device (NSK, Kanuma, Tochigi, Japan) at the power of 2. The spacer was slightly pre-curved and inserted to 1 mm short of the working length. The entire final irrigation protocol was the same as in Group 1. Three mL of sodium hypochlorite and 1.5 mm of EDTA were used (
Figure 2).
Group 5 (UX): The Ultramint X ultrasound was used (MK Life Medica and Dental Products, Porto Alegre, Brazil) with the X Blue flexible insert (#25, 0.02) according to the manufacturer's indications. The X Blue tip was pre-curved slightly and inserted 1 mm before the working length. The entire irrigation protocol was the same as in Group 1. Three mL of sodium hypochlorite and 1.5 mm of EDTA were used (
Figure 2).
Group 6 (CI): the canal was manually irrigated with a syringe and 30-G apical outlet needle (MKLIFE, Porto Alegre/RS, Brazil). The needle was inserted to 3 mm before the working length. The irrigation protocol consisted of 3 cycles of 0.5 mL each (flow rate of 0.5 ml/5s) of 2.5% NaOCl, with a 20-second resting interval between each cycle. A total of 1.5 mL of NaOCl was used. Next, 3 cycles of 17% EDTA irrigation were performed (each cycle of 0.5 mL, with a flow rate of 0.5 mL/5s), with a 20-second resting interval between each cycle, resulting in a total of 1.5 mL of 17% EDTA. Finally, 3 cycles of 2.5% sodium hypochlorite (each cycle of 0.5 mL, with a flow rate of 0.5 mL/5s) were performed with a 20-second resting interval between each cycle. Three mL of sodium hypochlorite and 1.5 mL of EDTA were used.
After irrigation, the dentin disks were removed and taken to the environmental scanning electron microscope and new images were taken of the central part of the disk with 500x magnification following the same parameters used for the baseline images.
The total area and the area with the smear layer were measured in the pre- and post-agitation images with the ImageJ 1.50 software (National Institutes of Health, NIH, Bethesda, MD, USA). The percentage of clean area of each specimen was then calculated according to the protocol described by Teves
et al. [
15], The areas of the smear layer were selected using the Photoshop SC software (Adobe Systems Incorporated, California, USA), and the image was saved for a further analysis using Image J 1.50 program (National Institutes of Health, NIH, Bethesda, MD, USA). The selected areas were expressed as a percentage of smear layer presence, which was used to calculate smear layer removal from original 500X ESEM (Environmental Scanning Electron Microscope) images. The number of specimens that were completely clean was recorded.
The data were tested for normality by the Shapiro-Wilks test, which revealed the absence of normality. For within-group comparisons, the Wilcoxon test was used; for between-group comparisons, the Kruskal-Wallis test was used for global comparisons, and the Dunn test was used for individual comparisons. The Chi-square test was used to compare the number of completely clean samples between the groups. The significance level adopted was 5%. The GraphPad Prism 8.0 program was used for statistical analysis.
RESULTS
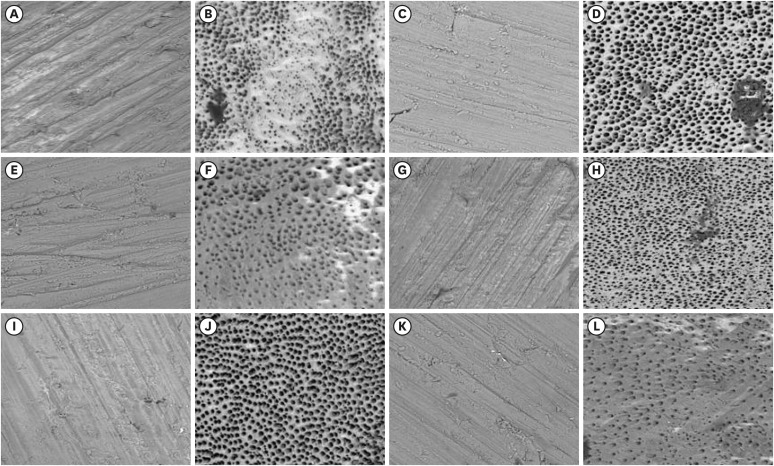
Table 1 shows the median, minimum, and maximum values of the pre- and post-agitation smear layer percentage of the groups studied and the percentage of clean specimens. The Ultramint X group had the lowest smear layer coverage (0%) with a statistically significant difference compared to the conventional (5.45%) and HBW ring (7.07%) groups (
p < 0.05). No significant differences were found in smear layer removal among Ultra-X, Easy Clean, and PUI groups (
p > 0.05). On the other hand, Ultramint X had the highest number of completely clean specimens and was different from the other groups (
p < 0.05).
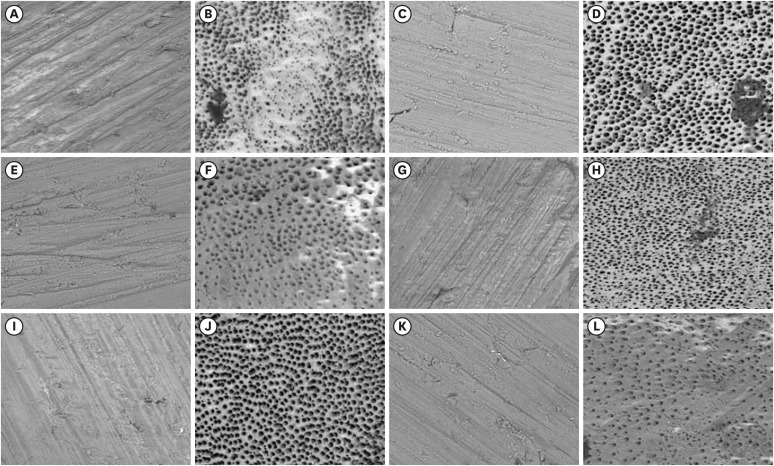
Figure 3 contains the representative images of each group studied pre- and post-agitation conditions.
Table 1 Values of the median, minimum and maximum values of the percentage of presence of smear layer pre and post agitations, and the percentage of completely clean specimens of the different studied groups
|
Group |
Pre |
Post |
% completely clean specimens |
|
PIK |
100 (100–100)aA
|
3.05 (0.11–34.56)abB
|
0b
|
|
PUI-Irrisonic |
100 (100–100)aA
|
3.30 (0–9.32)abB
|
10b
|
|
HBW |
100 (100–100)aA
|
7.07 (0–30.8)aB
|
10b
|
|
Easyclean |
100 (100–100)aA
|
1.43 (0.34–8.2)abB
|
0b
|
|
Ultramint X |
100 (100–100)aA
|
0 (0–0.7)bB
|
70a
|
|
Agulha e seringa |
100 (100–100)aA
|
5.45 (1.13–43.29)aB
|
0b
|
Figure 3 Representative scanning electron microscopy images before and after treatment in studied groups. (A and B) PIK pre and post; (C and D) PUI pre and post; (E and F) HBW pre and post; (G and H) Easyclean pre and post, (I and J) Ultramint X pre and post; (K and L); conventional pre and post (magnification 500× and 20 kV current).
DISCUSSION
Mechanical instrumentation of the root canal is a crucial phase in root canal therapy, yet it gives rise to a layer of organic and inorganic debris measuring 1 to 5 micrometers. This layer obstructs the dentinal tubules, hindering direct contact with the irrigant, intracanal medication, and filling cement. Moreover, this debris can potentially harbor bacteria, posing a risk of treatment failure [
16].
Bovine dentin disks featuring a smear layer were utilized to assess the efficacy of various irrigant agitation methods in cleaning. Bovine dentin was a surrogate for human dentin due to its availability, ease of procurement in optimal conditions, and consistent composition [
17]. Comparative studies investigating the characteristics of bovine and human dentin have demonstrated a similarity in mineral composition [
18]. Moreover, research has established that the concentration of chemical elements, such as calcium and phosphorus, remains comparable between bovine and human dentin. This is particularly noteworthy as the smear layer formed on both bovine and human substrates exhibits no significant differences in mineral concentration [
19]. A comprehensive study comparing the composition of bovine dentin with dentin from various other animals concluded that bovine dentin is the most suitable substitute for human dentin in laboratory studies [
20]. Consequently, bovine dentin has become a widely adopted standard for evaluating smear layer cleaning [
21].
Root anatomy has a crucial role in root canal cleaning and shaping. Smear layer cleaning studies often utilize uncurved or slightly curved teeth, where the challenges of cleaning and disinfection are comparatively less. In this study, the evaluation focused on apical cleaning of roots exhibiting a more intricate anatomy, characterized by a severe curvature (70°). Prototyped teeth with dentin disks were employed in the apical region across all groups to enhance standardization and reproducibility while minimizing bias and anatomical variations [
22]. The use of 3D-printed teeth allowed for standardization and presented an alternative to natural teeth [
23,
24]. In assessing smear layer removal in the apical portion, strategic 2 mm cavities were created on the inner wall of 3D-printed teeth. These cavities were meticulously positioned 2 mm from the root apex to facilitate the insertion of dentin discs. This meticulous preparation not only ensures uniformity in the location of dentin discs but also underscores the importance of precision in dental research. Establishing controlled conditions through the standardization of cavity locations enhances result reproducibility and allows for a more reliable and detailed assessment of smear layer removal efficacy in the apical region.
The use of environmental scanning electron microscopy (ESEM) facilitated the analysis of dentin both before and after the irrigation protocols without the need for metallization. This approach captured images from the same location, with the initial image as a control for each specimen. This before-and-after comparison of the same area was employed to enhance the precision and reliability of the assessment to standardize the same evaluation zone before and after the central region of the dentin disc, located 1 mm from the external edge of the disc, was utilized. Offering more robust information about the cleaning efficacy in a critical and standardized analysis of the groups [
25].
In this investigation, various methods of final irrigant agitation were scrutinized, considering the emergence of new and innovative instruments and techniques designed for the disinfection and cleaning of the smear layer in the root canal [
26]. The null hypothesis was rejected, as the analysis revealed a substantial difference between the employed methods.
This research conducted a comparative analysis between passive ultrasonic irrigation (PUI) and other agitation devices, specifically the PIK tip and the Ultramint X. Notably, these devices had yet to be previously studied, especially in roots characterized by pronounced curvatures. Across all groups, the agitation time, protocol, and irrigant volume were maintained consistently. The standardized protocol comprised 3 20-second irrigations with 2.5% sodium hypochlorite, followed by 3 20-second irrigations with 17% EDTA, and concluded with 3 20-second irrigations with 2.5% sodium hypochlorite; the final step involved saline irrigation, as prior studies suggested that this particular combination is optimal for effectively removing both organic and inorganic residues of the smear layer [
1,
9,
27].
The study’s results indicated that none of the agitation methods could entirely eliminate the smear layer from all specimens, aligning with findings from other studies highlighting the challenges in cleaning the apical third [
9,
12,
28]. The complexity of canal curvature could contribute to this difficulty. Ultramint X exhibited the most effective smear layer cleaning, although without a significant difference compared to Irrisonic-PUI, PIK, and Easy Clean in continuous rotation, demonstrating similar efficacy. The comparable results for Easy Clean and PUI align with a specific study but differ from another investigation, which reported Easy Clean superior to Irrisonic-PUI in mandibular molars' apical third [
19,
28]. This discrepancy may be attributed to the non-curvature of the insert. When evaluating the percentage of completely clean samples, Ultramint X exhibited superior performance compared to all other groups. This superiority can be attributed to the insert's greater flexibility, enabling excellent adaptation and conformation to the curvature of the root canal. Additionally, the higher frequency of the ultrasound device, approximately 45 KHz, likely contributed to its effectiveness in smear layer removal [
13]. Combining these factors facilitated optimal cleaning outcomes in the root canal treatment.
The conventional irrigation group demonstrated comparable cleanliness to all groups except Ultramint X. The similarity in results between the PUI and conventional irrigation groups can be attributed to the apical preparation with a 40/.04 instrument, which facilitates the penetration of the irrigant into the apical third. Similar findings have been observed in other studies that reported no significant differences between conventional irrigation and ultrasonic agitation [
29,
30].
The results of the PIK had high variability, possibly due to the power used and the polymer material of the insert, which promoted less agitation of the solution. There are no studies in the literature analyzing the PIK and the HBW devices with which to compare our results.
A limitation of our study is that we exclusively assessed the cleanliness of the smear layer on a single root canal wall. While this focus provides insights into a specific aspect, it doesn't negate the significance of understanding the conditions on that particular wall. Additionally, it's essential to acknowledge that the smear layer formation in our study involved the use of water sandpaper. In contrast, in actual root canals, it is typically a result of instrumentation. However, it's noteworthy that microscopic evaluation unveiled a smear layer pattern similar to that found in a root canal, adding relevance to our findings despite these limitations.
This laboratory study aimed to analyze the efficiency of different irrigant agitation methods in removing the smear layer from the apical third of mesial roots of molars with a 70° curvature. This study is one of the few that analyzed the cleaning of root canals with severe curvatures and with a new evaluation methodology. Further studies, especially clinical studies, are necessary to analyze the impact of different methods on treatment success.
CONCLUSIONS
None of the irrigant agitation methods removed the smear layer of all dentin disks placed in the apical third of root canals with accentuated curvature. Ultramint X was the agitation method that resulted in the most significant number of completely clean specimens, demonstrating its effectiveness.
-
Funding: The authors thank the CNPq proc n. 402754/2021-2, a Brazilian government agency for funding the research.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Preprint Policy: This article has been published in Restorative Dentistry and Endodontics after undergoing peer review and can also be viewed on the website at [https://doi.org/10.21203/rs.3.rs-3314693/v1].
-
Author Contributions:
Conceptualization: Cordova AT, Duarte MAH, Alcalde MP, Klymus ME, Bonjardim LR, Vivan RR.
Formal analysis: Cordova AT, Duarte MAH, Alcalde MP, Klymus ME, Bonjardim LR, Vivan RR.
Investigation: Cordova AT, Duarte MAH, Alcalde MP, Klymus ME, Bonjardim LR, Vivan RR.
Methodology: Cordova AT, Duarte MAH, Alcalde MP, Klymus ME, Bonjardim LR, Vivan RR.
Statistical analyses: Duarte MAH.
Writing original draft: Cordova AT, Duarte MAH, Alcalde MP.
Supervision: Duarte MAH, Alcalde MP.
REFERENCES
- 1. Zehnder M. Root canal irrigants. J Endod 2006;32:389-398.ArticlePubMed
- 2. Virdee SS, Seymour DW, Farnell D, Bhamra G, Bhakta S. Efficacy of irrigant activation techniques in removing intracanal smear layer and debris from mature permanent teeth: a systematic review and meta-analysis. Int Endod J 2018;51:605-621.ArticlePubMedPDF
- 3. Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:658-666.ArticlePubMed
- 4. Violich DR, Chandler NP. The smear layer in endodontics - a review. Int Endod J 2010;43:2-15.ArticlePubMed
- 5. Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. J Endod 2007;33:96-105.ArticlePubMed
- 6. Zancan RF, Di Maio A, Tomson PL, Duarte MA, Camilleri J. The presence of smear layer affects the antimicrobial action of root canal sealers. Int Endod J 2021;54:1369-1382.ArticlePubMedPDF
- 7. Portenier I, Haapasalo H, Rye A, Waltimo T, Ørstavik D, Haapasalo M. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J 2001;34:184-188.ArticlePubMedPDF
- 8. Urban K, Donnermeyer D, Schäfer E, Bürklein S. Canal cleanliness using different irrigation activation systems: a SEM evaluation. Clin Oral Investig 2017;21:2681-2687.ArticlePubMedPDF
- 9. Orlowski NB, Schimdt TF, Teixeira CD, Garcia LD, Savaris JM, Tay FR, et al. Smear layer removal using passive ultrasonic irrigation and different concentrations of sodium hypochlorite. J Endod 2020;46:1738-1744.ArticlePubMed
- 10. van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J 2007;40:415-426.ArticlePubMed
- 11. Duque JA, Duarte MA, Canali LC, Zancan RF, Vivan RR, Bernardes RA, et al. Comparative effectiveness of new mechanical irrigant agitating devices for debris removal from the canal and isthmus of mesial roots of mandibular molars. J Endod 2017;43:326-331.ArticlePubMed
- 12. Machado R, da Silva I, Comparin D, de Mattos BA, Alberton LR, da Silva Neto UX. Smear layer removal by passive ultrasonic irrigation and 2 new mechanical methods for activation of the chelating solution. Restor Dent Endod 2021;46:e11.ArticlePubMedPMCPDF
- 13. Güven Y, Ali A, Arslan H. Efficiency of Endosonic Blue, EDDY, Ultra X and EndoActivator in the removal of calcium hydroxide paste from root canals. Aust Endod J 2022;48:32-36.ArticlePubMedPDF
- 14. Coaguila-Llerena H, Lazo-Quezada G, Teves A, Zevallos-Chávez M, Faria G. Removal of separated instruments from unfavourable locations: case reports using the HBW ultrasonic ring or a surgical approach. Aust Endod J 2023;49:358-364.PubMed
- 15. Teves A, Blanco D, Casaretto M, Torres J, Alvarado DE, Coaguila-Llerena H, et al. Multispecies biofilm removal by XP-endo Finisher and passive ultrasonic irrigation: a scanning electron microscopy study. Aust Endod J 2022;48:91-97.ArticlePubMedPDF
- 16. Parente JM, Loushine RJ, Susin L, Gu L, Looney SW, Weller RN, et al. Root canal debridement using manual dynamic agitation or the EndoVac for final irrigation in a closed system and an open system. Int Endod J 2010;43:1001-1012.ArticlePubMed
- 17. Yassen GH, Platt JA, Hara AT. Bovine teeth as substitute for human teeth in dental research: a review of literature. J Oral Sci 2011;53:273-282.ArticlePubMed
- 18. Tanaka JL, Medici Filho E, Salgado JA, Salgado MA, Moraes LC, Moraes ME, et al. Comparative analysis of human and bovine teeth: radiographic density. Braz Oral Res 2008;22:346-351.ArticlePubMed
- 19. Falla-Sotelo FO, Rizzutto MA, Tabacniks MH, Added IN, Barbosa I, Markarian RA, et al. Analysis and discussion of trace elements in teeth of different animal species. Braz J Phys 2005;35:761-762.Article
- 20. Teruel JD, Alcolea A, Hernández A, Ruiz AJ. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch Oral Biol 2015;60:768-775.ArticlePubMed
- 21. Martins Justo A, Abreu da Rosa R, Santini MF, Cardoso Ferreira MB, Pereira JR, Húngaro Duarte MA, et al. Effectiveness of final irrigant protocols for debris removal from simulated canal irregularities. J Endod 2014;40:2009-2014.ArticlePubMed
- 22. Ordinola-Zapata R, Bramante CM, Duarte MA, Cavenago BC, Jaramillo D, Versiani MA. Shaping ability of Reciproc and TF adaptive systems in severely curved canals of rapid microCT-based prototyping molar replicas. J Appl Oral Sci 2014;22:509-515.ArticlePubMedPMC
- 23. Anderson J, Wealleans J, Ray J. Endodontic applications of 3D printing. Int Endod J 2018;51:1005-1018.ArticlePubMedPDF
- 24. Reymus M, Fotiadou C, Kessler A, Heck K, Hickel R, Diegritz C. 3D printed replicas for endodontic education. Int Endod J 2019;52:123-130.ArticlePubMedPDF
- 25. Boutsioukis C, Arias-Moliz MT, Chávez de Paz LE. A critical analysis of research methods and experimental models to study irrigants and irrigation systems. Int Endod J 2022;55(Supplement 2):295-329.ArticlePubMedPMCPDF
- 26. Gambarini G, Laszkiewicz J. A scanning electron microscopic study of debris and smear layer remaining following use of GT rotary instruments. Int Endod J 2002;35:422-427.ArticlePubMed
- 27. Rossi-Fedele G, Doğramaci EJ, Guastalli AR, Steier L, de Figueiredo JA. Antagonistic interactions between sodium hypochlorite, chlorhexidine, EDTA, and citric acid. J Endod 2012;38:426-431.ArticlePubMed
- 28. Kato AS, Cunha RS, da Silveira Bueno CE, Pelegrine RA, Fontana CE, de Martin AS. Investigation of the efficacy of passive ultrasonic irrigation versus irrigation with reciprocating activation: an environmental scanning electron microscopic study. J Endod 2016;42:659-663.ArticlePubMed
- 29. Rödig T, Koberg C, Baxter S, Konietschke F, Wiegand A, Rizk M. Micro-CT evaluation of sonically and ultrasonically activated irrigation on the removal of hard-tissue debris from isthmus-containing mesial root canal systems of mandibular molars. Int Endod J 2019;52:1173-1181.PubMed
- 30. Rödig T, Döllmann S, Konietschke F, Drebenstedt S, Hülsmann M. Effectiveness of different irrigant agitation techniques on debris and smear layer removal in curved root canals: a scanning electron microscopy study. J Endod 2010;36:1983-1987.ArticlePubMed
 , Murilo Priori Alcalde
, Murilo Priori Alcalde , Michel Espinosa Klymus
, Michel Espinosa Klymus , Leonardo Rigoldi Bonjardim
, Leonardo Rigoldi Bonjardim , Rodrigo Ricci Vivan
, Rodrigo Ricci Vivan , Marco Antonio Hungaro Duarte
, Marco Antonio Hungaro Duarte





 KACD
KACD
 ePub Link
ePub Link Cite
Cite

