Articles
- Page Path
- HOME > Restor Dent Endod > Volume 48(4); 2023 > Article
- Research Article Can different agents reduce the damage caused by bleaching gel to pulp tissue? A systematic review of basic research
-
Letícia Aparecida Silva Batista
 , Alexandre Henrique dos Reis-Prado
, Alexandre Henrique dos Reis-Prado , Hebertt Gonzaga dos Santos Chaves
, Hebertt Gonzaga dos Santos Chaves , Lara Cancella de Arantes
, Lara Cancella de Arantes , Luís Fernando Santos Alves Morgan
, Luís Fernando Santos Alves Morgan , Carolina Bosso André
, Carolina Bosso André , Thaís Yumi Suzuki
, Thaís Yumi Suzuki , Francine Benetti
, Francine Benetti
-
Restor Dent Endod 2023;48(4):e39.
DOI: https://doi.org/10.5395/rde.2023.48.e39
Published online: November 6, 2023
Department of Restorative Dentistry, Universidade Federal de Minas Gerais (UFMG), School of Dentistry, Belo Horizonte, MG, Brazil.
- Correspondence to Francine Benetti, PhD. Department of Restorative Dentistry, Universidade Federal de Minas Gerais (UFMG), School of Dentistry, R. Prof. Moacir Gomes de Freitas, 688 – Pampulha, Belo Horizonte, MG 31270-901, Brazil. francine-benetti@ufmg.br
Copyright © 2023. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives This study aimed to investigate the effectiveness of different topical/systemic agents in reducing the damage caused by bleaching gel to pulp tissue or cells.
-
Materials and Methods Electronic searches were performed in July 2023. In vivo and in vitro studies evaluating the effects of different topical or systemic agents on pulp inflammation or cytotoxicity after exposure to bleaching agents were included. The risk of bias was assessed.
-
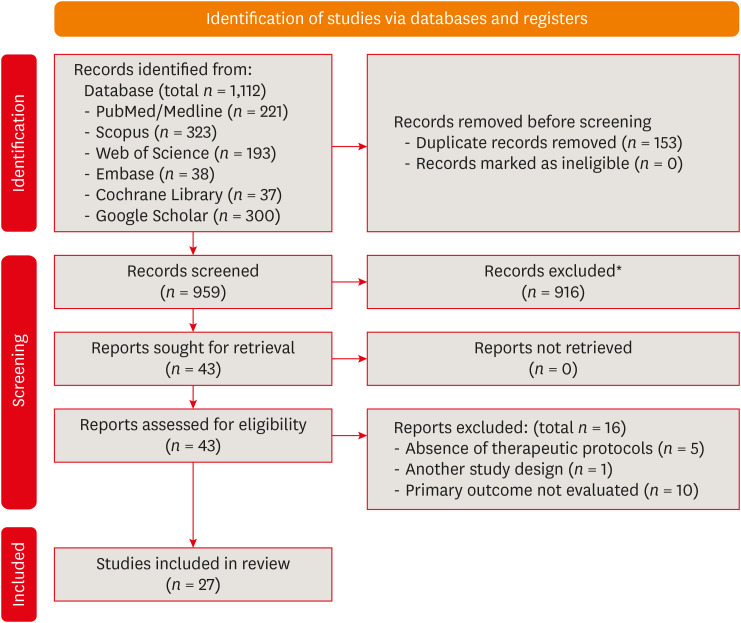
Results Out of 1,112 articles, 27 were included. Nine animal studies evaluated remineralizing/anti-inflammatories agents in rat molars subjected to bleaching with 35%–38% hydrogen peroxide (HP). Five of these studies demonstrated a significant reduction in inflammation caused by HP when combined with bioglass or MI Paste Plus (GC America), or following KF-desensitizing or Otosporin treatment (n = 3). However, orally administered drugs did not reduce pulp inflammation (n = 4). Cytotoxicity (n = 17) was primarily assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay on human dental pulp cells and mouse dental papilla Cell-23 cells. Certain substances, including sodium ascorbate, butein, manganese chloride, and peroxidase, were found to reduce cytotoxicity, particularly when applied prior to bleaching. The risk of bias was high in animal studies and low in laboratory studies.
-
Conclusions Few in vivo studies have evaluated agents to reduce the damage caused by bleaching gel to pulp tissue. Within the limitations of these studies, it was found that topical agents were effective in reducing pulp inflammation in animals and cytotoxicity. Further analyses with human pulp are required to substantiate these findings.
-
Trial Registration PROSPERO Identifier: CRD42022337192
INTRODUCTION
MATERIALS AND METHODS
RESULTS
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart.
Main characteristics and effects of protocols on pulp tissue using in vivo models
| Author | Experimental model | Groups | Bleaching gel protocol | Additional protocol | Period of analysis | Methods for outcome assessment | Main results |
|---|---|---|---|---|---|---|---|
| Moura et al. 2022 [61] | Upper and lower molars of rats | Control: untreated, Ble: 37.5% HP, Ble+Ibu-Gel: 37.5%HP and Ibu-Gel, Ble+Ibu-Neg: 37.5%HP and Ibu-Neg150 | 37.5% HP 24 min (3 × 8 min) | 0.01 mL per hemi-arch: 10 min before and after HP. | 24 h and 14 d | Inflammation and tissue organization: HE; Immunolabeling of IL-10, IL-1β, SP, COX-2 and Bradykinin: IHC | Ble+Ibu-Neg group decreased inflammation and necrotic areas at 24 h, without significant differences at 14 d. At 14 d, Ble+Ibu-Neg increased IL-10 immunolabeling, while Ble+Ibu-Gel decreased IL-1β at 24 h. There were no significant differences in the other markers. |
| Barbosa et al. 2020 [1] | Upper molars of rats | Control: untreated, Ble: 35% HP, Ble-Rem: 35% HP before MI Paste Plus, Rem-Ble: MI Paste Plus before 35% HP, Rem-Ble-Rem: MI Paste Plus before and after HP, Ble+Rem: MI Paste Plus mixed with 35% HP | 35% HP gel for 30 min | 0.01 mL MI Paste Plus (topically): for 20 min, before and/or after HP; MI Paste mixed with HP (topically): for 30 min | 2 and 30 d | Inflammation and pulp chamber area: HE | MI Paste Plus mixed with HP reduced the inflammation at 2 d, and tertiary dentin formation at 30 d. |
| Carminatti et al. 2020 [12] | Upper molars of rats | Control: placebo gel, Ble: 35% HP, Ble-BS: 35% HP before BS, BS-Ble: BS before 35% HP, BS/7d-Ble: BS for 7 d before 35% HP, Ble+BS: HP mixed with BS | 35% HP gel for 30 min | BS (topically): rubbed for 30 s and remained for 20 min, after or before HP; HP mixed with BS (topically): for 30 min | 2 and 30 d | Inflammation and pulp chamber area: HE | A single BS-based gel application beforehand or BS mixed with HP reduced inflammation, while BS mixed with HP minimized tertiary dentin formation at 30 d. |
| da Silva et al. 2020 [25] | Upper molars of rats | Control: placebo gel, 38% HP, Ibu, Ibu before and after HP, 2% Des KF, 2% Des KF before HP | 38% HP gel for 40 min | 0.2 mL Ibu (orally): for 30 min before and after HP, and every 12 h until the analysis; 0.01 mL Des KF (topically): for 10 min, 30 min before HP | 0, 24 and 48 h | Inflammation: HE; Immunolabeling of SP and CGRP: IHC | 2% Des KF reduced inflammation at 24 h, and decreased SP and CGRP immunolabeling at 24 and 48 h. |
| Ferreira et al. 2020 [58] | Upper molars of rats | Control: placebo gel, Ble: 35% HP, Ble-O: 35% HP before Oto, Ble-C: 35% HP before Curcumin, Ble-I: 35% HP before Ibu | 35% HP gel for 30 min | Oto or Curcumin (topically): for 10 min, after HP; 50 mg/Kg Ibu (orally): after HP, for 2 days, every 12h | 2 d | Inflammation: HE | Otosporin significantly reduced inflammation in all thirds of the coronary pulp. |
| Gallinari et al. 2019 [3] | Upper molars of rats | Placebo gel, 35% HP, Placebo gel before Oto, 35% HP before Oto, Placebo after Tyl, 35% HP after Tyl | 35% HP gel for 45 min | Oto (topically): for 10 min, after HP; Tyl (orally): 30 min before HP, and every 12 h until the analysis | 0, 24 and 48 h | Inflammation: HE; Immunolabeling of SP and CGRP: IHC | Otosporin reduced inflammation, mainly at 48 h. Otosporin and Tylenol reduced SP immunolabeling, mainly at 0 and 24 h, without significant influence on CGRP immunolabeling. |
| Louzada et al. 2019 [26] | Upper molars of rats | Control: untreated, 35% HP, 35% HP before 2.5% carvedilol gel | 35% HP gel for 30 min | 0.01 mL Carvedilol gel (topically): for 10 min, immediately after HP | 2 and 30 d | Inflammation and pulp chamber area: HE | 2.5% Carvedilol gel did not influence inflammatory reaction and tertiary dentin formation. |
| Benetti et al. 2018 [11] | Upper molars of rats | Control: placebo gel, 35% HP, 35% HP before Oto | 35% HP gel for 30 min | 0.01 mL Oto (topically): for 10 min, after HP | 2 d | Inflammation: HE; Immunolabeling of TNF-α, IL-6, IL-17: IHC | Otosporin reduced pulp inflammation in the occlusal and middle thirds, and decreased the immunolabeling of TNF-α. There was no influence on IL-6 and IL-17. |
| Lima et al. 2016 [17] | Lower molars of rats | Control: untreated, DW before 35% HP, AA before 35% HP | 35% HP gel for 10 min (2 × 5 min) | DW or AA (orally): 90 min before HP | 6 h, 24 h, 3 d and 7 d | Inflammation and tissue organization: HE | Ascorbic acid prior to HP did not influence inflammation, but enhanced tissue organization at 24 h. |
Main characteristics and effects of protocols on cell viability/cytotoxicity, morphology, and mineralization
| Author | Experimental model | Groups | Bleaching gel protocol | Additional protocol | Period of analysis | Methods for outcome assessment | Main results | |
|---|---|---|---|---|---|---|---|---|
| In vitro studies using enamel/dentin discs and MSCs | ||||||||
| Dias et al. 2023 [62] | 5.6 × 2.3 mm enamel/dentin discs, and MDPC-23 cells | Negative control, PCP, 10% HP, 10% HP + PCP, 20% HP, 20% HP + PCP, 35% HP, 35% HP + PCP | 10%, 20% and 35% HP for 45 min | 10 μL of PCP (topically): before HP | 4 h for cell viability, 1 h for cell morphology | Cell viability: Alamar Blue and fluorescence; Cell morphology: SEM | Coating enamel with PCP before the bleaching protocols minimized the cytotoxic effects and morphological changes caused by HP, independent of its concentration. | |
| de Oliveira Ribeiro et al. 2022 [60] | 5.6 × 2.3 mm enamel/dentin discs, and MDPC-23 cells | Negative control, 35% HP, 10% HP, 10% HP + 2 mg/mL MnO2, 10% HP + 6 mg/mL MnO2, 10% HP + 10 mg/mL MnO2 | 10% HP for 45 min | 20 μL of 10% HP mixed with 2-10 mg/mL MnO2 (topically): 45 min (3 applications of 15 min) | 1 h | Cell viability: MTT and live/dead | Higher concentrations of MnO2 applied to the gel reduced cytotoxicity, especially 10 mg/mL of MnO2. | |
| Ribeiro et al. 2022 [59] | 5.6 × 2.3 mm bovine enamel/dentin discs, and MDPC-23 cells | CG: untreated; G1: 35% HP; G2: 35% HP + 2 mg/mL MnO2; G3: 35% HP + 6 mg/mL MnO2; G4: 35% HP + 10 mg/mL MnO2 | 35% HP for 45 min (3 × 15 min) | 2, 6, and 10 mg/mL of MnO2 incorporated into the bleaching gel: for 45 min (3 × 15 min) | 1 h | Cell viability: MTT; Cytotoxicity: live/dead assay; Cell morphology: SEM | 6 and 10 mg/mL of MnO2 incorporated into the bleaching gel increased cell viability. | |
| Ortecho-Zuta et al. 2019 [57] | 5.6 × 2.3 mm enamel/dentin discs, and MDPC-23 cells | Untreated, HP: 35% HP, 35% HP + HRP | 35% HP for 45 min (3 applications of 15 min) | 1 mL of 35% HP mixed with 10 mg of HRP (topically): for 45 min (3 applications of 15 min) | 1 h | Cell viability: MTT; cell morphology: SEM; cytotoxicity: live/dead assay | HRP associated with HP increased cell viability compared to 35% HP alone, in addition to showing less impact on morphology. | |
| Soares et al. 2019 [23] | 5.6 ± 3.5 mm enamel/dentin discs, and MDPC-23 cells | Untreated, HP: 35% HP, 35% HP + FS, 35% HP + MC, 35% HP + PR, 35% HP + CT | 35% HP for 45 min (3 applications of 15 min) | 40 mL of 35% HP mixed with 1 mg of FS, MC, PR, or CT (topically): for 45 min (3 applications of 15 min) | 1 and 24 h | Cell viability: MTT | All chemically activated groups increased cell viability, mainly in the HP + PR group. | |
| de Oliveira Duque et al. 2014 [45] | 5.6 ± 3.5 mm enamel/dentin discs, HDPCs and MDPC-23 cells | Untreated, 10% CP, 35% HP, 35% HP + FS | 10% CP for 4 h, and 35% HP for 45 min (3 applications of 15 min) | 0.004 g FS mixed with 35% HP (topically): 45 min (3 applications of 15 min) | 1 h | Cell viability: MTT | Chemical activation of HP by FS had no significant protective effect against cytotoxicity, decreasing cell viability compared to CP. | |
| Soares et al. 2013 [34] | 5.6 × 3.5 mm enamel/dentin discs, and MDPC-23 cells | Control: DW, 1 d 16% CP, 7 d 16% CP, 14 d 16% CP, 7 d 16% CP + 0,05% fluoride, 14 d 16% CP + 0.05% fluoride, 7 d 16% CP + 0.2% fluoride, 14 d 16% CP + 0.2% fluoride | 16% CP for 8 h/d | 0.05 % or 0.2% fluoride (topically): for 1 min after 16% CP | 1, 7 and 14 d | Cell viability: MTT; ALP activity: colorimetric endpoint assay; cell membrane damage: flow cytometry | Fluoride solutions cannot prevent the toxic effects of a 16% CP bleaching applied on enamel, in addition to having no impact on ALP activity. | |
| Lima et al. 2010 [47] | 0.5 mm dentin discs and MDPC-23 cells | Control: untreated, 10% sodium ascorbate, 10% CP, 10% sodium ascorbate + 10% CP, 16% CP, 10% sodium ascorbate + 16% CP | 10% and 16% CP for 6 h | Sodium ascorbate 10% (topically): for 6 h, before CP | 6 h | Cell viability: MTT; cell morphology: SEM | 10% sodium ascorbate on the dentin discs before the use of the CP reduced the cytotoxic effects of these products on cells. | |
| In vitro studies using MSCs | ||||||||
| Huang et al. 2019 [56] | HDPCs | NC: negative control, HP (50, 150, 250, 350 μM), HP+NAC: HP (250 μM) + NAC (2.5 mM), HP+CsA: HP (250 μM) + CsA (2 μM), siRNA-CypD: CypD siRNA targeting human PPIF, siRNA-CypD+HP: CypD siRNA targeting human PPIF + HP (250 μM) | 250 μM HP for 24 h | NAC or CsA reagents for 24 h; CypD siRNA-PPIF for 24 h | 1, 3, 6, 12, 24 and 48 h for MTT, 24 h for the other analyses. | Cell viability: MTT; measurement of cell death: FITC and TUNEL assays; intercellular ATP level: ATP detection kit; detection of Ca2+: fluorescence microscope | NAC, CsA and CypD siRNA-PPIF were able to preserve the cell viability, mitigate cell death, decrease the intracellular Ca2+ and enhance the ATP level. | |
| Kim et al. 2017 [54] | HDPCs | Control: untreated, 180 μM HP, HP + 50 μM IAA, HP + 100 μM IAA, HP + 150 μM IAA, HP + 200 μM IAA, HP + 250 μM IAA, HP + 300 μM IAA | 180 μM HP for 24 h | IAA: ranging from 1 to 300 μM | 24 h | Cell viability: MTS | IAA treatment increased cell viability. | |
| Kim et al. 2017 [55] | HDPCs | Control: untreated, HP: 300 µM HP, CA: 20 µM CA, CA + HP: 20 µM CA and then 300 µM HP, CoPP: 20 µM CoPP, CoPP+HP: 20 µM CoPP and then 300 µM HP | 300 µM HP for 24 h | 20 µM CA: for 24 h, before HP | 24 h | Cell viability: MTT; cytotoxicity: LDH activity assay | Pre-treatment with CA effectively prevented HP-induced cell death. | |
| Vargas et al. 2014 [53] | MDPC-23 cells | Negative control: DMEM + 5% DMSO, positive control: 0.018% HP, 1 mM α-T, 3 mM α-T, 5 mM α-T: 10 mM α-T, 1mM α-T + 0.018% HP, 3 mM α-T + 0.018% HP, G9: 5 mM α-T followed by 0.018% HP, G10: 10 mM α-T followed by 0.018% HP | 0.018% HP for 30 min | 1, 3, 5, or 10 mM α-T: for 60 min before HP | 60 min | Cell viability: MTT | Pretreatment with vitamin E α-T isomer increased cell viability of MDPC-23 pulp cells, especially using 5 and 10 mM α-T. | |
| Vargas et al. 2014 [52] | MDPC-23 cells | 1 mM α-T, 1 mM α-T + 0.018% HP, 3 mM α-T, 3 mM α-T + 0.018% HP, 5 mM α-T, 5 mM α-T +, 0.018% HP, 10 mM α-T, 10 mM α-T + 0.018% HP, negative control: DMEM + 5% DMSO, positive control: 0.018% HP | 0.018 % HP for 30 min | 1, 3, 5, or 10 mM α-T: for 1, 4, 8 and 24 h before HP | 1, 4, 8 and 24 h | Cell viability: MTT | Vitamin E alpha-tocopherol isomer showed a protective effect against HP cytotoxicity, especially using 1 and 3 mM α-T for 24 h. | |
| Jeong et al. 2010 [46] | HDPCs | 1 mM HP, HP + 5 μM sappanchalcone, HP + 10 μM sappanchalcone, HP + 20 μM sappanchalcone, HP + 40 μM sappanchalcone, HP + 40 μM sappanchalcone + 100 μM SnPP, 100 μM SnPP, HP + 20 μM CoPP, positive control: 20 μM | 1 mM HP for 12 h | 5–40 μM sappanchalcone: 12 h | 12 h | Cell viability: MTT | Copp and 20 and 40 μM sappanchalcone reduced HP-induced cytotoxicity. | |
| Lee et al. 2013 [24] | HDPCs | Control: untreated, 1mM HP, 1mM HP + 2.5 μM butein, 1mM HP + 5 μM butein, 1mM HP + 10 μM butein, 1mM HP + 20 μM butein, 1mM HP + 20 μM CoPP, 1mM HP+ 100 μM SnPP | 1 mM HP for 12 h | Butein, CoPP and SnPP: for 8 h or until 24 h | 8 h | Cell viability: MTT | Butein inhibited HP-induced cytotoxicity. | |
| Lee et al. 2013 [49] | HDPCs | HP+Ad/PPARγ: HP+Ad PPARγ, HP+Ad/LacZ: control, HP | 150 μmol HP for 12 d | Ad/PPARγ virus: a dose of 100 MOI for 24 h | 12 d | Cell viability: MTT; dentin mineralization: alizarin red stain and ALP activity assay. | PPARγ in pulp cells increased cell viability, odontoblastic differentiation and dentin mineralization. | |
| Lee et al. 2013 [50] | HDPCs | Control: untreated, HP: 500 µM HP, 500 µM HP + 5 µM sulfuretin, 500 µM HP + 10 µM sulfuretin, 500 µM HP + 20 µM sulfuretin, 500 µM HP + 40 µM sulfuretin, sulfuretin: 5-40 µM sulfuretin, HP+CoPP: 500 µM HP + 20 µM CoPP, positive control: 20 µM CoPP | 500 µM HP for 12 h | 5-40 µM sulfuretin: for 12 h, before HP | 12 h | Cell viability: MTT | Pre-treatment with sulfuretin increased cell viability, presumably through HO-1 expression. | |
| Lee et al. 2013 [51] | HDPCs | HP: 150 µM HP, 150 µM HP + 15 µM pachymic acid, 150 µM HP + 5 µM pachymic acid, untreated cells | 150 µM HP for 1, 3, 5, 7 and 12 d | 15 µM pachymic acid: before 1 h prior to incubation with HP | 1, 3, 5, 7, and 12 d | Cell viability: MTT; odontoblast differentiation level: ALP activity and alizarin red S staining | Pachymic acid increased cell viability and mineralization. | |
| Choi et al. 2012 [48] | hDPSCs | HP: 200 µM HP, 200 µM HP + 2 µM SOD1, 200 µM HP + 2 µM LMWP-SOD1, 2 µM SOD1, 2 µM LMWP-SOD1, untreated | 200 µM HP for 2 h | 2 µM LMWP-SOD1: for 3 h, before HP | 3 and 28 d | Cell viability: MTT; matrix mineralization: alizarin red S staining; expression of odontogenic markers: PCR | LMWP-SOD1 did not influence cell viability and the calcified area. However, it reverses HP inhibition of osteogenic markers. | |
Main characteristics and effects of protocols on oxidative stress and the expression of other proteins
| Author | Experimental model | Groups | Bleaching gel protocol | Additional protocol | Period of analysis | Methods for outcome assessment | Main results | |
|---|---|---|---|---|---|---|---|---|
| In vitro studies using enamel/dentin discs and MSCs | ||||||||
| Dias et al. 2023 [62] | Enamel/dentin discs, and MDPC-23 cells | Negative control, PCP, 10% HP, 10% HP + PCP, 20% HP, 20% HP + PCP, 35% HP, 35% HP + PCP | 10%, 20% and 35% HP for 45 min | 10 μL of PCP (topically): before HP | 1 h | Oxidative stress: carboxy-H2DCFDA fluorescence | The groups where PCP was used before applying the bleaching gels showed lower oxidative stress in MDPC-23 cells. | |
| de Oliveira Ribeiro et al. 2022 [60] | Enamel/dentin discs, and MDPC-23 cells | Negative control, 35% HP, 10% HP, 10% HP + 2 mg/mL MnO2, 10% HP + 6 mg/mL MnO2, 10% HP + 10 mg/mL MnO2 | 10% HP for 45 min | 20 μL of 10% HP mixed with 2 mg/mL, 6 mg/mL, or 10 mg/mL MnO2 (topically): 45 min (3 applications of 15 min) | 30 min | Oxidative stress: carboxy-H2DCFDA fluorescence | MnO2 increased the degradation kinetics of the HP molecule, consequently reducing the cellular oxidative stress index. | |
| Ribeiro et al. 2022 [59] | Bovine enamel and dentin discs, and MDPC-23 cells | CG: untreated; G1: 35% HP; G2: 35% HP + 2 mg/mL MnO2; G3: 35% HP + 6 mg/mL MnO2; G4: 35% HP + 10 mg/mL MnO2 | 45 min (3 applications of 15 min) | 2, 6, and 10 mg/mL of MnO2 was incorporated into the bleaching gel: for 45 min (3 × 15 min) | Immediately | Oxidative stress: carboxy-H2DCFDA fluorescence; | The addition of MnO2 to 35% HP reduced oxidative stress. | |
| Ortecho-Zuta et al. 2019 [57] | Enamel/dentin discs, and MDPC-23 cells | Untreated, HP: 35% HP, 35% HP + HRP | 35% HP for 45 min (3 applications of 15 min) | 1 mL of 35% HP mixed with 10 mg HRP (topically): for 45 min (3 applications of 15 min) | 1 h | Oxidative stress: carboxy-H2DCFDA fluorescence | HRP combined with HP reduced oxidative stress. | |
| Soares et al. 2019 [23] | Enamel/dentin discs, and MDPC-23 cells | Untreated, HP: 35% HP, 35% HP + FS, 35% HP + MC, 35% HP + PR, 35% HP + CT | 35% HP for 45 min (3 applications of 15 min) | 40 mL of 35% HP mixed with 1 mg FS, MC, PR, or CT (topically): for 45 min (3 applications of 15 min) | 1 and 24 h | Oxidative stress: carboxy-H2DCFDA fluorescence | All chemically activated groups showed reduced oxidative stress. | |
| In vitro studies using MSCs | ||||||||
| Huang et al. 2019 [56] | HDPCs | NC: negative control, HP (50, 150, 250, 350 μM), HP+NAC: HP (250 μM) + NAC (2.5 mM), HP+CsA: HP (250 μM) + CsA (2 μM), siRNA-CypD: CypD siRNA targeting human PPIF, siRNA-CypD+HP: CypD siRNA targeting human PPIF + HP (250 μM) | 250 μM HP for 24 h | NAC or CsA reagents for 24 h; CypD siRNA-PPIF for 24 h | 24 h | Bockade of CypD: western lot analyses | NAC, CsA, and CypD siRNA-PPIF decreased the CypD expression, increasing mitochondrial membrane potential. | |
| Kim et al. 2017 [54] | HDPCs | Control: untreated, 180 μM HP, HP + 50 μM IAA, HP + 100 μM IAA, HP + 150 μM IAA, HP + 200 μM IAA, HP + 250 μM IAA, HP + 300 μM IAA | 180 μM HP for 24 h | IAA: ranging from 1 to 300 μM | 6 h | Expression of apoptotic (BAX and p53) and antiapoptotic (BCL-2 and ATF5) genes: PCR; ROS detection: fluorescence; Nrf2 and HO-1 expression: densitometric analysis; cell cycle: flow cytometry | IAA treatment protected HDPCs against HP-induced oxidative stress via increased expression of Nrf2 and HO-1. Moreover, IAA treatment rescued cell cycle and prevented apoptosis. | |
| Kim et al. 2017 [55] | HDPCs | Control: untreated, HP: 300 µM HP, CA: 20 µM CA, CA + HP: 20 µM CA and then 300 µM HP, CoPP: 20 µM CoPP, CoPP+HP: 20 µM CoPP and then 300 µM HP | 300 µM HP for 24 h | 20 µM CA: for 24 h, before HP | 6 h | ROS measurement: fluorescence analysis; expression of proteins (HO-1, Nrf2): western blot | Pre-treatment with CA protected HDPCs against HP-induced oxidative stress by enhancing the expression of HO-1 through the Nrf2 signaling pathway. | |
| Jeong et al. 2010 [46] | HDPCs | 1 mM HP, HP + 5 μM sappanchalcone, HP + 10 μM sappanchalcone, HP + 20 μM sappanchalcone, HP + 40 μM sappanchalcone, HP + 40 μM sappanchalcone + 100 μM SnPP, 100 μM SnPP, HP + 20 μM CoPP, positive control: 20 μM | 1 mM HP for 12 h | 5–40 μM sappanchalcone: 12 h | 12 h | ROS measurement: fluorescence | CoPP and 20 and 40 μM sappanchalcone inhibited ROS production. | |
| Lee et al. 2013 [24] | HDPCs | Control: untreated, 1mM HP, 1mM HP + 2.5 μM butein, 1mM HP + 5 μM butein, 1mM HP + 10 μM butein, 1mM HP + 20 μM butein, 1mM HP + 20 μM CoPP, 1mM HP+ 100 μM SnPP | 1 mM HP for 12 h | Butein, CoPP and SnPP: for 8 h or until 24 h | 8 h for ROS detection; 0, 3, 6, 12, 18 and 24 h for HO-1 expression | ROS measurement: fluorescence; HO-1 expression: western blot; Nrf2 expression: immunofluorescence, western blot | Butein inhibited HP-induced ROS production, presumably through JNK Nrf2/ARE-dependent HO-1 expression. | |
| Lee et al. 2013 [49] | HDPCs | HP+Ad/PPARγ: HP+Ad PPARγ, HP+Ad/LacZ: control, HP | 150 μmol HP for 12 d | Ad/PPARγ virus: a dose of 100 multiplicity of infection (MOI) for 24 h | 12 d | ROS measurement: flow cytometry; expression of antioxidant molecules: western blot | PPARγ in pulp cells removed cellular ROS under oxidative stress. | |
| Lee et al. 2013 [50] | HDPCs | Control: untreated, HP: 500 µM HP, 500 µM HP + 5 µM sulfuretin, 500 µM HP + 10 µM sulfuretin, 500 µM HP + 20 µM sulfuretin, 500 µM HP + 40 µM sulfuretin, sulfuretin: 5-40 µM sulfuretin, HP+CoPP: 500 µM HP + 20 µM CoPP, positive control: 20 µM CoPP | 500 µM HP for 12 h | 5-40 µM sulfuretin: for 12 h, before HP | 12 h | ROS measurement: fluorescence; expression of HO-1: western blot | Pre-treatment with sulfuretin suppressed cellular damage from oxidation caused by HP in HDPCs, presumably through HO-1 expression. | |
| Lee et al. 2013 [51] | HDPCs | HP: 150 µM HP, 150 µM HP + 15 µM pachymic acid, 150 µM HP + 5 µM pachymic acid, untreated cells | 150 µM HP for 1, 3, 5, 7 and 12 d | 15 µM pachymic acid: before 1 h prior to incubation with HP | 1, 3, 5, 7 and 12 d | Expression of inflammatory molecules and odontoblast differentiation level: western blot | The pachymic acid showed anti-inflammatory function and odontoblast differentiation via HO-1 pathway. | |
| Choi et al. 2012 [48] | hDPSCs | HP: 200 µM HP, 200 µM HP + 2 µM SOD1, 200 µM HP + 2 µM LMWP-SOD1, 2 µM SOD1, 2 µM LMWP-SOD1, untreated | 200 µM HP for 2 h | 2 µM LMWP-SOD1: for 3 h, before HP | 3 and 28 d | Transduction of LMWP-SOD1 and quantification of p53 and p21Cip1: western blot | LMWP-SOD1 conjugates were effective for attenuating cellular senescence and reversing osteoblastic differentiation of hDPSCs caused by oxidative stress inhibition. | |
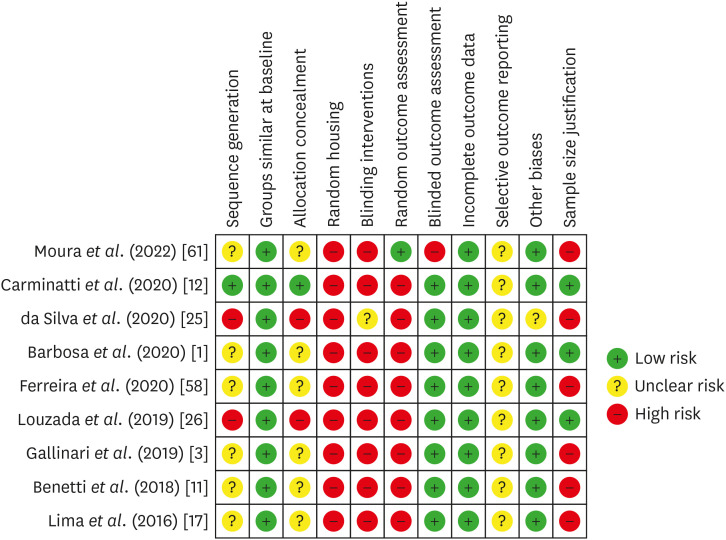
Risk of bias in individual animal studies according to the SYRCLE’s RoB tool for assessing risk of bias.
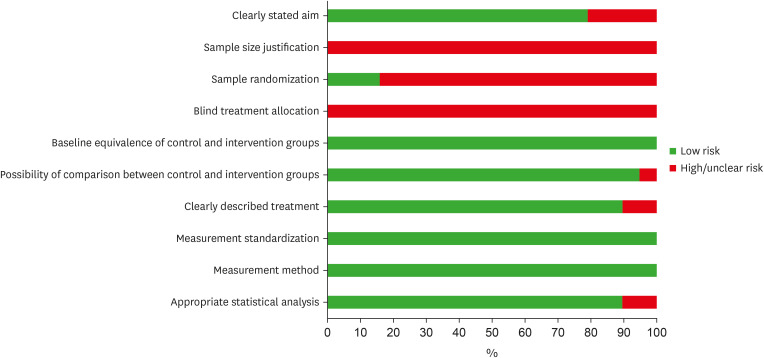
Risk of bias assessment of the eligible in vitro studies, by the percentage of the scores attributed to each evaluated study (Joanna Briggs Institute’s Critical Appraisal Checklist).
DISCUSSION
CONCLUSIONS
-
Funding: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES, 88887.649870/2021-00; 88887.712700/2022-00) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 128044/2022-5).
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Morgan LFSA, André CB, Suzuki TY, Benetti F.
Data curation: Batista LAS, Chaves HGS, Reis-Prado AH, Arantes LC, Benetti F.
Methodology: Reis-Prado AH, Chaves HGS, André CB, Suzuki TY, Benetti F.
Project administration: Benetti F, Reis-Prado AH, Morgan LFSA.
Resources: Morgan LFSA, Benetti F.
Supervision: André CB, Suzuki TY, Benetti F.
Validation: Batista LAS, Reis-Prado AH, Arantes LC.
Visualization: Batista LAS, Chaves HGS, Arantes LC.
Writing - original draft: Batista LAS, Reis-Prado AH, Chaves HGS, Arantes LC.
Writing - review & editing: Morgan LFSA, André CB, Suzuki TY, Benetti F.
SUPPLEMENTARY MATERIALS
- 1. Barbosa JG, Benetti F, de Oliveira Gallinari M, Carminatti M, da Silva ABD, Lopes INI, et al. Bleaching gel mixed with MI Paste Plus reduces penetration of H2O2 and damage to pulp tissue and maintains bleaching effectiveness. Clin Oral Investig 2020;24:1299-1309.ArticlePubMedPDF
- 2. Vladislavic NZ, Tadin A, Gavic L, Jerkovic D, Franic I, Verzak Z. In vivo evaluation of whitening toothpaste efficiency and patient treatment satisfaction: a randomized controlled trial. Clin Oral Investig 2022;26:739-750.PubMed
- 3. Gallinari MO, Cintra LTÂ, Benetti F, Rahal V, Ervolino E, Briso ALF. Pulp response of rats submitted to bleaching and the use of different anti-inflammatory drugs. PLoS One 2019;14:e0210338.ArticlePubMedPMC
- 4. Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:e59-e64.Article
- 5. Cintra LT, Benetti F, da Silva Facundo AC, Ferreira LL, Gomes-Filho JE, Ervolino E, et al. The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 2013;39:1576-1580.ArticlePubMed
- 6. Soares DG, Ribeiro AP, Lima AF, Sacono NT, Hebling J, de Souza Costa CA. Effect of fluoride-treated enamel on indirect cytotoxicity of a 16% carbamide peroxide bleaching gel to pulp cells. Braz Dent J 2013;24:121-127.ArticlePubMed
- 7. Reis-Prado AH, Grossi IR, Chaves HGS, André CB, Morgan LFSA, Briso ALF, et al. Influence of hydrogen peroxide on mineralization in dental pulp cells: a systematic review. Front Dent Med 2021;2:689537.Article
- 8. Favoreto MW, Vochikovski L, Terra RMO, Campos VS, Santos ME, Meireles SS, et al. Topical application of Otosporin® before in-office bleaching: a split mouth, triple-blind, multicenter randomized clinical trial. Clin Oral Investig 2022;26:2555-2564.ArticlePubMedPDF
- 9. Vochikovski L, Favoreto MW, Rezende M, Terra RMO, da Silva KL, Farago PV, et al. Effect of an experimental desensitizing gel on bleaching-induced tooth sensitivity after in-office bleaching-a double-blind, randomized controlled trial. Clin Oral Investig 2023;27:1567-1576.ArticlePubMedPDF
- 10. Rezende M, Loguercio AD, Kossatz S, Reis A. Predictive factors on the efficacy and risk/intensity of tooth sensitivity of dental bleaching: a multi regression and logistic analysis. J Dent 2016;45:1-6.ArticlePubMed
- 11. Benetti F, Briso ALF, Ferreira LL, Carminatti M, Álamo L, Ervolino E, et al. In vivo study of the action of a topical anti-inflammatory drug in rat teeth submitted to dental bleaching. Braz Dent J 2018;29:555-561.ArticlePubMed
- 12. Carminatti M, Benetti F, Siqueira RL, Zanotto ED, Briso ALF, Chaves-Neto AH, et al. Experimental gel containing bioactive glass-ceramic to minimize the pulp damage caused by dental bleaching in rats. J Appl Oral Sci 2020;28:e20190384.ArticlePubMedPMC
- 13. Santana Jorge O, Noronha Ferraz de Arruda C, Tonani Torrieri R, Geng Vivanco R, de Carvalho Panzeri Pires-de-Souza F. Over-the-counter bleaching agents can help with tooth whitening maintenance. J Esthet Restor Dent 2022;34:328-334.ArticlePubMedPDF
- 14. Souza RO, Lombardo GH, Pereira SM, Zamboni SC, Valera MC, Araujo MA, et al. Analysis of tooth enamel after excessive bleaching: a study using scanning electron microscopy and energy dispersive X-ray spectroscopy. Int J Prosthodont 2010;23:29-32.PubMed
- 15. Deng M, Wen HL, Dong XL, Li F, Xu X, Li H, et al. Effects of 45S5 bioglass on surface properties of dental enamel subjected to 35% hydrogen peroxide. Int J Oral Sci 2013;5:103-110.ArticlePubMedPMCPDF
- 16. Yang SY, Han AR, Kim KM, Kwon JS. Effects of incorporating 45S5 bioactive glass into 30% hydrogen peroxide solution on whitening efficacy and enamel surface properties. Clin Oral Investig 2022;26:5301-5312.ArticlePubMedPDF
- 17. Lima AF, Marques MR, Soares DG, Hebling J, Marchi GM, de Souza Costa CA. Antioxidant therapy enhances pulpal healing in bleached teeth. Restor Dent Endod 2016;41:44-54.ArticlePubMedPMCPDF
- 18. Benetti F, Gomes-Filho JE, Ferreira LL, Ervolino E, Briso ALF, Sivieri-Araújo G, et al. Hydrogen peroxide induces cell proliferation and apoptosis in pulp of rats after dental bleaching in vivo: effects of the dental bleaching in pulp. Arch Oral Biol 2017;81:103-109.PubMed
- 19. Benetti F, Gomes-Filho JE, Ferreira LL, Sivieri-Araújo G, Ervolino E, Briso ALF, et al. Concentration-dependent effect of bleaching agents on the immunolabelling of interleukin-6, interleukin-17 and CD5-positive cells in the dental pulp. Int Endod J 2018;51:789-799.ArticlePubMedPDF
- 20. Benetti F, Briso ALF, Carminatti M, de Araújo Lopes JM, Barbosa JG, Ervolino E, et al. The presence of osteocalcin, osteopontin and reactive oxygen species-positive cells in pulp tissue after dental bleaching. Int Endod J 2019;52:665-675.ArticlePubMedPDF
- 21. Benetti F, Briso ALF, de Araújo Lopes JM, Carminatti M, Conti LC, Gallinari MO, et al. In vivo analysis of the presence of heme oxygenase-1, transcription factor Jun-D and CD90+/CD73+/CD105+/CD45- cells in the pulp of bleached teeth. Int Endod J 2019;52:1723-1737.PubMed
- 22. Cintra LT, Benetti F, Ferreira LL, Gomes-Filho JE, Ervolino E, Gallinari MO, et al. Penetration capacity, color alteration and biological response of 2 in-office bleaching protocols. Braz Dent J 2016;27:169-175.ArticlePubMed
- 23. Soares DG, Marcomini N, Duque CCO, Bordini EAF, Zuta UO, Basso FG, et al. Increased whitening efficacy and reduced cytotoxicity are achieved by the chemical activation of a highly concentrated hydrogen peroxide bleaching gel. J Appl Oral Sci 2019;27:e20180453.ArticlePubMedPMC
- 24. Lee DS, Li B, Kim KS, Jeong GS, Kim EC, Kim YC. Butein protects human dental pulp cells from hydrogen peroxide-induced oxidative toxicity via Nrf2 pathway-dependent heme oxygenase-1 expressions. Toxicol In Vitro 2013;27:874-881.ArticlePubMed
- 25. da Silva LMAV, Cintra LTA, Gallinari MO, Benetti F, Rahal V, Ervolino E, et al. Influence of pain-relieving therapies on inflammation and the expression of proinflammatory neuropeptides after dental bleaching treatment. Restor Dent Endod 2020;45:e20.PubMedPMC
- 26. Louzada LM, Briso ALF, Benetti F, Vieira LB, de Castilho Jacinto R, Dezan-Júnior E, et al. Anti-inflammatory potential of a carvedilol gel in the pulpal tissue of rats after dental bleaching: a histopathological evaluation. J Investig Clin Dent 2019;10:e12401.ArticlePubMedPDF
- 27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 2021;134:103-112.ArticlePubMed
- 28. Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of google scholar in evidence reviews and its applicability to grey literature searching. PLoS One 2015;10:e0138237.ArticlePubMedPMC
- 29. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-174.ArticlePubMed
- 30. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43.ArticlePubMedPMCPDF
- 31. Dos Reis-Prado AH, Abreu LG, Tavares WLF, Peixoto IFDC, Viana ACD, de Oliveira EMC, et al. Comparison between immediate and delayed post space preparations: a systematic review and meta-analysis. Clin Oral Investig 2021;25:417-440.ArticlePubMedPDF
- 32. Schulte JR, Morrissette DB, Gasior EJ, Czajewski MV. The effects of bleaching application time on the dental pulp. J Am Dent Assoc 1994;125:1330-1335.ArticlePubMed
- 33. Soares DG, Ribeiro AP, Sacono NT, Coldebella CR, Hebling J, Costa CA. Transenamel and transdentinal cytotoxicity of carbamide peroxide bleaching gels on odontoblast-like MDPC-23 cells. Int Endod J 2011;44:116-125.ArticlePubMed
- 34. Soares DG, Ribeiro AP, da Silveira Vargas F, Hebling J, de Souza Costa CA. Efficacy and cytotoxicity of a bleaching gel after short application times on dental enamel. Clin Oral Investig 2013;17:1901-1909.ArticlePubMedPDF
- 35. Soares DG, Basso FG, Hebling J, de Souza Costa CA. Immediate and late analysis of dental pulp stem cells viability after indirect exposition to alternative in-office bleaching strategies. Clin Oral Investig 2015;19:1013-1020.ArticlePubMedPDF
- 36. Guazzo R, Gardin C, Bellin G, Sbricoli L, Ferroni L, Ludovichetti FS, et al. Graphene-based nanomaterials for tissue engineering in the dental field. Nanomaterials (Basel) 2018;8:349.ArticlePubMedPMC
- 37. Babich H, Reisbaum AG, Zuckerbraun HL. In vitro response of human gingival epithelial S-G cells to resveratrol. Toxicol Lett 2000;114:143-153.PubMed
- 38. Soares DG, Basso FG, Pontes EC, Garcia LF, Hebling J, de Souza Costa CA. Effective tooth-bleaching protocols capable of reducing H2O2 diffusion through enamel and dentine. J Dent 2014;42:351-358.ArticlePubMed
- 39. Kwon SR, Dawson DV, Schenck DM, Fiegel J, Wertz PW. Spectrophotometric evaluation of potassium nitrate penetration into the pulp cavity. Oper Dent 2015;40:614-621.ArticlePubMedPDF
- 40. Kwon SR, Dawson DV, Wertz PW. Time course of potassium nitrate penetration into the pulp cavity and the effect of penetration levels on tooth whitening efficacy. J Esthet Restor Dent 2016;28(Supplement 1):S14-S22.ArticlePubMedPDF
- 41. Kwon SR, Pallavi F, Shi Y, Oyoyo U, Mohraz A, Li Y. Effect of bleaching gel viscosity on tooth whitening efficacy and pulp chamber penetration: an in vitro study. Oper Dent 2018;43:326-334.PubMed
- 42. Balladares L, Alegría-Acevedo LF, Montenegro-Arana A, Arana-Gordillo LA, Pulido C, Salazar-Gracez MT, et al. Effects of pH and application technique of in-office bleaching gels on hydrogen peroxide penetration into the pulp chamber. Oper Dent 2019;44:659-667.ArticlePubMedPDF
- 43. Ma Q, Chen J, Xu X, Wang T. Impact of transparent tray-based application of bioactive glasses desensitizer on the permeability of enamel and dentin to hydrogen peroxide: an in vitro study. BMC Oral Health 2020;20:103.PubMedPMC
- 44. Parreiras SO, Favoreto MW, Lenz RE, Serra ME, Borges CPF, Loguercio AD, et al. Effect of prior application of desensitizing agent on the teeth submitted to in-office bleaching. Braz Dent J 2020;31:236-243.ArticlePubMed
- 45. Duque CC, Soares DG, Basso FG, Hebling J, de Souza Costa CA. Bleaching effectiveness, hydrogen peroxide diffusion, and cytotoxicity of a chemically activated bleaching gel. Clin Oral Investig 2014;18:1631-1637.PubMed
- 46. Jeong GS, Lee DS, Li B, Lee HJ, Kim EC, Kim YC. Effects of sappanchalcone on the cytoprotection and anti-inflammation via heme oxygenase-1 in human pulp and periodontal ligament cells. Eur J Pharmacol 2010;644:230-237.ArticlePubMed
- 47. Lima AF, Lessa FC, Mancini MN, Hebling J, Costa CA, Marchi GM. Transdentinal protective role of sodium ascorbate against the cytopathic effects of H2O2 released from bleaching agents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:e70-e76.Article
- 48. Choi YJ, Lee JY, Chung CP, Park YJ. Cell-penetrating superoxide dismutase attenuates oxidative stress-induced senescence by regulating the p53-p21(Cip1) pathway and restores osteoblastic differentiation in human dental pulp stem cells. Int J Nanomedicine 2012;7:5091-5106.PubMedPMC
- 49. Lee YH, Kang YM, Heo MJ, Kim GE, Bhattarai G, Lee NH, et al. The survival role of peroxisome proliferator-activated receptor gamma induces odontoblast differentiation against oxidative stress in human dental pulp cells. J Endod 2013;39:236-241.ArticlePubMed
- 50. Lee DS, Kim KS, Ko W, Keo S, Jeong GS, Oh H, et al. Cytoprotective effects of sulfuretin from Rhus verniciflua through regulating of heme oxygenase-1 in human dental pulp cells. Nat Prod Sci 2013;19:54-60.
- 51. Lee YH, Lee NH, Bhattarai G, Kim GE, Lee IK, Yun BS, et al. Anti-inflammatory effect of pachymic acid promotes odontoblastic differentiation via HO-1 in dental pulp cells. Oral Dis 2013;19:193-199.PubMed
- 52. Vargas FS, Soares DG, Basso FG, Hebling J, Costa CA. Dose-response and time-course of α-tocoferol mediating the cytoprotection of dental pulp cells against hydrogen peroxide. Braz Dent J 2014;25:367-371.ArticlePubMed
- 53. da Silveira Vargas F, Soares DG, Ribeiro AP, Hebling J, De Souza Costa CA. Protective effect of alpha-tocopherol isomer from vitamin E against the H2O2 induced toxicity on dental pulp cells. BioMed Res Int 2014;2014:895049.PubMedPMC
- 54. Kim D, Kim H, Kim K, Roh S. The protective effect of indole-3-acetic acid (IAA) on H2O2-damaged human dental pulp stem cells is mediated by the AKT pathway and involves increased expression of the transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) and its downstream target heme oxygenase 1 (HO-1). Oxid Med Cell Longev 2017;2017:8639485.ArticlePubMedPMCPDF
- 55. Kim NY, Ahn SG, Kim SA. Cinnamaldehyde protects human dental pulp cells against oxidative stress through the Nrf2/HO-1-dependent antioxidant response. Eur J Pharmacol 2017;815:73-79.ArticlePubMed
- 56. Huang S, Zheng B, Jin X, Yu Q, Zhang X, Sun X, et al. Blockade of cyclophilin D attenuates oxidative stress-induced cell death in human dental pulp cells. Oxid Med Cell Longev 2019;2019:1729013.ArticlePubMedPMCPDF
- 57. Ortecho-Zuta U, de Oliveira Duque CC, Leite ML, Bordini E, Basso FG, Hebling J, et al. Effects of enzymatic activation of bleaching gels on hydrogen peroxide degradation rates, bleaching effectiveness, and cytotoxicity. Oper Dent 2019;44:414-423.ArticlePubMedPDF
- 58. Ferreira L, Benetti F, Álamo L, Bosisio AC, Proença A, Rahal V, et al. Otosporin reduces pulp inflammatory reactions after dental bleaching of rat molars. Dent Press Endod 2020;10:54-61.
- 59. Ribeiro R, de Oliveira Duque CC, Ortecho-Zuta U, Leite ML, Hebling J, Soares DG, et al. Influence of manganese oxide on the esthetic efficacy and toxicity caused by conventional in-office tooth bleaching therapy. Oper Dent 2022;47:425-436.ArticlePubMedPDF
- 60. de Oliveira Ribeiro RA, Zuta UO, Soares IPM, Anselmi C, Soares DG, Briso ALF, et al. Manganese oxide increases bleaching efficacy and reduces the cytotoxicity of a 10% hydrogen peroxide bleaching gel. Clin Oral Investig 2022;26:7277-7286.ArticlePubMedPDF
- 61. Moura SKSCF, dos Santos MLV, do Nascimento LA, da Silva MFA, de França GM, da Costa LM, et al. Design of a thermosensitive ibuprofen-loaded nanogel as smart material applied as anti-inflammatory in tooth bleaching: an in vivo study. J Drug Deliv Sci Technol 2022;68:103123.
- 62. Dias MF, Martins BV, de Oliveira Ribeiro RA, Leite ML, Ortecho-Zuta U, Hebling J, et al. A new approach for professional dental bleaching using a polymeric catalyst primer. J Esthet Restor Dent 2023;35:406-415.ArticlePubMedPDF
- 63. Cintra LT, Benetti F, Ferreira LL, Rahal V, Ervolino E, Jacinto RC, et al. Evaluation of an experimental rat model for comparative studies of bleaching agents. J Appl Oral Sci 2016;24:95-104.ArticlePubMedPMC
- 64. Benetti F, Lemos CAA, de Oliveira Gallinari M, Terayama AM, Briso ALF, de Castilho Jacinto R, et al. Influence of different types of light on the response of the pulp tissue in dental bleaching: a systematic review. Clin Oral Investig 2018;22:1825-1837.ArticlePubMedPDF
- 65. Caviedes-Bucheli J, Ariza-García G, Restrepo-Méndez S, Ríos-Osorio N, Lombana N, Muñoz HR. The effect of tooth bleaching on substance P expression in human dental pulp. J Endod 2008;34:1462-1465.ArticlePubMed
- 66. Paula E, Kossatz S, Fernandes D, Loguercio A, Reis A. The effect of perioperative ibuprofen use on tooth sensitivity caused by in-office bleaching. Oper Dent 2013;38:601-608.ArticlePubMedPDF
- 67. Galler KM, Weber M, Korkmaz Y, Widbiller M, Feuerer M. Inflammatory response mechanisms of the dentine-pulp complex and the periapical tissues. Int J Mol Sci 2021;22:1480.ArticlePubMedPMC
- 68. Vaseenon S, Srisuwan T, Chattipakorn N, Chattipakorn SC. Lipopolysaccharides and hydrogen peroxide induce contrasting pathological conditions in dental pulpal cells. Int Endod J 2023;56:179-192.ArticlePubMedPDF
- 69. Panzarini SR, Trevisan CL, Brandini DA, Poi WR, Sonoda CK, Luvizuto ER, et al. Intracanal dressing and root canal filling materials in tooth replantation: a literature review. Dent Traumatol 2012;28:42-48.ArticlePubMed
- 70. Marinho AC, Polay AR, Gomes BP. Accuracy of turbidimetric limulus amebocyte lysate assay for the recovery of endotoxin interacted with commonly used antimicrobial agents of endodontic therapy. J Endod 2015;41:1653-1659.ArticlePubMed
- 71. Markovic L, Jordan RA, Lakota N, Gaengler P. Micromorphology of enamel surface after vital tooth bleaching. J Endod 2007;33:607-610.ArticlePubMed
- 72. Jurema AL, Claudino ES, Torres CR, Bresciani E, Caneppele TM. Effect of over-the-counter whitening products associated or not with 10% carbamide peroxide on color change and microhardness: in vitro study. J Contemp Dent Pract 2018;19:359-366.PubMed
- 73. Bayrak S, Tunc ES, Sonmez IS, Egilmez T, Ozmen B. Effects of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) application on enamel microhardness after bleaching. Am J Dent 2009;22:393-396.PubMed
- 74. Yassin O, Milly H. Effect of CPP-ACP on efficacy and postoperative sensitivity associated with at-home vital tooth bleaching using 20% carbamide peroxide. Clin Oral Investig 2019;23:1555-1559.ArticlePubMedPDF
- 75. Pintado-Palomino K, Tirapelli C. The effect of home-use and in-office bleaching treatments combined with experimental desensitizing agents on enamel and dentin. Eur J Dent 2015;9:66-73.ArticlePubMedPMC
- 76. Charakorn P, Cabanilla LL, Wagner WC, Foong WC, Shaheen J, Pregitzer R, et al. The effect of preoperative ibuprofen on tooth sensitivity caused by in-office bleaching. Oper Dent 2009;34:131-135.ArticlePubMedPDF
- 77. de Souza Costa CA, Hebling J, Scheffel DL, Soares DG, Basso FG, Ribeiro AP. Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater 2014;30:769-784.ArticlePubMed
- 78. de Oliveira Duque CC, Soares DG, Basso FG, Hebling J, de Souza Costa CA. Influence of enamel/dentin thickness on the toxic and esthetic effects of experimental in-office bleaching protocols. Clin Oral Investig 2017;21:2509-2520.ArticlePubMedPDF
- 79. Llena C, Martínez-Galdón O, Forner L, Gimeno-Mallench L, Rodríguez-Lozano FJ, Gambini J. Hydrogen peroxide diffusion through enamel and dentin. Materials (Basel) 2018;11:1694.ArticlePubMedPMC
- 80. Sato C, Rodrigues FA, Garcia DM, Vidal CM, Pashley DH, Tjäderhane L, et al. Tooth bleaching increases dentinal protease activity. J Dent Res 2013;92:187-192.ArticlePubMedPDF
- 81. Garcia EJ, Mena-Serrano A, de Andrade AM, Reis A, Grande RH, Loguercio AD. Immediate bonding to bleached enamel treated with 10% sodium ascorbate gel: a case report with one-year follow-up. Eur J Esthet Dent 2012;7:154-162.PubMed
- 82. The Council of the European Union. Council directive 2011/84/EU of 20 September 2011. Amending directive 76/768/EEC, concerning cosmetic products, for the purpose of adapting Annex III thereto to technical progress. Off J Eur Union 2011;283:36-38.
REFERENCES
Tables & Figures
REFERENCES
Citations

- Clareamento dental e TikTok: avaliação da qualidade do conteúdo em mídia social
Rafaele T Costa, Thayna Silva do Carmo Tavares, André Walsh-Monteiro
Ciência ET Praxis.2025; 21(36): 111. CrossRef - Synthesis, characterization and evaluation of novel bleaching gels containing bioactive glass and nano-hydroxyapatite on hydrogen peroxide diffusion, bleaching efficacy and enamel protection
Adrieli Burey, Byron Carpio-Salvatierra, Michael Favoretto, María Luján Méndez Bauer, Viviane Hass, Alessandra Reis, Alessandro D. Loguercio, Paulo Vitor Farago
Clinical Oral Investigations.2025;[Epub] CrossRef - Cytotoxicity of Bleaching Products: A Systematic Review
Mireia Montaner, José Luis Sanz, Carmen Llena, María Melo, Clara Puig-Herreros, James Ghilotti
Applied Sciences.2024; 14(9): 3680. CrossRef
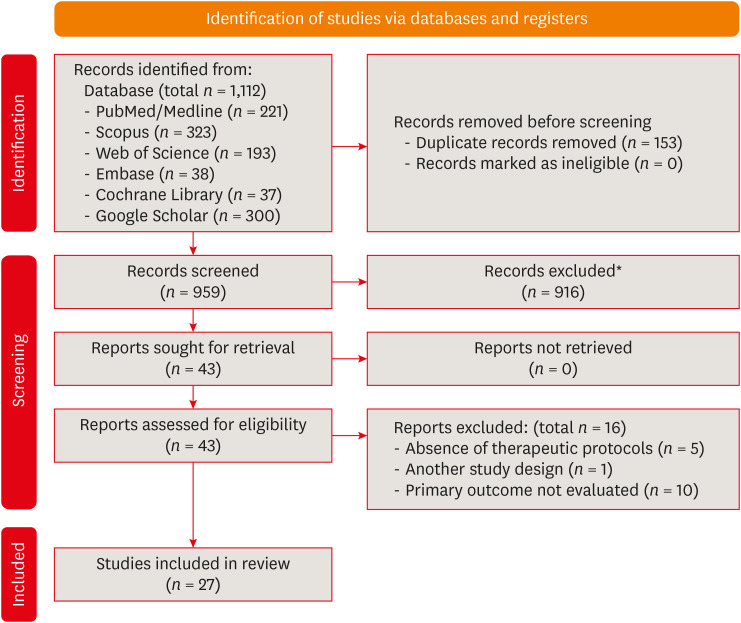
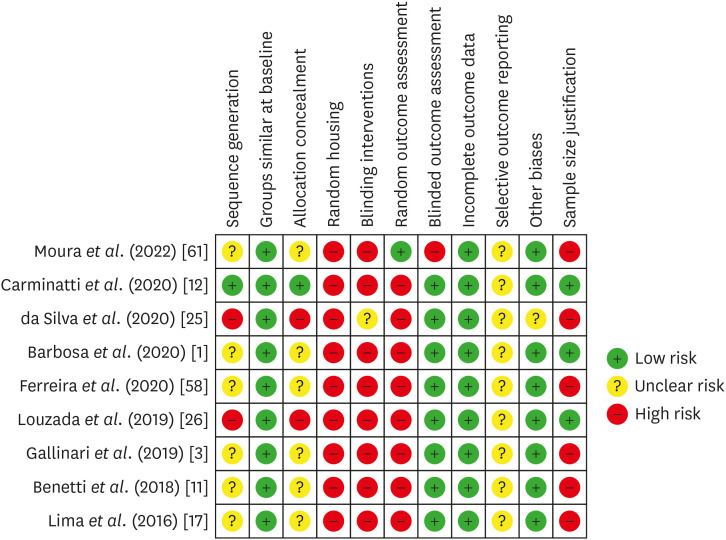
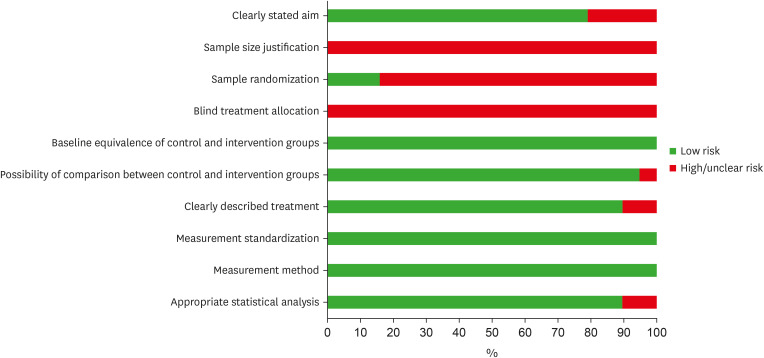
Figure 1
Figure 2
Figure 3
Main characteristics and effects of protocols on pulp tissue using in vivo models
| Author | Experimental model | Groups | Bleaching gel protocol | Additional protocol | Period of analysis | Methods for outcome assessment | Main results |
|---|---|---|---|---|---|---|---|
| Moura | Upper and lower molars of rats | Control: untreated, Ble: 37.5% HP, Ble+Ibu-Gel: 37.5%HP and Ibu-Gel, Ble+Ibu-Neg: 37.5%HP and Ibu-Neg150 | 37.5% HP 24 min (3 × 8 min) | 0.01 mL per hemi-arch: 10 min before and after HP. | 24 h and 14 d | Inflammation and tissue organization: HE; Immunolabeling of IL-10, IL-1β, SP, COX-2 and Bradykinin: IHC | Ble+Ibu-Neg group decreased inflammation and necrotic areas at 24 h, without significant differences at 14 d. At 14 d, Ble+Ibu-Neg increased IL-10 immunolabeling, while Ble+Ibu-Gel decreased IL-1β at 24 h. There were no significant differences in the other markers. |
| Barbosa | Upper molars of rats | Control: untreated, Ble: 35% HP, Ble-Rem: 35% HP before MI Paste Plus, Rem-Ble: MI Paste Plus before 35% HP, Rem-Ble-Rem: MI Paste Plus before and after HP, Ble+Rem: MI Paste Plus mixed with 35% HP | 35% HP gel for 30 min | 0.01 mL MI Paste Plus (topically): for 20 min, before and/or after HP; MI Paste mixed with HP (topically): for 30 min | 2 and 30 d | Inflammation and pulp chamber area: HE | MI Paste Plus mixed with HP reduced the inflammation at 2 d, and tertiary dentin formation at 30 d. |
| Carminatti | Upper molars of rats | Control: placebo gel, Ble: 35% HP, Ble-BS: 35% HP before BS, BS-Ble: BS before 35% HP, BS/7d-Ble: BS for 7 d before 35% HP, Ble+BS: HP mixed with BS | 35% HP gel for 30 min | BS (topically): rubbed for 30 s and remained for 20 min, after or before HP; HP mixed with BS (topically): for 30 min | 2 and 30 d | Inflammation and pulp chamber area: HE | A single BS-based gel application beforehand or BS mixed with HP reduced inflammation, while BS mixed with HP minimized tertiary dentin formation at 30 d. |
| da Silva | Upper molars of rats | Control: placebo gel, 38% HP, Ibu, Ibu before and after HP, 2% Des KF, 2% Des KF before HP | 38% HP gel for 40 min | 0.2 mL Ibu (orally): for 30 min before and after HP, and every 12 h until the analysis; 0.01 mL Des KF (topically): for 10 min, 30 min before HP | 0, 24 and 48 h | Inflammation: HE; Immunolabeling of SP and CGRP: IHC | 2% Des KF reduced inflammation at 24 h, and decreased SP and CGRP immunolabeling at 24 and 48 h. |
| Ferreira | Upper molars of rats | Control: placebo gel, Ble: 35% HP, Ble-O: 35% HP before Oto, Ble-C: 35% HP before Curcumin, Ble-I: 35% HP before Ibu | 35% HP gel for 30 min | Oto or Curcumin (topically): for 10 min, after HP; 50 mg/Kg Ibu (orally): after HP, for 2 days, every 12h | 2 d | Inflammation: HE | Otosporin significantly reduced inflammation in all thirds of the coronary pulp. |
| Gallinari | Upper molars of rats | Placebo gel, 35% HP, Placebo gel before Oto, 35% HP before Oto, Placebo after Tyl, 35% HP after Tyl | 35% HP gel for 45 min | Oto (topically): for 10 min, after HP; Tyl (orally): 30 min before HP, and every 12 h until the analysis | 0, 24 and 48 h | Inflammation: HE; Immunolabeling of SP and CGRP: IHC | Otosporin reduced inflammation, mainly at 48 h. Otosporin and Tylenol reduced SP immunolabeling, mainly at 0 and 24 h, without significant influence on CGRP immunolabeling. |
| Louzada | Upper molars of rats | Control: untreated, 35% HP, 35% HP before 2.5% carvedilol gel | 35% HP gel for 30 min | 0.01 mL Carvedilol gel (topically): for 10 min, immediately after HP | 2 and 30 d | Inflammation and pulp chamber area: HE | 2.5% Carvedilol gel did not influence inflammatory reaction and tertiary dentin formation. |
| Benetti | Upper molars of rats | Control: placebo gel, 35% HP, 35% HP before Oto | 35% HP gel for 30 min | 0.01 mL Oto (topically): for 10 min, after HP | 2 d | Inflammation: HE; Immunolabeling of TNF-α, IL-6, IL-17: IHC | Otosporin reduced pulp inflammation in the occlusal and middle thirds, and decreased the immunolabeling of TNF-α. There was no influence on IL-6 and IL-17. |
| Lima | Lower molars of rats | Control: untreated, DW before 35% HP, AA before 35% HP | 35% HP gel for 10 min (2 × 5 min) | DW or AA (orally): 90 min before HP | 6 h, 24 h, 3 d and 7 d | Inflammation and tissue organization: HE | Ascorbic acid prior to HP did not influence inflammation, but enhanced tissue organization at 24 h. |
AA, ascorbic acid; Ble, bleached; BS, Biosilicate; COX-2, cyclooxygenase 2; CGRP, calcitonin gene-related peptide; Des, desensitizing agent; DW, distilled water; HE, hematoxylin-eosin; HP, hydrogen peroxide; Ibu, ibuprofen; Ibu-Gel, ibuprofen-loaded hydrogel; Ibu-Neg, ibuprofen-loaded nanogel; IHC, immunohistochemistry; IL, interleukin; KF, potassium nitrate and sodium fluoride; Oto, Otosporin; Rem, remineralizer; SP, substance P; TNF, tumor necrosis factor; Tyl, Tylenol.
Main characteristics and effects of protocols on cell viability/cytotoxicity, morphology, and mineralization
| Author | Experimental model | Groups | Bleaching gel protocol | Additional protocol | Period of analysis | Methods for outcome assessment | Main results | |
|---|---|---|---|---|---|---|---|---|
| Dias | 5.6 × 2.3 mm enamel/dentin discs, and MDPC-23 cells | Negative control, PCP, 10% HP, 10% HP + PCP, 20% HP, 20% HP + PCP, 35% HP, 35% HP + PCP | 10%, 20% and 35% HP for 45 min | 10 μL of PCP (topically): before HP | 4 h for cell viability, 1 h for cell morphology | Cell viability: Alamar Blue and fluorescence; Cell morphology: SEM | Coating enamel with PCP before the bleaching protocols minimized the cytotoxic effects and morphological changes caused by HP, independent of its concentration. | |
| de Oliveira Ribeiro | 5.6 × 2.3 mm enamel/dentin discs, and MDPC-23 cells | Negative control, 35% HP, 10% HP, 10% HP + 2 mg/mL MnO2, 10% HP + 6 mg/mL MnO2, 10% HP + 10 mg/mL MnO2 | 10% HP for 45 min | 20 μL of 10% HP mixed with 2-10 mg/mL MnO2 (topically): 45 min (3 applications of 15 min) | 1 h | Cell viability: MTT and live/dead | Higher concentrations of MnO2 applied to the gel reduced cytotoxicity, especially 10 mg/mL of MnO2. | |
| Ribeiro | 5.6 × 2.3 mm bovine enamel/dentin discs, and MDPC-23 cells | CG: untreated; G1: 35% HP; G2: 35% HP + 2 mg/mL MnO2; G3: 35% HP + 6 mg/mL MnO2; G4: 35% HP + 10 mg/mL MnO2 | 35% HP for 45 min (3 × 15 min) | 2, 6, and 10 mg/mL of MnO2 incorporated into the bleaching gel: for 45 min (3 × 15 min) | 1 h | Cell viability: MTT; Cytotoxicity: live/dead assay; Cell morphology: SEM | 6 and 10 mg/mL of MnO2 incorporated into the bleaching gel increased cell viability. | |
| Ortecho-Zuta | 5.6 × 2.3 mm enamel/dentin discs, and MDPC-23 cells | Untreated, HP: 35% HP, 35% HP + HRP | 35% HP for 45 min (3 applications of 15 min) | 1 mL of 35% HP mixed with 10 mg of HRP (topically): for 45 min (3 applications of 15 min) | 1 h | Cell viability: MTT; cell morphology: SEM; cytotoxicity: live/dead assay | HRP associated with HP increased cell viability compared to 35% HP alone, in addition to showing less impact on morphology. | |
| Soares | 5.6 ± 3.5 mm enamel/dentin discs, and MDPC-23 cells | Untreated, HP: 35% HP, 35% HP + FS, 35% HP + MC, 35% HP + PR, 35% HP + CT | 35% HP for 45 min (3 applications of 15 min) | 40 mL of 35% HP mixed with 1 mg of FS, MC, PR, or CT (topically): for 45 min (3 applications of 15 min) | 1 and 24 h | Cell viability: MTT | All chemically activated groups increased cell viability, mainly in the HP + PR group. | |
| de Oliveira Duque | 5.6 ± 3.5 mm enamel/dentin discs, HDPCs and MDPC-23 cells | Untreated, 10% CP, 35% HP, 35% HP + FS | 10% CP for 4 h, and 35% HP for 45 min (3 applications of 15 min) | 0.004 g FS mixed with 35% HP (topically): 45 min (3 applications of 15 min) | 1 h | Cell viability: MTT | Chemical activation of HP by FS had no significant protective effect against cytotoxicity, decreasing cell viability compared to CP. | |
| Soares | 5.6 × 3.5 mm enamel/dentin discs, and MDPC-23 cells | Control: DW, 1 d 16% CP, 7 d 16% CP, 14 d 16% CP, 7 d 16% CP + 0,05% fluoride, 14 d 16% CP + 0.05% fluoride, 7 d 16% CP + 0.2% fluoride, 14 d 16% CP + 0.2% fluoride | 16% CP for 8 h/d | 0.05 % or 0.2% fluoride (topically): for 1 min after 16% CP | 1, 7 and 14 d | Cell viability: MTT; ALP activity: colorimetric endpoint assay; cell membrane damage: flow cytometry | Fluoride solutions cannot prevent the toxic effects of a 16% CP bleaching applied on enamel, in addition to having no impact on ALP activity. | |
| Lima | 0.5 mm dentin discs and MDPC-23 cells | Control: untreated, 10% sodium ascorbate, 10% CP, 10% sodium ascorbate + 10% CP, 16% CP, 10% sodium ascorbate + 16% CP | 10% and 16% CP for 6 h | Sodium ascorbate 10% (topically): for 6 h, before CP | 6 h | Cell viability: MTT; cell morphology: SEM | 10% sodium ascorbate on the dentin discs before the use of the CP reduced the cytotoxic effects of these products on cells. | |
| Huang | HDPCs | NC: negative control, HP (50, 150, 250, 350 μM), HP+NAC: HP (250 μM) + NAC (2.5 mM), HP+CsA: HP (250 μM) + CsA (2 μM), siRNA-CypD: CypD siRNA targeting human PPIF, siRNA-CypD+HP: CypD siRNA targeting human PPIF + HP (250 μM) | 250 μM HP for 24 h | NAC or CsA reagents for 24 h; CypD siRNA-PPIF for 24 h | 1, 3, 6, 12, 24 and 48 h for MTT, 24 h for the other analyses. | Cell viability: MTT; measurement of cell death: FITC and TUNEL assays; intercellular ATP level: ATP detection kit; detection of Ca2+: fluorescence microscope | NAC, CsA and CypD siRNA-PPIF were able to preserve the cell viability, mitigate cell death, decrease the intracellular Ca2+ and enhance the ATP level. | |
| Kim | HDPCs | Control: untreated, 180 μM HP, HP + 50 μM IAA, HP + 100 μM IAA, HP + 150 μM IAA, HP + 200 μM IAA, HP + 250 μM IAA, HP + 300 μM IAA | 180 μM HP for 24 h | IAA: ranging from 1 to 300 μM | 24 h | Cell viability: MTS | IAA treatment increased cell viability. | |
| Kim | HDPCs | Control: untreated, HP: 300 µM HP, CA: 20 µM CA, CA + HP: 20 µM CA and then 300 µM HP, CoPP: 20 µM CoPP, CoPP+HP: 20 µM CoPP and then 300 µM HP | 300 µM HP for 24 h | 20 µM CA: for 24 h, before HP | 24 h | Cell viability: MTT; cytotoxicity: LDH activity assay | Pre-treatment with CA effectively prevented HP-induced cell death. | |
| Vargas | MDPC-23 cells | Negative control: DMEM + 5% DMSO, positive control: 0.018% HP, 1 mM α-T, 3 mM α-T, 5 mM α-T: 10 mM α-T, 1mM α-T + 0.018% HP, 3 mM α-T + 0.018% HP, G9: 5 mM α-T followed by 0.018% HP, G10: 10 mM α-T followed by 0.018% HP | 0.018% HP for 30 min | 1, 3, 5, or 10 mM α-T: for 60 min before HP | 60 min | Cell viability: MTT | Pretreatment with vitamin E α-T isomer increased cell viability of MDPC-23 pulp cells, especially using 5 and 10 mM α-T. | |
| Vargas | MDPC-23 cells | 1 mM α-T, 1 mM α-T + 0.018% HP, 3 mM α-T, 3 mM α-T + 0.018% HP, 5 mM α-T, 5 mM α-T +, 0.018% HP, 10 mM α-T, 10 mM α-T + 0.018% HP, negative control: DMEM + 5% DMSO, positive control: 0.018% HP | 0.018 % HP for 30 min | 1, 3, 5, or 10 mM α-T: for 1, 4, 8 and 24 h before HP | 1, 4, 8 and 24 h | Cell viability: MTT | Vitamin E alpha-tocopherol isomer showed a protective effect against HP cytotoxicity, especially using 1 and 3 mM α-T for 24 h. | |
| Jeong | HDPCs | 1 mM HP, HP + 5 μM sappanchalcone, HP + 10 μM sappanchalcone, HP + 20 μM sappanchalcone, HP + 40 μM sappanchalcone, HP + 40 μM sappanchalcone + 100 μM SnPP, 100 μM SnPP, HP + 20 μM CoPP, positive control: 20 μM | 1 mM HP for 12 h | 5–40 μM sappanchalcone: 12 h | 12 h | Cell viability: MTT | Copp and 20 and 40 μM sappanchalcone reduced HP-induced cytotoxicity. | |
| Lee | HDPCs | Control: untreated, 1mM HP, 1mM HP + 2.5 μM butein, 1mM HP + 5 μM butein, 1mM HP + 10 μM butein, 1mM HP + 20 μM butein, 1mM HP + 20 μM CoPP, 1mM HP+ 100 μM SnPP | 1 mM HP for 12 h | Butein, CoPP and SnPP: for 8 h or until 24 h | 8 h | Cell viability: MTT | Butein inhibited HP-induced cytotoxicity. | |
| Lee | HDPCs | HP+Ad/PPARγ: HP+Ad PPARγ, HP+Ad/LacZ: control, HP | 150 μmol HP for 12 d | Ad/PPARγ virus: a dose of 100 MOI for 24 h | 12 d | Cell viability: MTT; dentin mineralization: alizarin red stain and ALP activity assay. | PPARγ in pulp cells increased cell viability, odontoblastic differentiation and dentin mineralization. | |
| Lee | HDPCs | Control: untreated, HP: 500 µM HP, 500 µM HP + 5 µM sulfuretin, 500 µM HP + 10 µM sulfuretin, 500 µM HP + 20 µM sulfuretin, 500 µM HP + 40 µM sulfuretin, sulfuretin: 5-40 µM sulfuretin, HP+CoPP: 500 µM HP + 20 µM CoPP, positive control: 20 µM CoPP | 500 µM HP for 12 h | 5-40 µM sulfuretin: for 12 h, before HP | 12 h | Cell viability: MTT | Pre-treatment with sulfuretin increased cell viability, presumably through HO-1 expression. | |
| Lee | HDPCs | HP: 150 µM HP, 150 µM HP + 15 µM pachymic acid, 150 µM HP + 5 µM pachymic acid, untreated cells | 150 µM HP for 1, 3, 5, 7 and 12 d | 15 µM pachymic acid: before 1 h prior to incubation with HP | 1, 3, 5, 7, and 12 d | Cell viability: MTT; odontoblast differentiation level: ALP activity and alizarin red S staining | Pachymic acid increased cell viability and mineralization. | |
| Choi | hDPSCs | HP: 200 µM HP, 200 µM HP + 2 µM SOD1, 200 µM HP + 2 µM LMWP-SOD1, 2 µM SOD1, 2 µM LMWP-SOD1, untreated | 200 µM HP for 2 h | 2 µM LMWP-SOD1: for 3 h, before HP | 3 and 28 d | Cell viability: MTT; matrix mineralization: alizarin red S staining; expression of odontogenic markers: PCR | LMWP-SOD1 did not influence cell viability and the calcified area. However, it reverses HP inhibition of osteogenic markers. | |
α-T, alpha-tocopherol; Ad, adenovirus; ALP, alkaline phosphatase; ATP, adenosine triphosphate; CA, cinnamaldehyde; CG, control group; CoPP, cobalt protoporphyrin; CP, carbamide peroxide; CsA, cyclosporine A; CT, catalase; DMEM, Dulbecco’s Modified Eagle’s Medium; DMSO, dimethyl sulfoxide; DW, deionized water; FITC, fluorescein isothiocyanate; FS, ferrous sulfate; G, group; HDPCs, human dental pulp cells; hDPSCs, human dental pulp stem cells; HO-1, heme oxygenase 1; HP, hydrogen peroxide; HRP, horseradish peroxidase; IAA, indole-3-acetic acid; LDH, lactate dehydrogenase; LMWP, low-molecular weight protamine; MC, manganese chloride; MDPC-23, mouse dental papilla Cell-23; MnO2, manganese dioxide; MOI, multiplicity of infection; MSCs, mesenchymal stem cells; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NAC, N-acetylcysteine; NC, negative control; PCP, polymeric catalyst primer; PCR, polymerase chain reaction; PPARγ, proliferator-activated receptor gamma; PPIF, peptidylprolyl isomerase F; PR, peroxidase; SEM, scanning electron microscopy; siRNA-CypD, cyclophilin D small interfering ribonucleic acid; SnPP, tin protoporphyrin; SOD1, superoxide dismutase 1; TUNEL, terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling.
Main characteristics and effects of protocols on oxidative stress and the expression of other proteins
| Author | Experimental model | Groups | Bleaching gel protocol | Additional protocol | Period of analysis | Methods for outcome assessment | Main results | |
|---|---|---|---|---|---|---|---|---|
| Dias | Enamel/dentin discs, and MDPC-23 cells | Negative control, PCP, 10% HP, 10% HP + PCP, 20% HP, 20% HP + PCP, 35% HP, 35% HP + PCP | 10%, 20% and 35% HP for 45 min | 10 μL of PCP (topically): before HP | 1 h | Oxidative stress: carboxy-H2DCFDA fluorescence | The groups where PCP was used before applying the bleaching gels showed lower oxidative stress in MDPC-23 cells. | |
| de Oliveira Ribeiro | Enamel/dentin discs, and MDPC-23 cells | Negative control, 35% HP, 10% HP, 10% HP + 2 mg/mL MnO2, 10% HP + 6 mg/mL MnO2, 10% HP + 10 mg/mL MnO2 | 10% HP for 45 min | 20 μL of 10% HP mixed with 2 mg/mL, 6 mg/mL, or 10 mg/mL MnO2 (topically): 45 min (3 applications of 15 min) | 30 min | Oxidative stress: carboxy-H2DCFDA fluorescence | MnO2 increased the degradation kinetics of the HP molecule, consequently reducing the cellular oxidative stress index. | |
| Ribeiro | Bovine enamel and dentin discs, and MDPC-23 cells | CG: untreated; G1: 35% HP; G2: 35% HP + 2 mg/mL MnO2; G3: 35% HP + 6 mg/mL MnO2; G4: 35% HP + 10 mg/mL MnO2 | 45 min (3 applications of 15 min) | 2, 6, and 10 mg/mL of MnO2 was incorporated into the bleaching gel: for 45 min (3 × 15 min) | Immediately | Oxidative stress: carboxy-H2DCFDA fluorescence; | The addition of MnO2 to 35% HP reduced oxidative stress. | |
| Ortecho-Zuta | Enamel/dentin discs, and MDPC-23 cells | Untreated, HP: 35% HP, 35% HP + HRP | 35% HP for 45 min (3 applications of 15 min) | 1 mL of 35% HP mixed with 10 mg HRP (topically): for 45 min (3 applications of 15 min) | 1 h | Oxidative stress: carboxy-H2DCFDA fluorescence | HRP combined with HP reduced oxidative stress. | |
| Soares | Enamel/dentin discs, and MDPC-23 cells | Untreated, HP: 35% HP, 35% HP + FS, 35% HP + MC, 35% HP + PR, 35% HP + CT | 35% HP for 45 min (3 applications of 15 min) | 40 mL of 35% HP mixed with 1 mg FS, MC, PR, or CT (topically): for 45 min (3 applications of 15 min) | 1 and 24 h | Oxidative stress: carboxy-H2DCFDA fluorescence | All chemically activated groups showed reduced oxidative stress. | |
| Huang | HDPCs | NC: negative control, HP (50, 150, 250, 350 μM), HP+NAC: HP (250 μM) + NAC (2.5 mM), HP+CsA: HP (250 μM) + CsA (2 μM), siRNA-CypD: CypD siRNA targeting human PPIF, siRNA-CypD+HP: CypD siRNA targeting human PPIF + HP (250 μM) | 250 μM HP for 24 h | NAC or CsA reagents for 24 h; CypD siRNA-PPIF for 24 h | 24 h | Bockade of CypD: western lot analyses | NAC, CsA, and CypD siRNA-PPIF decreased the CypD expression, increasing mitochondrial membrane potential. | |
| Kim | HDPCs | Control: untreated, 180 μM HP, HP + 50 μM IAA, HP + 100 μM IAA, HP + 150 μM IAA, HP + 200 μM IAA, HP + 250 μM IAA, HP + 300 μM IAA | 180 μM HP for 24 h | IAA: ranging from 1 to 300 μM | 6 h | Expression of apoptotic (BAX and p53) and antiapoptotic (BCL-2 and ATF5) genes: PCR; ROS detection: fluorescence; Nrf2 and HO-1 expression: densitometric analysis; cell cycle: flow cytometry | IAA treatment protected HDPCs against HP-induced oxidative stress via increased expression of Nrf2 and HO-1. Moreover, IAA treatment rescued cell cycle and prevented apoptosis. | |
| Kim | HDPCs | Control: untreated, HP: 300 µM HP, CA: 20 µM CA, CA + HP: 20 µM CA and then 300 µM HP, CoPP: 20 µM CoPP, CoPP+HP: 20 µM CoPP and then 300 µM HP | 300 µM HP for 24 h | 20 µM CA: for 24 h, before HP | 6 h | ROS measurement: fluorescence analysis; expression of proteins (HO-1, Nrf2): western blot | Pre-treatment with CA protected HDPCs against HP-induced oxidative stress by enhancing the expression of HO-1 through the Nrf2 signaling pathway. | |
| Jeong | HDPCs | 1 mM HP, HP + 5 μM sappanchalcone, HP + 10 μM sappanchalcone, HP + 20 μM sappanchalcone, HP + 40 μM sappanchalcone, HP + 40 μM sappanchalcone + 100 μM SnPP, 100 μM SnPP, HP + 20 μM CoPP, positive control: 20 μM | 1 mM HP for 12 h | 5–40 μM sappanchalcone: 12 h | 12 h | ROS measurement: fluorescence | CoPP and 20 and 40 μM sappanchalcone inhibited ROS production. | |
| Lee | HDPCs | Control: untreated, 1mM HP, 1mM HP + 2.5 μM butein, 1mM HP + 5 μM butein, 1mM HP + 10 μM butein, 1mM HP + 20 μM butein, 1mM HP + 20 μM CoPP, 1mM HP+ 100 μM SnPP | 1 mM HP for 12 h | Butein, CoPP and SnPP: for 8 h or until 24 h | 8 h for ROS detection; 0, 3, 6, 12, 18 and 24 h for HO-1 expression | ROS measurement: fluorescence; HO-1 expression: western blot; Nrf2 expression: immunofluorescence, western blot | Butein inhibited HP-induced ROS production, presumably through JNK Nrf2/ARE-dependent HO-1 expression. | |
| Lee | HDPCs | HP+Ad/PPARγ: HP+Ad PPARγ, HP+Ad/LacZ: control, HP | 150 μmol HP for 12 d | Ad/PPARγ virus: a dose of 100 multiplicity of infection (MOI) for 24 h | 12 d | ROS measurement: flow cytometry; expression of antioxidant molecules: western blot | PPARγ in pulp cells removed cellular ROS under oxidative stress. | |
| Lee | HDPCs | Control: untreated, HP: 500 µM HP, 500 µM HP + 5 µM sulfuretin, 500 µM HP + 10 µM sulfuretin, 500 µM HP + 20 µM sulfuretin, 500 µM HP + 40 µM sulfuretin, sulfuretin: 5-40 µM sulfuretin, HP+CoPP: 500 µM HP + 20 µM CoPP, positive control: 20 µM CoPP | 500 µM HP for 12 h | 5-40 µM sulfuretin: for 12 h, before HP | 12 h | ROS measurement: fluorescence; expression of HO-1: western blot | Pre-treatment with sulfuretin suppressed cellular damage from oxidation caused by HP in HDPCs, presumably through HO-1 expression. | |
| Lee | HDPCs | HP: 150 µM HP, 150 µM HP + 15 µM pachymic acid, 150 µM HP + 5 µM pachymic acid, untreated cells | 150 µM HP for 1, 3, 5, 7 and 12 d | 15 µM pachymic acid: before 1 h prior to incubation with HP | 1, 3, 5, 7 and 12 d | Expression of inflammatory molecules and odontoblast differentiation level: western blot | The pachymic acid showed anti-inflammatory function and odontoblast differentiation via HO-1 pathway. | |
| Choi | hDPSCs | HP: 200 µM HP, 200 µM HP + 2 µM SOD1, 200 µM HP + 2 µM LMWP-SOD1, 2 µM SOD1, 2 µM LMWP-SOD1, untreated | 200 µM HP for 2 h | 2 µM LMWP-SOD1: for 3 h, before HP | 3 and 28 d | Transduction of LMWP-SOD1 and quantification of p53 and p21Cip1: western blot | LMWP-SOD1 conjugates were effective for attenuating cellular senescence and reversing osteoblastic differentiation of hDPSCs caused by oxidative stress inhibition. | |
Ad, adenovirus; ARE, antioxidant response element; ATF-5, activating transcription factor 5; BAX, BCL2 associated X; BCL-2, B-cell lymphoma protein 2; CA, cinnamaldehyde; CG, control group; CoPP, cobalt protoporphyrin; CsA, cyclosporine A; CT, catalase; FS, ferrous sulfate; G, group; HDPCs, human dental pulp cells; hDPSCs, human dental pulp stem cells; HO-1, heme oxygenase 1; HP, hydrogen peroxide; HRP, horseradish peroxidase; IAA, indole-3-acetic acid; JNK, c-Jun NH2-terminal kinase; LMWP, low-molecular weight protamine; MC, manganese chloride; MDPC-23, mouse dental papilla Cell-23; MOI, multiplicity of infection; MnO2, manganese oxide; MSCs, mesenchymal stem cells; NAC, N-acetylcysteine; NC, negative control; Nrf2, nuclear factor erythroid 2-related factor 2; p 53, PCP, polymeric catalyst primer; PCR, polymerase chain reaction; PPARγ, proliferator-activated receptor gamma; PPIF, peptidylprolyl isomerase F; PR, peroxidase; ROS, reactive oxygen species; siRNA-CypD, cyclophilin D small interfering ribonucleic acid; SnPP, tin protoporphyrin; SOD1, superoxide dismutase 1.
AA, ascorbic acid; Ble, bleached; BS, Biosilicate; COX-2, cyclooxygenase 2; CGRP, calcitonin gene-related peptide; Des, desensitizing agent; DW, distilled water; HE, hematoxylin-eosin; HP, hydrogen peroxide; Ibu, ibuprofen; Ibu-Gel, ibuprofen-loaded hydrogel; Ibu-Neg, ibuprofen-loaded nanogel; IHC, immunohistochemistry; IL, interleukin; KF, potassium nitrate and sodium fluoride; Oto, Otosporin; Rem, remineralizer; SP, substance P; TNF, tumor necrosis factor; Tyl, Tylenol.
α-T, alpha-tocopherol; Ad, adenovirus; ALP, alkaline phosphatase; ATP, adenosine triphosphate; CA, cinnamaldehyde; CG, control group; CoPP, cobalt protoporphyrin; CP, carbamide peroxide; CsA, cyclosporine A; CT, catalase; DMEM, Dulbecco’s Modified Eagle’s Medium; DMSO, dimethyl sulfoxide; DW, deionized water; FITC, fluorescein isothiocyanate; FS, ferrous sulfate; G, group; HDPCs, human dental pulp cells; hDPSCs, human dental pulp stem cells; HO-1, heme oxygenase 1; HP, hydrogen peroxide; HRP, horseradish peroxidase; IAA, indole-3-acetic acid; LDH, lactate dehydrogenase; LMWP, low-molecular weight protamine; MC, manganese chloride; MDPC-23, mouse dental papilla Cell-23; MnO2, manganese dioxide; MOI, multiplicity of infection; MSCs, mesenchymal stem cells; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NAC, N-acetylcysteine; NC, negative control; PCP, polymeric catalyst primer; PCR, polymerase chain reaction; PPARγ, proliferator-activated receptor gamma; PPIF, peptidylprolyl isomerase F; PR, peroxidase; SEM, scanning electron microscopy; siRNA-CypD, cyclophilin D small interfering ribonucleic acid; SnPP, tin protoporphyrin; SOD1, superoxide dismutase 1; TUNEL, terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling.
Ad, adenovirus; ARE, antioxidant response element; ATF-5, activating transcription factor 5; BAX, BCL2 associated X; BCL-2, B-cell lymphoma protein 2; CA, cinnamaldehyde; CG, control group; CoPP, cobalt protoporphyrin; CsA, cyclosporine A; CT, catalase; FS, ferrous sulfate; G, group; HDPCs, human dental pulp cells; hDPSCs, human dental pulp stem cells; HO-1, heme oxygenase 1; HP, hydrogen peroxide; HRP, horseradish peroxidase; IAA, indole-3-acetic acid; JNK, c-Jun NH2-terminal kinase; LMWP, low-molecular weight protamine; MC, manganese chloride; MDPC-23, mouse dental papilla Cell-23; MOI, multiplicity of infection; MnO2, manganese oxide; MSCs, mesenchymal stem cells; NAC, N-acetylcysteine; NC, negative control; Nrf2, nuclear factor erythroid 2-related factor 2; p 53, PCP, polymeric catalyst primer; PCR, polymerase chain reaction; PPARγ, proliferator-activated receptor gamma; PPIF, peptidylprolyl isomerase F; PR, peroxidase; ROS, reactive oxygen species; siRNA-CypD, cyclophilin D small interfering ribonucleic acid; SnPP, tin protoporphyrin; SOD1, superoxide dismutase 1.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

