Abstract
-
Objectives
The purpose of this study was to evaluate the influence of endodontic access cavities design on the removal of calcium hydroxide medication of the apical third of mandibular incisor root canal walls and dentinal tubules with different cleaning protocols: EDDY sonic activation, Er,Cr:YSGG laser-activated irrigation, or conventional irrigation with IrriFlex.
-
Materials and Methods
Seventy-eight extracted human mandibular incisors were assigned to 6 experimental groups (n = 13) according to the endodontic access cavity and cleaning protocol for calcium hydroxide removal: traditional access cavity (TradAC)/EDDY; ultraconservative access cavity performed in the incisal edge (UltraAC.Inc)/EDDY; TradAC/Er,Cr:YSGG; UltraAC.Inc/Er,Cr:YSGG; TradAC/IrriFlex; or UltraAC.Inc/IrriFlex. Confocal laser scanning microscopy images were used to measure the non-penetration percentage, maximum residual calcium hydroxide penetration depth, and penetration area at 2 and 4 mm from the apex. Data were statistically analyzed using Shapiro-Wilk and WRS2 package for 2-way comparison of non-normally distributed parameters (depth of penetration, area of penetration, and percentage of non-penetration) according to cavity and cleaning protocol with the significance level set at 5%.
-
Results
The effect of cavity and cleaning protocol interactions on penetration depth, penetration area and non-penetration percentage was not found statistically significant at 2 and 4 mm levels (p > 0.05).
-
Conclusions
The present study demonstrated that TradAC or UltraAC.Inc preparations with different cleaning protocols in extracted mandibular incisors did not influence the remaining calcium hydroxide at 2 and 4 mm from the apex.
-
Keywords: Access cavity; Calcium hydroxide; Confocal laser scanning microscopy; Irrigation; Laser
INTRODUCTION
Access cavity preparation is the foremost technical step of root canal treatment requiring extensive knowledge of the internal and external anatomy of the teeth. The traditional access cavity (TradAC) preparation focuses on three key points: 1) complete unroofing of the pulp chamber with the exposure of the pulp horns, 2) creation of a smooth unimpeded pathway to the root canal orifices, and 3) preservation of sound structure of the tooth [
1,
2,
3,
4]. In an attempt to minimize the removal of tooth structure, the conservative access cavity (ConsAC) and ultraconservative access cavity (UltraAC) preparation emerged targeting the maintenance of the pulp chamber roof and the pericervical dentine as much as possible in order to ultimately improve the tooth’s survival [
5,
6,
7,
8]. While some studies demonstrated an improved fracture resistance in teeth with ConsAC and UltraAC, the majority of studies failed to demonstrate such an effect [
5,
9,
10,
11,
12,
13]. In addition, it has been reported that such minimally invasive access cavities might compromise proper instrumentation, cleaning, and disinfection of root canals [
6].
Calcium hydroxide has been extensively used in endodontics to improve root canal disinfection [
14]. One important concern regarding its use is related to difficulties in removing from the root canal system [
15,
16]. As calcium hydroxide residues can affect the penetration of filling material into the dentinal tubules and increase apical leakage a complete removal is desired. Therefore, the combination of chemical effect and mechanical activation of the irrigant has been proposed to ensure favorable results [
17,
18].
The EDDY (VDW, Munich, Germany) tip is made from flexible polyamide with a size of 25.04 to prevent it from cutting dentin and changing root canal morphology during sonic activation at high frequency (6,000 Hz). According to the manufacturer, it allows efficient cleaning of complex root canal systems without the limitations of ultrasound-activated devices [
19]. Studies on the effectiveness of EDDY in removing calcium hydroxide from root canals indicated that it is successful in the apical region [
20,
21,
22]. Laser-activated irrigation (LAI) using Er,Cr:YSGG has been used as an alternative method to release the irrigant more deeply, thereby increasing the cleaning ability inside the root canal system [
23]. LAI increases debris, smear layer and calcium hydroxide removal from the apical third of the root canal system [
24,
25,
26]. IrriFlex needle (Produits Dentaires SA, Vevey, Switzerland) is a flexible root canal irrigation needle. It has 2 side vents at the tip, back-to-back with a size of 30 G (0.3 mm) and 0.04 taper (
www.pd-irriflex.com). This unique characteristic gives balanced irrigant expulsion, via 2 accurate jets, oriented directly to the dentinal walls. According to the manufacturer, the flexibility of the polypropylene body allows the needle to access the apical region without resistance or damage to the dentinal walls. The tapered shape of the needle adapts to the shape of the canal. The flow thickness of the irrigant is therefore constant as the fluid moves to the coronal area, which maximizes shear forces and the elimination of debris, smear layer and biofilm [
27].
This study aimed to assess the influence of access cavity preparation on calcium hydroxide removal from the apical third of mandibular incisor root canals using different cleaning protocols: sonic activation, Er,Cr:YSGG LAI and conventional irrigation with IrriFlex. The null hypotheses of the present study were as follows:
MATERIALS AND METHODS
The local ethics committee approved the experimental protocol of this study (No: DÜ/2021-12). The sample size of 13 samples per group was determined after a pilot study (power = 0.95, effect size = 0.66, alpha-type error = 0.05).
Seventy-eight extracted human mandibular incisors with straight and fully formed roots, with single root canals, similar general dimensions related to length with a minimum tooth length of 18 mm and pulp chamber dimensions and no previous treatments were selected from a random collection of extracted teeth. Radiographs of all teeth were obtained from bucco-lingual and mesio-distal projections and used to select teeth with a single oval-shaped canal [
7]. The teeth were numbered and randomly allocated (
http://www.random.org) into 2 groups (
n = 39) according to the following access cavity designs.
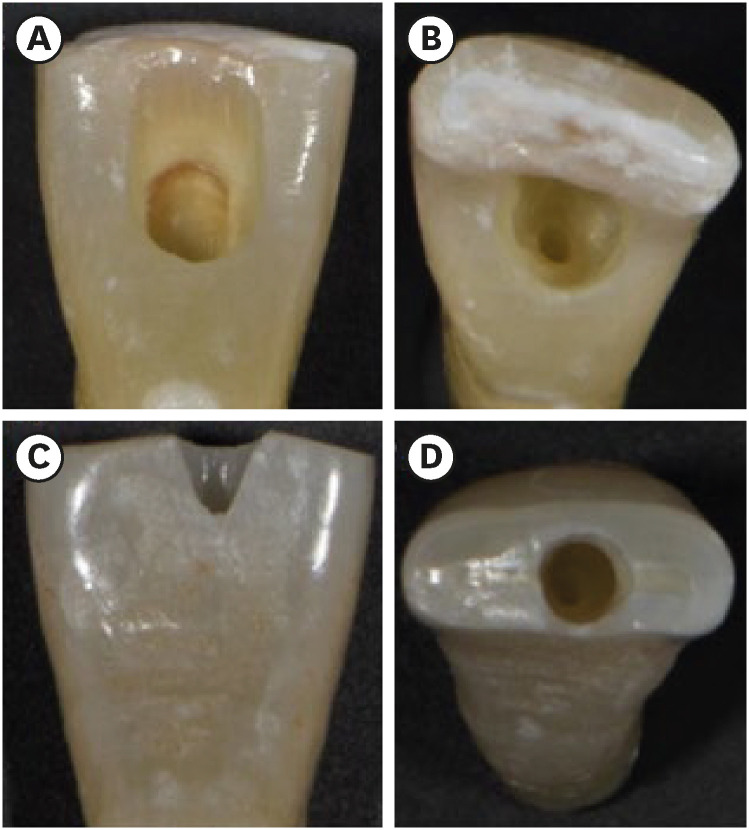
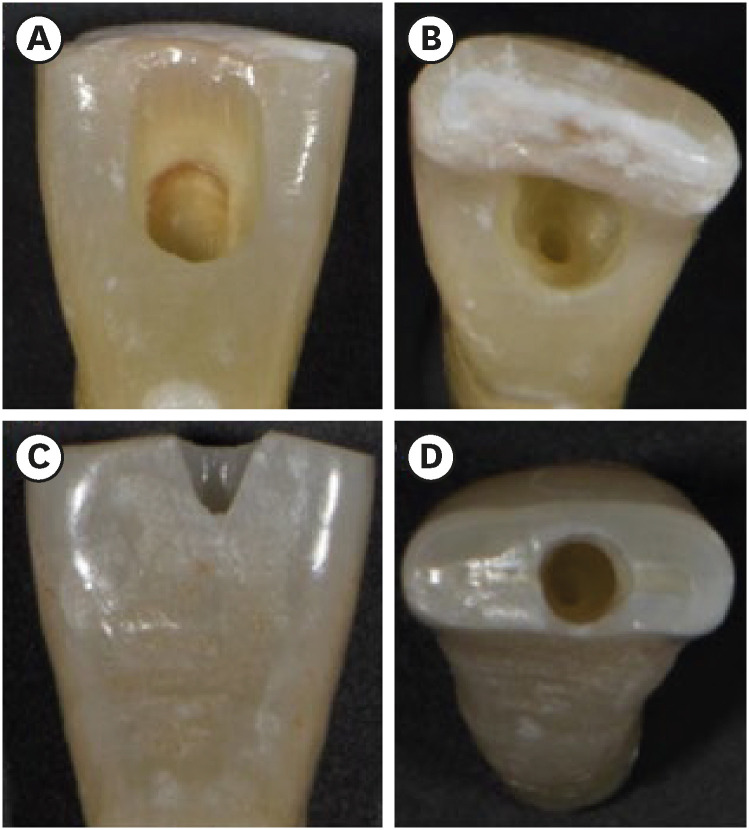
TradAC was prepared following conventional guidelines for outline size and using round 1014 HL and Endo-Z burs (Dentsply Maillefer, Ballaigues, Switzerland) at high speed under water cooling (
Figure 1A and 1B) [
3].
Figure 1Photographs demonstrating the cavity designs. (A, B) Traditional access cavity and (C, D) ultraconservative access cavity performed in the incisal edge.
Ultraconservative access cavity performed in the incisal edge (UltraAC.Inc)
UltraAC.Inc was drilled with diamond burs (802L 314-012; Dr. Hopf GmbH & Co. KG, Langenhagen, Germany) under water cooling at high speed. Incisors were accessed 1 mm palatal to the incisal edge, and cavities extended apically along the long axis. Canals were located while minimizing mesial-distal, buccal-lingual, and circumferential pericervical dentin removal (
Figure 1C and 1D) [
28].
A specialist in Endodontics with 6 years of clinical experience performed all access cavities procedures and root canal preparations. All procedures were performed under microscope magnification and high illumination (OMS 2380; Zumax, Suzhou, China). New burs were used for each access cavity preparation.
After access cavity preparation, the root canals were instrumented using TruNatomy Glider (17, 0.02v taper), Small (20, 0.04v taper), Prime (26, 0.04v taper) instruments at 500 rpm and 1.5 Ncm torque values. A new set of instruments were used for 4 root canal preparations. During the canal preparation, the root canal was irrigated with 2.5 mL 2.5% NaOCl solution after each instrument. After completion of the mechanical preparation, a final irrigation was applied using 5 mL 17% ethylenediaminetetraacetic acid (EDTA) followed by 5 mL 2.5% NaOCl, and 5 mL distilled water. Then, the canal was dried with paper points.
The root canals were filled with a paste made of calcium hydroxide powder (Kalsin; Spot Dis Deposu A.S., Izmir, Turkey) mixed with saline solution and 0.1% rhodamine B dye (Sigma-Aldrich, St Louis, MO, USA). Two radiographs (mesiodistal and buccopalatal direction) were taken to confirm the complete filling of the canals with the calcium hydroxide paste. The access cavities were sealed with Cavit (3M ESPE, Seefeld, Germany), and the specimens were stored for 1 week at 37°C in 100% relative humidity to simulate the clinical situation when calcium hydroxide is used as an intermediate root filling between 2 treatment visits.
After this period, 2 coats of colored nail polish were applied to the specimens, including the apical foramen, to prevent leakage of the irrigant. The specimens were fixed in Eppendorf vials with silicone impression material (Optosil; Heraeus Kulzer, Hanau, Germany). These procedures were performed to create a closed system, simulating the vapor lock effect [
29]. The roots were divided into 3 subgroups according to the irrigation protocol, as follows.
Before each cycle of activation, 2.5 mL 17% EDTA (Cerkamed, Stalowa, Poland) was applied to the root canal with a syringe. The irrigant was activated with a frequency of 6,000 Hz and an amplitude of 160 mm using an air scaler (TA-200-S4H; MICRON Co., Tokyo, Japan). The EDDY tip was placed 2 mm short of the working length (WL), and in-and-out movements with an amplitude of 5 mm were performed.
Laser-activated irrigation with Er,Cr:YSGG
The root canals were filled with 2.5 mL 17% EDTA and activated with Er,Cr:YSGG laser (Waterlase MD; Biolase Technology Inc., San Clemente, CA, USA) using the RFT2 tip (275 microns in diameter and 21 mm length), placed 2 mm short of the WL. The parameters of the laser used were output power of 3 W energy, pulse frequency of 20 Hz (pulses per second), using 10% air and 10% water [
30]. The irrigant was not aspirated from the canal.
A 2.5 mL syringe with a 30-gauge IrriFlex needle was placed 2 mm short of the WL into the canal, and in-and-out movements with an amplitude of 5 mm were performed; 17% EDTA was applied over 30 seconds.
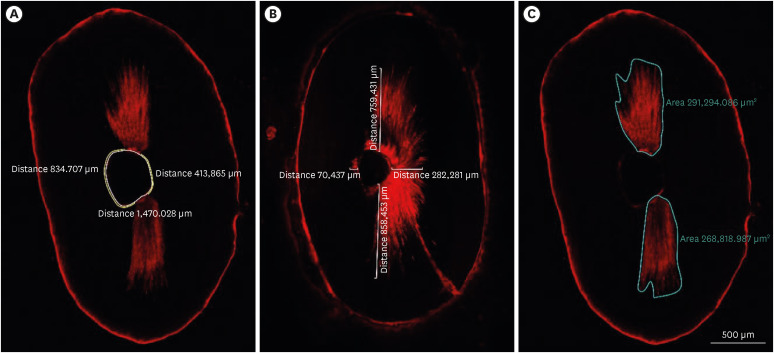
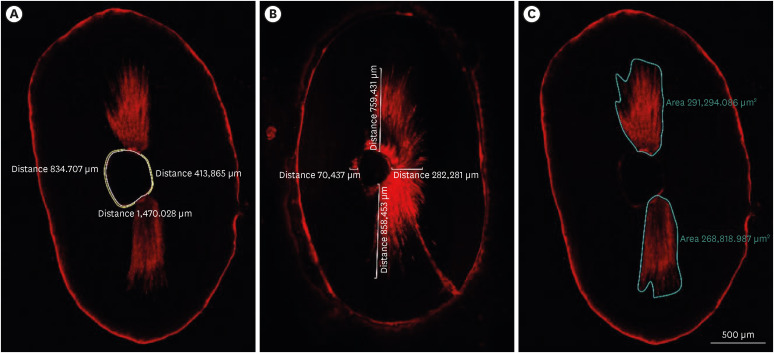
In all groups, irrigation activation was repeated 2 times for 30 seconds, resulting in a total of 1 minute of irrigation with 5 mL EDTA. Then, final irrigation with 5 mL 2.5% NaOCl and 5 mL distilled water was performed with an IrriFlex needle. After removal from the impression material, each specimen was embedded in a circular self-cure acrylic resin mold. Two 1-mm thick sections of each tooth were obtained at distances of 2 and 4 mm from the root apex using a slow-speed, water-cooled 0.3-mm microtome saw (Isomet 5000; Buehler, Lake Bluff, IL, USA). Slices were taken for confocal laser scanning microscopy (CLSM) (Zeiss LSM 800; Carl Zeiss, Jena, Germany) at ×5 magnification with a laser wavelength of 561 nm. The excitation wavelength was set at 543 nm, and the emission wavelength was set at 565 nm. These images were used to measure the non-penetration percentage, maximum residual calcium hydroxide penetration depth and penetration area (
Figure 2). The method proposed to measure the penetration percentage by Moon
et al. [
31] was adapted to measure the percentage of clean root canal walls. First, in each sample, the total perimeter of the root canal was measured with the Zeiss Zen software (Carl Zeiss) measuring tool. Then, the perimeter along the root canal walls where there was no evidence of residual calcium hydroxide was measured (
Figure 2A). Next, this value was divided by the perimeter of the root canal and this result was multiplied by 100 to calculate the percentage. For the maximum depth of residual calcium hydroxide into the dentinal tubules, the point from the root canal wall to the deepest point where residual calcium hydroxide could be observed was measured at 4 predetermined points (mesial, distal, buccal and lingual). The mean depth of residual calcium hydroxide was measured by averaging these 4 values for each specimen (
Figure 2B). The penetration area of residual calcium hydroxide was measured using the area calculating tool of the software (
Figure 2C). These measurements were made by an investigator who was blind to the experimental groups (B.G).
Figure 2Image analyses procedures. (A) The non-penetration percentage, (B) maximum residual calcium hydroxide penetration depth, and (C) penetration area of the calcium hydroxide inside the dentinal tubules.
Statistical analysis
The data were analyzed with the R Project program (version 3.5.0; R Project for Statistical Computing, Vienna, Austria). To assess intraobserver agreement, Cohen’s kappa statistics were calculated for measurements made by the same evaluator with a 1-week interval between measurements. Conformity to normal distribution was evaluated using the Shapiro-Wilk test. WRS2 package [
32] was used for 2-way comparison of non-normally distributed parameters (depth of penetration, area of penetration and percentage of non-penetration) according to cavity and cleaning protocol. The significance level was taken as
p < 0.05.
RESULTS
The kappa test results indicated no significant differences between intra-examiner values for measuring the penetration depth, penetration area, and nonpenetration percentage (kappa value = 0.818, 0.850, 0.838, respectively). None of the experimental groups totally removed the calcium hydroxide from the root canals. No differences were found in terms of penetration depth, penetration area and non-penetration percentage for the different access cavity designs (TradAC and UltraAC.Inc) and the different cleaning protocols (EDDY, Er,Cr:YSGG, and IrriFlex) in none of the 2 different assessment levels (2 and 4 mm) (
p > 0.05).
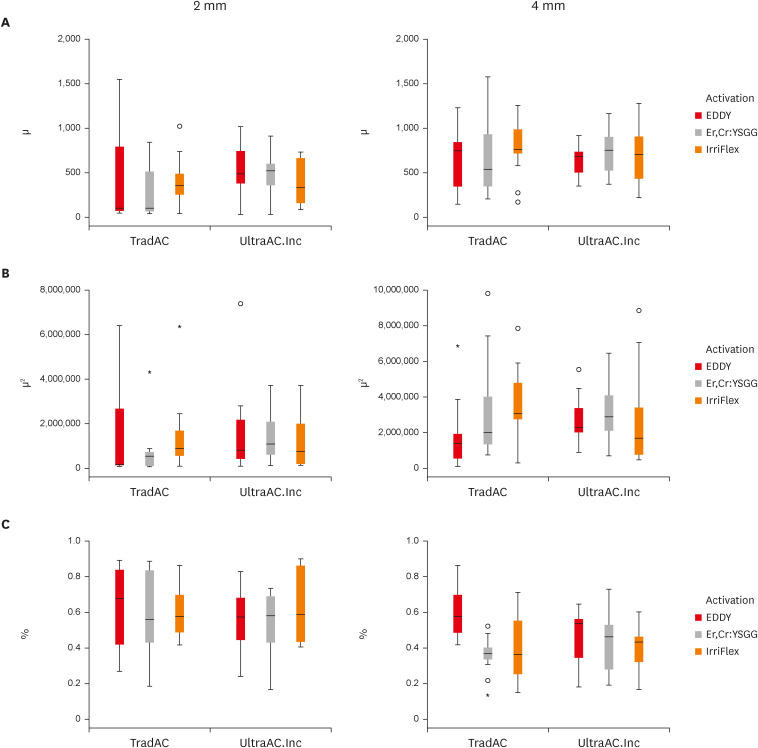
Figure 3 shows a box plot presentation of penetration depth, penetration area and non-penetration percentages, respectively, measured at 2 and 4 mm levels from the apex of the different access cavity design and different cleaning protocols.
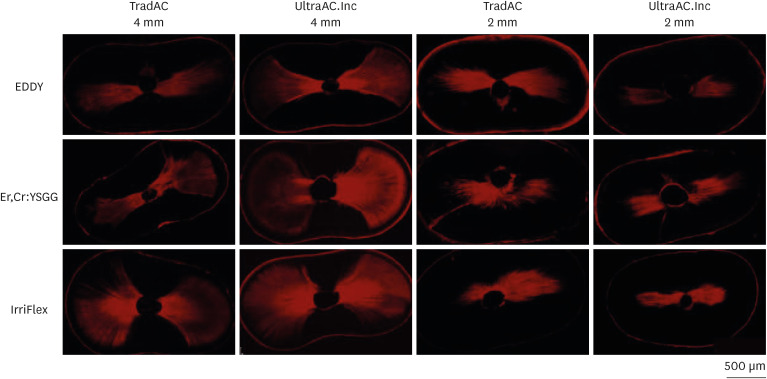
Figure 4 shows representative images of the apical root third in the 2 different assessment levels of the different tested groups.
Figure 3
Box plot presentation of (A) penetration depth, (B) penetration area, and (C) non-penetration percentages measured at 2 and 4 mm levels from the apex for comparing TradAC and UltraAC.Inc with different cleaning protocols.
TradAC, traditional access cavity; UltraAC.Inc, ultraconservative access cavity performed in the incisal edge.
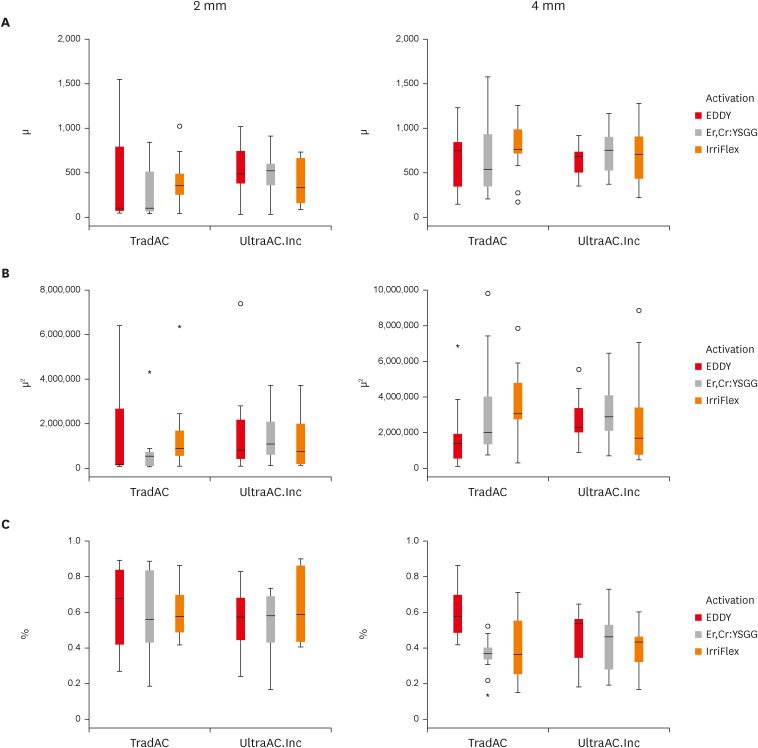
Figure 4
Confocal laser scanning microscopy representative images of the calcium hydroxide penetration of TradAC and UltraAC.Inc groups in the apical root third (4 and 2 mm) of specimens. The effect of access cavity preparation and cleaning protocols was not found statistically significant for both 2 and 4 mm level.
TradAC, traditional access cavity; UltraAC.Inc, ultraconservative access cavity performed in the incisal edge.

DISCUSSION
The influence of minimally invasive access cavities on different outcomes has been extensively evaluated in recent years, with indefinite conclusions mainly those related to fracture resistance [
8]. However, the effect such access cavities design might have on the removal of calcium hydroxide from root canals was not addressed up to now. In light of the potential negative impact of calcium hydroxide remnants on root canal filling procedures, and considering the absence of such type of studies in Endodontic literature, the present study aimed to assess how access cavity preparation may influence such outcomes. The results of the present study showed no significant differences among the tested groups, regardless of the access cavity preparation or cleaning protocols used. As a result, the first and second null hypotheses were accepted.
Complete removal calcium hydroxide of from the apical third of root canals was not achieved in any of the access cavity preparation designs or any of the cleaning protocols. Removing calcium hydroxide is essential to improve the quality of root canal filling, especially sealer penetration [
17]. Several authors observed that the apical third of the root canal is the most affected by remnants of calcium hydroxide [
29,
33]. Gokturk
et al. [
34] demonstrated that the apical third exhibited higher amounts of residual calcium hydroxide than the coronal and middle thirds of the root canals. For this reason, 2 and 4 mm sections from the apical third were examined in this study.
In the literature, many techniques have been proposed to evaluate
in vitro the calcium hydroxide remnants, such as scanning electron microscopy, cone beam computed tomography, stereomicroscope, laser scanning microscope, and micro–computed tomographic images [
20,
22,
34,
35,
36,
37]. In this study, CLSM was chosen as the preferred technique due to its ability to provide high-resolution intratubular analysis. Additionally, CLSM avoids the possibility of confusing calcium hydroxide residues with smear layer or dentin debris, which can occur in scanning electron microscopy evaluations. As the residues of calcium hydroxide have small particle size, they might penetrate into dentinal tubules [
38]. Analyzing with CLSM, penetration of the calcium hydroxide can be assessed more quickly, and a detailed view of the specimen can be obtained at lower magnifications using fluorescent Rhodamine B dye [
39]. However, due to areas with less dentin tubule density or even absence in the apical third, the tubule penetration of calcium hydroxide may not be homogenous along the entire perimeter of the canal [
40]. For this reason, we evaluated 3 parameters, penetration depth, penetration area, and non-penetration percentage, as the method used by Moon
et al. [
31].
In the present study, the different designs of the endodontic access cavity of mandibular incisors did not interfere with removing calcium hydroxide outcomes, regardless of the irrigation techniques used. Previous studies demonstrated no influence of minimally invasive endodontic access cavities in the shaping ability of mandibular incisors [
7,
41]. In the current study, similar to the previously published studies, the selected mandibular incisors had only 1 root canal, with small dimensions, and absence of curvatures. Undoubtedly, such simplistic anatomy might corroborate the results. Moreover, the straight-line access may have contributed to the current results. Similar to the present results, Küçükkaya Eren and Uzunoĝlu Özyürek [
36] reported that the conservative and traditional endodontic cavities did not affect the removal of calcium hydroxide from artificial groves in the coronal and apical portions of mandibular premolars. However, these authors used stereomicroscope methodology, which does not allow the evaluate calcium hydroxide medication inside dentinal tubules. Future studies should be performed aiming to evaluate calcium hydroxide removal in more complex root canal anatomies to evaluate potential differences in outcomes when compared to simpler counterparts. Given the potential challenges associated with removing calcium hydroxide from root canal systems, further evaluation of these anatomies is necessary to better understand the effectiveness and limitations of current removal techniques, especially under different access designs.
The tested irrigation techniques have different fluid dynamics characteristics, and no studies compared these techniques before. Previously published studies compared the calcium hydroxide removal efficiency of EDDY with different irrigation techniques and demonstrated better results for removal using EDDY [
20,
21,
22]. When compared to the present, these different results might be explained by differences in the methodology. In these 3 studies, the evaluation of calcium hydroxide removal was performed in artificial grooves with a laser scanning microscope, optical microscopy and scanning electron microscopy, and stereomicroscope [
20,
21,
22]. However, the lack of irregularities in artificial grooves prepared on root dentin probably facilitated the dissolution and removal of the calcium hydroxide. In addition, Rödig
et al. [
42] reported no differences when EDDY-activated irrigation was compared to manual irrigation on the ability to remove accumulated hard-tissue debris in the mesial root canal systems of mandibular molars. The authors emphasized that the absence of differences might be a result of the needle insertion depth at 2 mm from WL. In the present study, all irrigation device tips were placed 2 mm short of the WL. It is also important to emphasize that manual irrigation was performed with an IrriFlex needle, which has improved flexibility allowing access to the apical portion of the root canal.
LAI is a technique that uses the transmission of energy to produce transient cavitation in the liquid through optical breakdown [
43]. Previous studies have reported that using the Er,Cr:YSGG laser using 2,780 nm, 20 Hz, and 3.0 W is more effective in removing remnants than at lower output powers [
30,
44]. It was significantly more effective in removing the remnants than at the 1.5 W output power. Therefore, these parameters were used in the present study. Eymirli
et al. [
26] and Kuştarcı
et al. [
45] reported that none of the tested techniques completely removed calcium hydroxide, but LAI was more efficient than 27-G needle irrigation in artificial grooves evaluated with a stereomicroscope. In the current study, manual irrigation was performed with a 30-G IrriFlex needle, which might influence the absence of differences between LAI and manual irrigation. The Irriflex needle has a 30 G size with 0.04 taper and a flexible polypropylene body; these properties may have caused similar results in the apical region with other techniques.
Such results are consistent with previous studies evaluating the cleaning abilities of Er,Cr:YSGG laser, which has shown that the laser may not be an effective tool for removing residual materials and smear layer in the apical third of root canals. This may be due to the laser’s fiber tip moving in a circular motion in the coronal and middle thirds, but being moved parallel to the canal wall in the apical area without touching the residual materials and smear layer. Additionally, the presence of lateral and accessory canals in the apical region may have contributed to the laser´s limited effectiveness. Moreover, the size of the laser tips and the extent of apical preparation may also affect the depth of laser irradiation. In our study, we used an RFT2 tip with a diameter of 275 µm and a length of 21 mm, and the root canal preparation was performed using a 0.04v taper and 26 tip instrument, which might be considered low-level preparation. The decision to stop root canal preparation in such an instrument was based on the concept of minimally invasive endodontics, which is not only restricted to access cavities but also to root canal preparation procedures. Although there is no definitive conclusion regarding the effectiveness of this philosophy, ongoing debate emphasizes the need of conducting such evaluations to establish a body of evidence [
6,
46]. In the current study, the minimal apical preparation may have limited the removal of calcium hydroxide due to the limited volume of irrigant that could reach the apical third.
This study has also some limitations. In the current study design, calcium hydroxide removal efficiency was evaluated only in single-rooted teeth at 2 and 4 mm sections from the apical third. Further studies should investigate these outcomes on multi-rooted teeth and different root portions. Moreover, one may argue that other commercially available calcium hydroxide presentations should be also evaluated. However, as the intention of the present study was to evaluate the effect of cavity design and cleaning protocols we have decided to use only 1 type of calcium hydroxide presentation on the study. Future studies should be performed with other presentations. It is also important to emphasize that including too many variables in an experimental study can have several negative consequences, such as confounding effects, increased complexity, and reduced generalizability, among others. Therefore, it is generally recommended that such studies include only a limited number of carefully chosen variables, in order to minimize these potential issues and maximize the validity and generalizability of the results. Also, in the present study, calcium hydroxide paste remained in the root canals for only 7 days. In general, calcium hydroxide is used as an intracanal medication for a period of 1 to 4 weeks, although some studies have used shorter or longer time periods. The duration of calcium hydroxide application may depend on factors such as the severity of the infection, the size and shape of the canal, and the patient’s overall health and immune status. The optimal duration of calcium hydroxide application should be determined on a case-by-case basis, taking into account the specific clinical circumstances and treatment goals [
47]. Even so, future studies could evaluate if longer time points can jeopardize the calcium hydroxide removal from root canals. The findings of this study have clinical relevance, as they shed light on the challenges of completely removing calcium hydroxide from the apical third of mandibular incisors root canals using different cleaning protocols. The difficulty in achieving complete removal of calcium hydroxide in this area may have implications for the success of endodontic treatment and highlights the need for further research in this area.
CONCLUSION
It can be concluded that neither the access cavity nor the cleaning protocol had an impact on the removal of calcium hydroxide from root canals.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Hakan Begeç for endodontic access cavities design photos.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Falakaloğlu S, Yeniçeri Özata M.
Data curation: Güneş B.
Formal analysis: Falakaloğlu S.
Funding acquisition: Falakaloğlu S, Yeniçeri Özata M, Güneş B, Güçyetmez Topal B.
Investigation: Falakaloğlu S, Gündoğar M, Silva EJNL.
Methodology: Falakaloğlu S, Yeniçeri Özata M.
Project administration: Falakaloğlu S, Yeniçeri Özata M.
Resources: Falakaloğlu S, Yeniçeri Özata M, Güneş B, Güçyetmez Topal B.
Software: Yeniçeri Özata M, Güneş B.
Supervision: Gündoğar M, Silva EJNL.
Validation: Gündoğar M, Silva EJNL.
Visualization: Falakaloğlu S, Yeniçeri Özata M, Güneş B.
Writing - original draft: Falakaloğlu S.
Writing - review & editing: Falakaloğlu S, Gündoğar M, Silva EJNL.
REFERENCES
- 1. Ingle JI, Walton RE, Lambert GL, Lambert C, Taintor JF, Zidell JD, Beveridge EE. Preparation for endodontic therapy. In: Ingle JI, Taintor JF, editors. Endodontics. 3rd ed. Philadelphia, PA: Lea & Febiger; 1985. p. 54-101.
- 2. Korzen BH, Pulver WH. Endodontic access cavities--the first step to success. Ont Dent 1978;55:19-22.
- 3. LaTurno SA, Zillich RM. Straight-line endodontic access to anterior teeth. Oral Surg Oral Med Oral Pathol 1985;59:418-419.ArticlePubMed
- 4. Levin HJ. Access cavities. Dent Clin North Am 1967;11:701-710.Article
- 5. Silva AA, Belladonna FG, Rover G, Lopes RT, Moreira EJ, De-Deus G, Silva EJ. Does ultraconservative access affect the efficacy of root canal treatment and the fracture resistance of two-rooted maxillary premolars? Int Endod J 2020;53:265-275.ArticlePubMedPDF
- 6. Silva EJ, De-Deus G, Souza EM, Belladonna FG, Cavalcante DM, Simões-Carvalho M, Versiani MA. Present status and future directions - Minimal endodontic access cavities. Int Endod J 2022;55(Suppl 3):531-587.ArticlePDF
- 7. Vieira GC, Pérez AR, Alves FR, Provenzano JC, Mdala I, Siqueira JF Jr, Rôças IN. Impact of contracted endodontic cavities on root canal disinfection and shaping. J Endod 2020;46:655-661.ArticlePubMed
- 8. Silva EJ, Pinto KP, Ferreira CM, Belladonna FG, De-Deus G, Dummer PM, Versiani MA. Current status on minimal access cavity preparations: a critical analysis and a proposal for a universal nomenclature. Int Endod J 2020;53:1618-1635.ArticlePubMedPDF
- 9. Abou-Elnaga MY, Alkhawas MA, Kim HC, Refai AS. Effect of truss access and artificial truss restoration on the fracture resistance of endodontically treated mandibular first molars. J Endod 2019;45:813-817.ArticlePubMed
- 10. Santosh SS, Ballal S, Natanasabapathy V. Influence of minimally invasive access cavity designs on the fracture resistance of endodontically treated mandibular molars subjected to thermocycling and dynamic loading. J Endod 2021;47:1496-1500.ArticlePubMed
- 11. Augusto CM, Barbosa AF, Guimarães CC, Lima CO, Ferreira CM, Sassone LM, Silva EJ. A laboratory study of the impact of ultraconservative access cavities and minimal root canal tapers on the ability to shape canals in extracted mandibular molars and their fracture resistance. Int Endod J 2020;53:1516-1529.ArticlePubMedPDF
- 12. Barbosa AF, Silva EJ, Coelho BP, Ferreira CM, Lima CO, Sassone LM. The influence of endodontic access cavity design on the efficacy of canal instrumentation, microbial reduction, root canal filling and fracture resistance in mandibular molars. Int Endod J 2020;53:1666-1679.ArticlePubMedPDF
- 13. Maske A, Weschenfelder VM, Soares Grecca Vilella F, Burnett Junior LH, de Melo TA. Influence of access cavity design on fracture strength of endodontically treated lower molars. Aust Endod J 2021;47:5-10.ArticlePubMedPDF
- 14. Kim D, Kim E. Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review - Part II. in vivo studies. Restor Dent Endod 2015;40:97-103.ArticlePubMed
- 15. Taşdemir T, Çelik D, Er K, Yildirim T, Ceyhanli KT, Yeşilyurt C. Efficacy of several techniques for the removal of calcium hydroxide medicament from root canals. Int Endod J 2011;44:505-509.ArticlePubMed
- 16. Capar ID, Ozcan E, Arslan H, Ertas H, Aydinbelge HA. Effect of different final irrigation methods on the removal of calcium hydroxide from an artificial standardized groove in the apical third of root canals. J Endod 2014;40:451-454.ArticlePubMed
- 17. Kim SK, Kim YO. Influence of calcium hydroxide intracanal medication on apical seal. Int Endod J 2002;35:623-628.ArticlePubMed
- 18. Lambrianidis T, Kosti E, Boutsioukis C, Mazinis M. Removal efficacy of various calcium hydroxide/chlorhexidine medicaments from the root canal. Int Endod J 2006;39:55-61.ArticlePubMed
- 19. Innovative Sonic Powered Irrigation. cited March 1, 2023]. Available from: https://www.vdw-dental.com/.
- 20. Donnermeyer D, Wyrsch H, Bürklein S, Schäfer E. Removal of calcium hydroxide from artificial grooves in straight root canals: sonic activation using eddy versus passive ultrasonic irrigation and XPendo finisher. J Endod 2019;45:322-326.ArticlePubMed
- 21. Marques-da-Silva B, Alberton CS, Tomazinho FS, Gabardo MC, Duarte MA, Vivan RR, Baratto-Filho F. Effectiveness of five instruments when removing calcium hydroxide paste from simulated internal root resorption cavities in extracted maxillary central incisors. Int Endod J 2020;53:366-375.ArticlePubMedPDF
- 22. Güven Y, Ali A, Arslan H. Efficiency of Endosonic Blue, EDDY, Ultra X and Endoactivator in the removal of calcium hydroxide paste from root canals. Aust Endod J 2022;48:32-36.ArticlePubMedPDF
- 23. Blanken J, Verdaasdonk R. Cavitation as a working mechanism of the Er,Cr:YSGG laser in endodontics: a visualization study. J Oral Laser Appl 2007;7:97-106.
- 24. de Groot SD, Verhaagen B, Versluis M, Wu MK, Wesselink PR, van der Sluis LW. Laser-activated irrigation within root canals: cleaning efficacy and flow visualization. Int Endod J 2009;42:1077-1083.ArticlePubMed
- 25. DiVito E, Peters OA, Olivi G. Effectiveness of the erbium:YAG laser and new design radial and stripped tips in removing the smear layer after root canal instrumentation. Lasers Med Sci 2012;27:273-280.ArticlePubMedPDF
- 26. Eymirli A, Nagas E, Uyanik MO, Cehreli ZC. Effect of laser-activated ırrigation with ethylene diaminetetraacetic acid and phytic acid on the removal of calcium hydroxide and triple antibiotic paste from root dentin. Photomed Laser Surg 2017;35:43-48.PubMed
- 27. IrriFlex. IrriFlex brochure. cited July 6, 2022]. Available from: https://pd-irriflex.com/.
- 28. Krishan R, Paqué F, Ossareh A, Kishen A, Dao T, Friedman S. Impacts of conservative endodontic cavity on root canal instrumentation efficacy and resistance to fracture assessed in incisors, premolars, and molars. J Endod 2014;40:1160-1166.ArticlePubMed
- 29. Dias-Junior LCL, Castro RF, Fernandes AD, Guerreiro MYR, Silva EJNL, Brandão JMDS. Final endodontic irrigation with 70% ethanol enhanced calcium hydroxide removal from the apical third. J Endod 2021;47:105-111.ArticlePubMed
- 30. Abduljalil M, Kalender A. Efficacy of Er,Cr:YSGG laser with different output powers on removing smear layer after retreatment of two different obturation techniques. Photobiomodul Photomed Laser Surg 2020;38:84-90.ArticlePubMed
- 31. Moon YM, Shon WJ, Baek SH, Bae KS, Kum KY, Lee W. Effect of final irrigation regimen on sealer penetration in curved root canals. J Endod 2010;36:732-736.ArticlePubMed
- 32. Mair P, Wilcox R. Robust statistical methods in R using the WRS2 package. Behav Res Methods 2020;52:464-488.ArticlePubMedPDF
- 33. Yaylali IE, Kececi AD, Ureyen Kaya B. Ultrasonically activated irrigation to remove calcium hydroxide from apical third of human root canal system: a systematic review of in vitro studies. J Endod 2015;41:1589-1599.PubMed
- 34. Gokturk H, Ozkocak I, Buyukgebiz F, Demir O. Effectiveness of various irrigation protocols for the removal of calcium hydroxide from artificial standardized grooves. J Appl Oral Sci 2017;25:290-298.ArticlePubMedPMC
- 35. Suresh N, Varghese A, Sundar S, Nagendrababu V, Velmurugan N. Do calcium chelators play a role in the removal of calcium hydroxide from root canals? A systematic review of laboratory studies. Eur Endod J 2022;7:11-19.ArticlePubMedPMC
- 36. Küçükkaya Eren S, Uzunoĝlu Özyürek E. Influence of cavity design on calcium hydroxide removal from root canal irregularities. Cumhur Dent J 2019;22:2146-2852.
- 37. Silva LJ, Pessoa OF, Teixeira MB, Gouveia CH, Braga RR. Micro-CT evaluation of calcium hydroxide removal through passive ultrasonic irrigation associated with or without an additional instrument. Int Endod J 2015;48:768-773.PubMed
- 38. Komabayashi T, D’souza RN, Dechow PC, Safavi KE, Spångberg LS. Particle size and shape of calcium hydroxide. J Endod 2009;35:284-287.ArticlePubMedPMC
- 39. Deniz Sungur D, Purali N, Coşgun E, Calt S. Push-out bond strength and dentinal tubule penetration of different root canal sealers used with coated core materials. Restor Dent Endod 2016;41:114-120.ArticlePubMedPMCPDF
- 40. Carrigan PJ, Morse DR, Furst ML, Sinai IH. A scanning electron microscopic evaluation of human dentinal tubules according to age and location. J Endod 1984;10:359-363.ArticlePubMed
- 41. Rover G, de Lima CO, Belladonna FG, Garcia LF, Bortoluzzi EA, Silva EJ, Teixeira CS. Influence of minimally invasive endodontic access cavities on root canal shaping and filling ability, pulp chamber cleaning and fracture resistance of extracted human mandibular incisors. Int Endod J 2020;53:1530-1539.ArticlePubMedPDF
- 42. Rödig T, Koberg C, Baxter S, Konietschke F, Wiegand A, Rizk M. Micro-CT evaluation of sonically and ultrasonically activated irrigation on the removal of hard-tissue debris from isthmus-containing mesial root canal systems of mandibular molars. Int Endod J 2019;52:1173-1181.PubMed
- 43. Blanken J, De Moor RJ, Meire M, Verdaasdonk R. Laser induced explosive vapor and cavitation resulting in effective irrigation of the root canal. Part 1: a visualization study. Lasers Surg Med 2009;41:514-519.ArticlePubMedPDF
- 44. Yavari HR, Rahimi S, Shahi S, Lotfi M, Barhaghi MH, Fatemi A, Abdolrahimi M. Effect of Er,Cr:YSGG laser irradiation on Enterococcus faecalis in infected root canals. Photomed Laser Surg 2010;28(Suppl 1):S91-S96.ArticlePubMed
- 45. Kuştarcı A, Er K, Siso SH, Aydın H, Harorlı H, Arslan D, Kirmali O. Efficacy of laser-activated irrigants in calcium hydroxide removal from the artificial grooves in root canals: an ex vivo study. Photomed Laser Surg 2016;34:205-210.PubMed
- 46. Silva EJ, Versiani MA, Souza EM, De-Deus G. Minimally invasive access cavities: does size really matter? Int Endod J 2021;54:153-155.ArticlePubMedPDF
- 47. Gulabivala K, Ng YL. Non-surgical root-canal treatment. Endodontics. 4th ed. Edinburgh: Mosby/Elsevier Ltd.; 2014. p. 174-236.
 , Merve Yeniçeri Özata2
, Merve Yeniçeri Özata2 , Betül Güneş3
, Betül Güneş3 , Emmanuel João Nogueira Leal Silva4
, Emmanuel João Nogueira Leal Silva4 , Mustafa Gündoğar5
, Mustafa Gündoğar5 , Burcu Güçyetmez Topal6
, Burcu Güçyetmez Topal6






 KACD
KACD
 ePub Link
ePub Link Cite
Cite

