Abstract
-
Objectives
This study aimed to evaluate in vitro the effects of the self-adhesive resin cements RelyX U200 (3M ESPE) and seT PP (SDI Limited) on murine macrophages and the interference of the photoactivation.
-
Materials and Methods
Cell viability assays, cell adherence, yeast phagocytosis of Saccharomyces boulardii and production of reactive oxygen species (ROS) were performed in the presence of capillaries containing the respective self-adhesive cement when photoactivated or not.
-
Results
After long periods of contact, both types of cements, when not photoactivated, are more cytotoxic for macrophages. The seT PP cement when only chemically activated seems to interfere more negatively in the process of phagocytosis of yeasts S. boulardii. Both types of cements interfere in the cell adhesion process, independent of photoactivation. None of the types of cements tested was able to induce the production of ROS.
-
Conclusions
Our results highlight the great importance of the photoactivation of self-adhesive resin cements in the dental clinic, since RelyX U200, when photoactivated, presented the best results within the evaluated parameters.
-
Keywords: Cytotoxicity; Macrophages; Dental cement; Resin cement
INTRODUCTION
Self-adhesive resin cements were recently introduced in dentistry with the promise of allying high performance in fixed prosthesis cementation and simplification of operative steps [
1]. In general, it is a consensus in the literature that resinous materials present a high cytotoxic and genotoxic potential in several cell types including fibroblasts, cells derived from bovine dental pulp, odontoblasts, cultures of lymphocytes, and macrophages [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11].
Adequate polymerization is essential to achieve excellence in the clinical performance of resinous materials. However, the maximum degree of conversion of monomers achieved during the polymerization is approximately 60%, due to the gradual increase of viscosity reached by the material during the reaction, and other factors, such as the diffusion and contact of free residual monomers can alter cellular metabolism, even at concentrations below the toxic threshold, and, consequently, induce adverse clinical effects [
10,
12,
13,
14].
Macrophages play a decisive role in the immune response and the tissue repair process. In the pulp and periapical tissues, macrophages are responsible for phagocytosis, presentation of antigens and production of inflammatory mediators, playing a central role in pulpal inflammation and repair of periapical changes [
15,
16].
Macrophages are essential in the elimination of pathogens during infections and in the healing process. Hence, the objective of this study was to evaluate the effect of the self-adhesive resin cements RelyX U200 (3M ESPE, St. Paul, MN, USA) and seT PP (SDI Limited, Victoria, Australia) on viability, cell adhesion, phagocytosis and production of reactive oxygen species (ROS) by murine macrophages. Additionally, the effects of the photoactivation of these types of cement for the same parameters were evaluated.
MATERIALS AND METHODS
Mice
A total of 30 female C57BL/6 mice, between 4–8 weeks of life were used in this study. The choice of the animals was based on previous studies from our group with C57BL/6 mice. The animals were obtained from the Biotersic Center of the Federal University of Minas Gerais (CEBIO-UFMG, Belo Horizonte, MG, Brazil), and were kept in a conventional animal house with barriers, temperature and light control. Food and water were offered ad libitum. The experimental protocol was approved by the Ethics Committee on the Use of Animals of the Federal University of Minas Gerais (CEUA-UFMG) under protocol No. 181/2017.
Isolation of macrophages
Macrophages were isolated from the peritoneal cavity of the mice 5 days after peritoneal injection of 2 mL thioglycolate 3% (Biobrás S.A., Montes Claros, MG, Brazil). The animals were sacrificed after the overdose of anesthetics. Cells were resuspended in complete medium: RPMI 1640 without phenol red (Sigma Chemicals Co., St. Louis, MO, USA), supplemented with 10% of fetal calf serum (Nutricell, Campinas, SP, Brazil), 0.1% of 0.05 mol L) 1 b-mercaptoethanol (Sigma Chemicals Co.), 0.2% of penicillin (100 U/mL)/streptomycin (0.1 mg/mL and 200 mmol/L) l-glutamine [
17].
The supernatant was discarded and the cells resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum (Nutricell) and 0.2% penicillin (100 U/mL)/streptomycin (0.1% mg/mL). More than 90% of the recovered cells had macrophage morphological characteristics when visualized by light microscopy.
Self-adhesive resin cement
The self-adhesive resin cement RelyX U200 (base paste: methacrylate monomers containing phosphoric acid groups, methacrylate monomers, silanated fillers, initiator components, stabilizers, rheological additives; catalyst paste: methacrylate monomers, alkaline [basic] fillers, silanated fillers, initiator components, stabilizers, pigments, rheological additives) and seT PP (35% by weight methacrylate ester, 65% by weight inorganic filler) were spatulated under sterile conditions in laminar flow hood, according to orientations of the respective manufacturers. The cement was then inserted into the ends of glass capillaries (Micron, Trianon Ind. And Com. Ltda, São Paulo, SP, Brazil) previously sectioned and sterilized. Half of the capillaries containing both types of cement were photoactivated with the aid of the BluePhase G2 apparatus (Ivoclar-Vivadent, Amherst, NY, USA) in the soft mode for 20 seconds, and the other half was not photoactivated. Empty glass capillaries were used as a control group. Later, the capillaries were sterilized with ethylene oxide. After sterilization, the groups were divided as follows: G1 - RelyX U200 without photoactivation, G2 - RelyX U200 photoactivated, G3 - seT PP without photoactivation, G4 - seT PP photoactivated and G5 - control group.
Cell viability
Cells were cultured for 24, 48, and 72 hours. Cellular viability was determined by the trypan blue exclusion assay [
18]. Briefly, 1 mL of cell suspension containing 5 × 10
5 cells was inoculated into 24-well plates (Nunclon, Nalge Nunc International, Naperville, IL, USA) and incubated in a humidified atmosphere containing 5% CO
2 at 37°C with capillaries containing photoactivated cement or not, in addition to empty capillaries. After each period, the capillaries were removed with the aid of a sterile tweezer and cell viability was determined. The living and dead cells were counted, and the results were expressed as percentages. Three trials were performed in triplicate.
Polypropylene tubes containing macrophages were incubated for 2 hours with capillaries (test and control groups) in an incubator with a humidified atmosphere containing 5% CO
2, at 37°C. Tubes were agitated in a vortex agitator for 5 seconds, at low speed. Twenty microliters of the cellular suspension were removed, placed into a Newbauer chamber and incubated for 1 hour at 37°C as above. The percentage of adherent and nonadherent macrophages was then established by counting under an optical microscope [
19]. The trial was performed twice in triplicate.
Cells (1 × 10
6 in 1 mL) were incubated for 2 hours in 24-well culture plates (Nunclon), onto round glass coverslips (Glasstécnica, São Paulo, SP, Brazil) in an incubator as above. Nonadherent cells were removed by washing with a warm complete medium, afterwards 10
7 colony-forming units of
Saccharomyces boulardii (Floratil, Merck S.A., Rio de Janeiro, RJ, Brazil) and capillaries with or without self-adhesive resin cement were added to the medium and plates were incubated for 1 hour. Unbound yeast cells were removed by washing with a complete medium, and the coverslips were covered for 1 minute with 1 mL of tannic acid at 1% (Merck S.A.) so that the distinction could be made between extracellular and intracellular yeast cells. One drop of fetal calf serum was applied onto each coverslip. The dried coverslips were stained with Panótico Rápido (Laborclin Ltd, Pinhais, PR, Brazil) and glued to microscope glass slides with Entellan (Merk) for observation under an optical microscope at ×1,000 magnification in oil immersion [
20]. To determine the percentages of macrophages with phagocyted yeast, the percentages of macrophages with adhered yeast, as well as the number of phagocyted yeast/cells, a minimum of 200 cells were counted [
21]. Two trials were performed in triplicate.
Cells were cultured in polypropylene tubes in an incubator as above. After 24 hours, 1 × 10
6 cells were transferred to a C96 White Maxisorp (Nalgene, Rochester, NY, USA) plate in 100 µL; 10
7 Zymosan A particles (Sigma Chemicals Co.) and 0.05 mmol L
−1 luminol in 1640 RPMI without phenol red were added to each well. Plates were read every 2 minutes for 118 minutes in a luminometer (LumiCount, Packard Instrument Company Inc., Downers Grove, IL, USA) [
22]. This procedure will measure the luminol-amplified chemiluminescence due to the production of superoxide, hydroxyl radical and oxygen peroxide in response to ingestion of zymosan particles by phagocytes [
22]. Results were expressed as the area under each of the curves obtained in the 118-minute period [
21]. Two trials were performed in triplicate.
All the results were analyzed in the GraphPad Prism program (GraphPad Software Inc., San Diego, CA, USA), in which the parametric analysis of variance (ANOVA) test (multiple comparisons) was used, and the proposed analyzes were considered significant when p < 0.05.
RESULTS
Cell viability
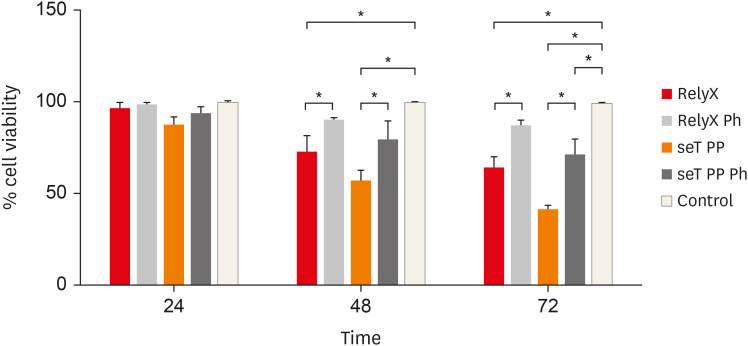
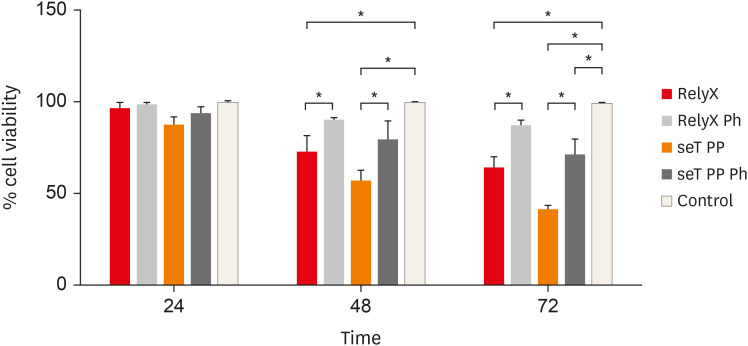
The cell viability of macrophages is shown in
Figure 1. The effects of photoactivation were noted at 48 and 72 hours. When only chemically activated, both types of cement had a significant reduction in the number of viable cells in relation to the photoactivated and control groups (
p < 0.05). In 72 hours, seT PP showed a significant reduction of viable cells independent of the photoactivation when compared to the control group (
p < 0.05).
Figure 1
Percentage of living macrophages from wt C57BL/6 mice after incubation in polypropylene tubes with capillaries containing self-adhesive resin cement from commercial sources. Bars represent the mean of three experiments in triplicate; lines stand for the standard error of the means.
*The statistical difference p < 0.05.
Cell adhesion
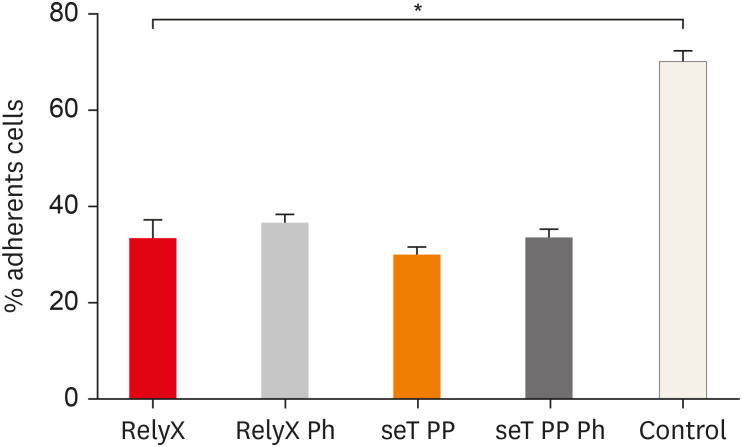
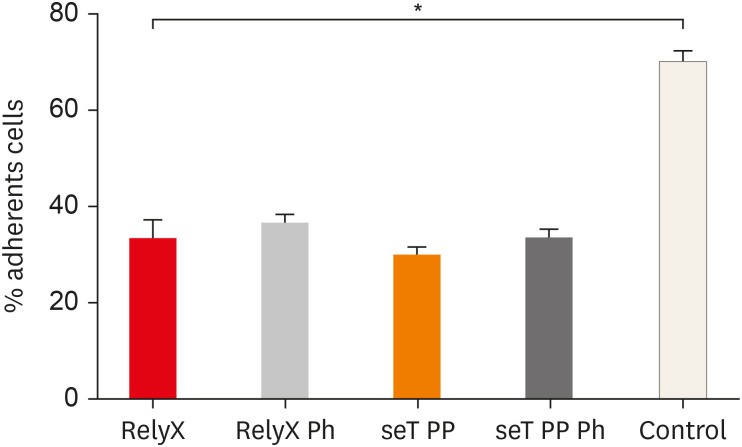
The adhesion of macrophages to the substrate is shown in
Figure 2. All experimental groups were able to significantly interfere in the macrophage adhesion process to the substrate, relative to the control group (
p < 0.05).
Figure 2
Percentage of adherent macrophages from wt C57BL/6 mice after incubation in culture plates with capillaries containing self-adhesive resin cement from both commercial sources. Bars stand for the mean results of two experiments made in triplicate; lines indicate standard error of the means.
*The statistical difference between control group and all experimental groups.
Phagocytosis assay
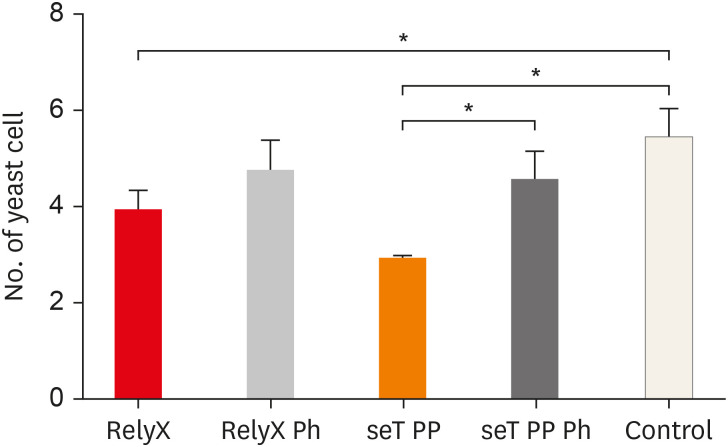
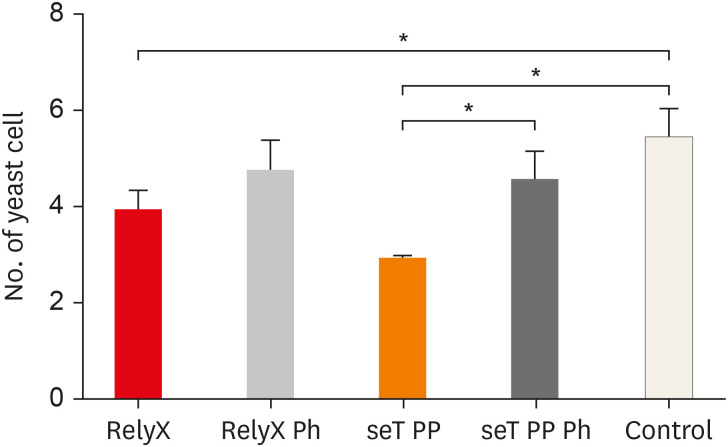
The mean number of yeasts phagocytosed by macrophages is shown in
Figure 3. Macrophages, when in the presence of chemically activated cement, presented a lower number of phagocytized yeasts, in relation to the control group (
p < 0.05). The cement seT PP, when not photoactivated (G3), presented a significant reduction in the mean number of phagocytized yeasts in relation to the photoactivated seT PP (
p < 0.05).
Figure 3
Numbers of yeast cells per macrophages are also shown. Bars stand for the mean results of two experiments made in Triplicates; lines indicate standard error of the means.
*The statistical difference p < 0.05.
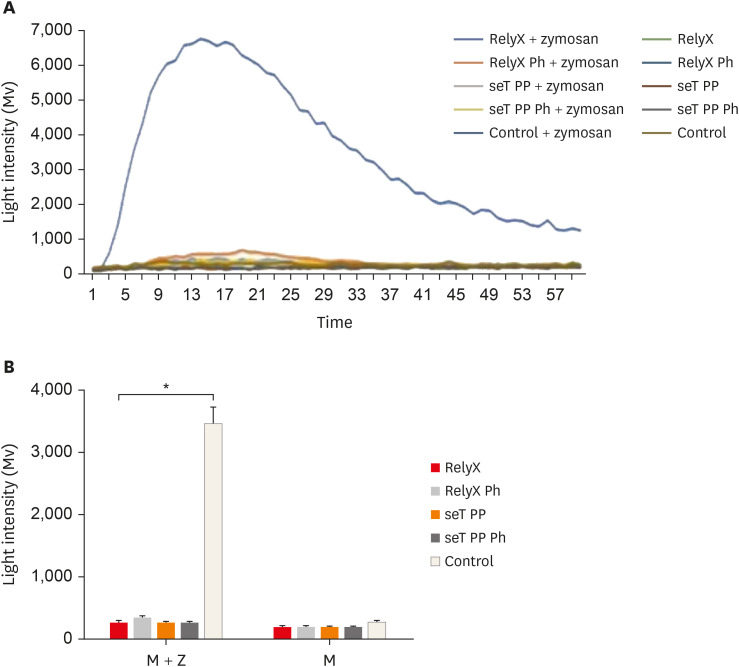
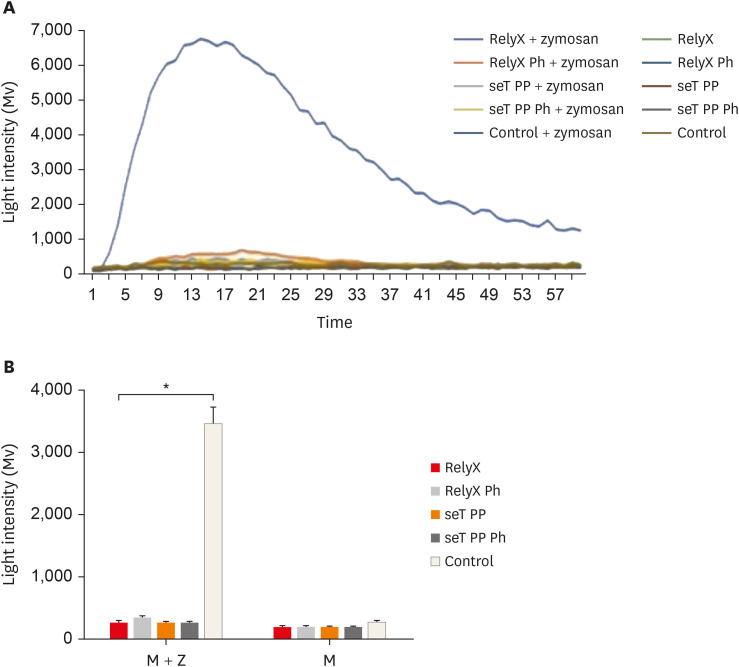
Reactive oxygen intermediates assay
None of the cell cultures was able to produce ROS, the mean luminous intensity being emitted by the experimental groups, similar to the negative control group. In the presence of the stimulus (zymosan A), all cell cultures exposed to resin cement had a significantly lower ROS production than the positive control group (
p < 0.05). The mean luminous intensity emitted and the kinetics of ROS production by macrophages are shown in
Figure 4.
Figure 4
Kinetic of reactive oxygen intermediates production by macrophages from wt C57BL/6 mice (A). Reactive oxygen species mean production by macrophages (B). Cells were cultured with capillaries containing self-adhesive resin cement and stimulated with zymosan. Bars stand for the mean results of two experiments made in triplicate; lines indicate standard error of the means.
*The statistical difference between control group and all experimental groups p < 0.05.
DISCUSSION
Self-adhesive resin cement was introduced in the dental market in 2002 to overcome the deficiencies of conventional cement used in the cementation of indirect restorations and at the same time reduce the number of operative steps [
23].
The degree of conversion of monomers into self-adhesive resin cement may vary from 37% when photoactivated for 20 seconds to 58% when photoactivated for 40 seconds, showing that the manufacturer’s recommended photoactivation time may often be insufficient [
24,
25]. The cytotoxicity of resinous materials, including self-adhesive cement, is strongly related to their composition, pH and polymerization [
26]. The interaction between resinous materials and cells, at the molecular level, may be responsible for several tissue reactions such as inflammation, immunological alterations, and genotoxicity, necrosis and cell apoptosis [
4,
27,
28,
29].
Our results demonstrated that, in general, both types of cements when not photoactivated are more cytotoxic to macrophages after long periods of contact and that RelyX U200 does not interfere significantly with cellular viability even in long periods. According to a previous study, macrophages in contact with RelyX Unicem photoactivated presented a superior survival rate concerning cultures exposed to chemically activated cement [
26]. Similar results were found in odontoblasts cultures exposed to RelyX U200 in relation to other commercial brands [
30]. It is known that RelyX U200 does not contain HEMA in its composition, and previous studies have shown that the absence of this monomer can result in lower cytotoxic effects in odontoblasts and cells derived from the dental pulp, in relation to those cement containing it in its composition [
5,
31]. According to the manufacturer, RelyX U200 has a considerable increase of pH during the photoactivation, approaching neutrality [
8]. This fact is confirmed by its predecessor RelyX Unicem which presented the highest pH values after photoactivation for 20 seconds than other brands of self-adhesive resin cement [
32]. On the other hand, recent studies have demonstrated a high cytotoxic and genotoxic potential of RelyX U200 in cells derived from dental pulp and fibroblasts [
4,
6]. This may be due to the high levels of camphorquinone in its composition which, when photoactivated inadequately, can induce high oxidative stress and damage to the DNA of gingival fibroblasts [
30,
33].
This study showed that, if only chemically activated, seT PP showed a lower rate of cell viability after long periods in contact with cultures of macrophages, besides a substantial interference in the process of phagocytosis of
S. boulardii yeasts by the same macrophages. Recent studies have evaluated the adhesion strength, pH and degree of conversion of monomers during the photoactivation of several commercial brands of self-reducing resin cement. It has been shown that among the commercial brands evaluated, the seT PP cement presents the lowest conversion rates of monomers and lower values, which may explain its negative performance in this study, when it does not show a high photo dependence after 20 seconds of photoactivation, in relation to other commercial photoactivated brands [
32].
Besides, the adhesion process and phagocytosis are highly influenced by the presence of dental materials, and endodontic cement [
18,
34]. On the other hand, cement considered to be highly biocompatible, such as mineral trioxide aggregate (MTA), does not appear to alter the phagocytosis capacity of macrophages [
21]. However, when associated with resinous components such as the Fillapex MTA, the phagocytic activity of both macrophages is substantially impaired [
35].
In this study, all cultures exposed to self-adhesive resin cement produced levels of ROS similar to the negative control group. However, when stimulated by zymosan A, the cultures of macrophages in contact with the self-adhesive resin cement presented lower levels of ROS than the positive control. The production of ROS in cell cultures exposed to methacrylate monomers is strongly associated with their mechanisms of cytotoxicity and cellular apoptosis [
36]. Receptor occupancy assays were performed after 24 hours of contact between macrophages and self-adhesive resin cement, and in this period none of the experimental groups was able to significantly reduce the number of viable cells, possibly explaining the basal production of the non-stimulated cultures. Some studies have also evaluated the interference in the production of ROS against resin cement. In one of these studies, extracts of RelyX Unicem presented similar results to the negative control group, being unable to induce ROS production in cells derived from the bovine dental pulp, however, in cells derived from the human dental pulp the same extracts were able to induce high production of ROS [
5]. Another study demonstrated that extracts of triethylene glycol dimethacrylate, a monomer present in RelyX U200, strongly induced the production of ROS in a salivary gland cell line [
37].
CONCLUSIONS
Within the limitations of this study, our results highlight the great importance of the photoactivation of self-adhesive resin cements in the dental clinic. Therefore, the RelyX U200 and seT PP cements when photoactivated showed less interference in the viability and activity of murine macrophages.
-
Funding: This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq) and Pró-Reitoria de Pesquisa da UFMG (PRPq).
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: da Silva DC, Tavares WLF, Sobrinho APR.
Data curation: da Silva DC, Tavares WLF, de Oliveira RR, Sobrinho APR.
Formal analysis: da Silva DC, Tavares WLF, Vaz LG.
Funding acquisition: Tavares WLF, Vieira LQ, Sobrinho APR.
Investigation: da Silva DC, Tavares WLF, Vaz LG, Vieira LQ, de Oliveira RR, Sobrinho APR.
Methodology: da Silva DC, Tavares WLF, de Oliveira RR.
Project administration: Tavares WLF, de Oliveira RR.
REFERENCES
- 1. Ferracane JL, Stansbury JW, Burke FJ. Self-adhesive resin cements – chemistry, properties and clinical considerations. J Oral Rehabil 2011;38:295-314.ArticlePubMed
- 2. Schedle A, Franz A, Rausch-Fan X, Spittler A, Lucas T, Samorapoompichit P, Sperr W, Boltz-Nitulescu G. Cytotoxic effects of dental composites, adhesive substances, compomers and cements. Dent Mater 1998;14:429-440.ArticlePubMed
- 3. Schmid-Schwap M, Franz A, König F, Bristela M, Lucas T, Piehslinger E, Watts DC, Schedle A. Cytotoxicity of four categories of dental cements. Dent Mater 2009;25:360-368.ArticlePubMed
- 4. Kurt A, Altintas SH, Kiziltas MV, Tekkeli SE, Guler EM, Kocyigit A, Usumez A. Evaluation of residual monomer release and toxicity of self-adhesive resin cements. Dent Mater J 2018;37:40-48.ArticlePubMed
- 5. Ülker HE, Hiller KA, Schweikl H, Seidenader C, Sengun A, Schmalz G. Human and bovine pulp-derived cell reactions to dental resin cements. Clin Oral Investig 2012;16:1571-1578.ArticlePubMedPDF
- 6. Arslan Malkoç M, Demir N, Şengün A, Bozkurt ŞB, Hakki SS. Cytotoxicity evaluation of luting resin cements on bovine dental pulp-derived cells (bDPCs) by real-time cell analysis. Dent Mater J 2015;34:154-160.ArticlePubMed
- 7. Pontes EC, Soares DG, Hebling J, Costa CA. Cytotoxicity of resin-based luting cements to pulp cells. Am J Dent 2014;27:237-244.PubMed
- 8. da Fonseca Roberti Garcia L, Pontes EC, Basso FG, Hebling J, de Souza Costa CA, Soares DG. Transdentinal cytotoxicity of resin-based luting cements to pulp cells. Clin Oral Investig 2016;20:1559-1566.ArticlePubMedPDF
- 9. Bakopoulou A, Mourelatos D, Tsiftsoglou AS, Giassin NP, Mioglou E, Garefis P. Genotoxic and cytotoxic effects of different types of dental cement on normal cultured human lymphocytes. Mutat Res 2009;672:103-112.ArticlePubMed
- 10. Porto IC, Oliveira DC, Raele RA, Ribas KH, Montes MA, De Castro CM. Cytotoxicity of current adhesive systems: in vitro testing on cell cultures of primary murine macrophages. Dent Mater 2011;27:221-228.PubMed
- 11. Gong Y, Yang RQ, Yang HL. Effects of composite resin and glass-ionomer cements on proliferation and functional activity of human macrophages. Shanghai Kou Qiang Yi Xue 2013;22:414-417.PubMed
- 12. Moraes RR, Brandt WC, Naves LZ, Correr-Sobrinho L, Piva E. Light- and time-dependent polymerization of dual-cured resin luting agent beneath ceramic. Acta Odontol Scand 2008;66:257-261.ArticlePubMed
- 13. Altintas SH, Usumez A. Evaluation of TEGDMA leaching from four resin cements by HPLC. Eur J Dent 2012;6:255-262.ArticlePubMedPMC
- 14. Ulker HE, Sengun A. Cytotoxicity evaluation of self adhesive composite resin cements by dentin barrier test on 3D pulp cells. Eur J Dent 2009;3:120-126.PubMedPMC
- 15. Trowbridge HO. Immunological aspects of chronic inflammation and repair. J Endod 1990;16:54-61.ArticlePubMed
- 16. Kamal AM, Okiji T, Kawashima N, Suda H. Defense responses of dentin/pulp complex to experimentally induced caries in rat molars: an immunohistochemical study on kinetics of pulpal Ia antigen-expressing cells and macrophages. J Endod 1997;23:115-120.PubMed
- 17. Rezende TM, Vargas DL, Cardoso FP, Sobrinho AP, Vieira LQ. Effect of mineral trioxide aggregate on cytokine production by peritoneal macrophages. Int Endod J 2005;38:896-903.ArticlePubMed
- 18. de Oliveira Mendes ST, Ribeiro Sobrinho AP, de Carvalho AT, de Souza Côrtes MI, Vieira LQ.
In vitro evaluation of the cytotoxicity of two root canal sealers on macrophage activity. J Endod 2003;29:95-99.PubMed
- 19. Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol 1993;54:283-288.ArticlePubMedPDF
- 20. Giaimis J, Lombard Y, Makaya-Kumba M, Fonteneau P, Poindron P. A new and simple method for studying the binding and ingestion steps in the phagocytosis of yeasts. J Immunol Methods 1992;154:185-193.ArticlePubMed
- 21. Rezende TM, Vieira LQ, Cardoso FP, Oliveira RR, de Oliveira Mendes ST, Jorge ML, Ribeiro Sobrinho AP. The effect of mineral trioxide aggregate on phagocytic activity and production of reactive oxygen, nitrogen species and arginase activity by M1 and M2 macrophages. Int Endod J 2007;40:603-611.ArticlePubMed
- 22. Trusk MA, Wilson ME, Dyke KV. The generation of chemiluminescence by phagocytic cells. In: Deluca MA, editor. Methods in enzymology. London: Academic Press; 1978. p. 462-493.
- 23. Radovic I, Monticelli F, Goracci C, Vulicevic ZR, Ferrari M. Self-adhesive resin cements: a literature review. J Adhes Dent 2008;10:251-258.PubMed
- 24. Kumbuloglu O, Lassila LV, User A, Vallittu PK. A study of the physical and chemical properties of four resin composite luting cements. Int J Prosthodont 2004;17:357-363.PubMed
- 25. Vrochari AD, Eliades G, Hellwig E, Wrbas KT. Curing efficiency of four self-etching, self-adhesive resin cements. Dent Mater 2009;25:1104-1108.ArticlePubMed
- 26. Monteiro GQ, Souza FB, Pedrosa RF, Sales GC, Castro CM, Fraga SN, Galvão BH, Braz R.
In vitro biological response to a self-adhesive resin cement under different curing strategies. J Biomed Mater Res B Appl Biomater 2010;92:317-321.PubMed
- 27. Rakich DR, Wataha JC, Lefebvre CA, Weller RN. Effect of dentin bonding agents on the secretion of inflammatory mediators from macrophages. J Endod 1999;25:114-117.ArticlePubMed
- 28. Accorinte ML, Loguercio AD, Reis A, Muench A, de Araújo VC. Adverse effects of human pulps after direct pulp capping with the different components from a total-etch, three-step adhesive system. Dent Mater 2005;21:599-607.ArticlePubMed
- 29. Paranjpe A, Bordador LC, Wang MY, Hume WR, Jewett A. Resin monomer 2-hydroxyethyl methacrylate (HEMA) is a potent inducer of apoptotic cell death in human and mouse cells. J Dent Res 2005;84:172-177.ArticlePubMedPDF
- 30. D’Alpino PHP, Moura GEDD, Barbosa SCA, Marques LA, Eberlin MN, Nascimento FD, Tersariol ILDS. Differential cytotoxic effects on odontoblastic cells induced by self-adhesive resin cements as a function of the activation protocol. Dent Mater 2017;33:1402-1415.ArticlePubMed
- 31. de Mendonça AA, Souza PP, Hebling J, Costa CA. Cytotoxic effects of hard-setting cements applied on the odontoblast cell line MDPC-23. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:e102-e108.ArticlePubMed
- 32. Almeida CM, Meereis CTW, Leal FB, Ogliari AO, Piva E, Ogliari FA. Evaluation of long-term bond strength and selected properties of self-adhesive resin cements. Braz Oral Res 2018;32:e15.ArticlePubMed
- 33. Volk J, Ziemann C, Leyhausen G, Geurtsen W. Non-irradiated campherquinone induces DNA damage in human gingival fibroblasts. Dent Mater 2009;25:1556-1563.ArticlePubMed
- 34. Segura JJ, Jiménez-Rubio A, Pulgar R, Olea N, Guerrero JM, Calvo JR.
In vitro effect of the resin component bisphenol A on substrate adherence capacity of macrophages. J Endod 1999;25:341-344.PubMed
- 35. Braga JM, Oliveira RR, de Castro Martins R, Vieira LQ, Sobrinho AP. Assessment of the cytotoxicity of a mineral trioxide aggregate-based sealer with respect to macrophage activity. Dent Traumatol 2015;31:390-395.ArticlePubMed
- 36. Schweikl H, Spagnuolo G, Schmalz G. Genetic and cellular toxicology of dental resin monomers. J Dent Res 2006;85:870-877.ArticlePubMedPDF
- 37. Samuelsen JT, Dahl JE, Karlsson S, Morisbak E, Becher R. Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation of the MAP-kinases p38, JNK and ERK. Dent Mater 2007;23:34-39.ArticlePubMed
 , Leonardo Gomes Vaz2
, Leonardo Gomes Vaz2 , Warley Luciano Fonseca Tavares1
, Warley Luciano Fonseca Tavares1 , Leda Quercia Vieira2
, Leda Quercia Vieira2 , Ricardo Reis de Oliveira1
, Ricardo Reis de Oliveira1 , Antônio Paulino Ribeiro Sobrinho1
, Antônio Paulino Ribeiro Sobrinho1






 KACD
KACD
 ePub Link
ePub Link Cite
Cite

