Abstract
-
Objectives
This randomized clinical trial aimed to assess the effectiveness of buccal infiltration with piroxicam on the anesthetic efficacy of inferior alveolar nerve block (IANB) with buccal infiltration in irreversible pulpitis, with pain assessed using the Heft-Parker visual analogue scale (HP-VAS).
-
Materials and Methods
This study included 56 patients with irreversible pulpitis in mandibular molars, randomly distributed between 2 groups (n = 28). After evaluating the initial pain score with the HP-VAS, each patient received IANB followed by buccal infiltration of 2% lignocaine with adrenaline (1:80,000). Five minutes later, the patients in groups 1 and 2 were given buccal infiltration with 40 mg/2 mL of piroxicam or normal saline, respectively. An access opening procedure (AOP) was performed 15 minutes post-IANB once the individual showed signs of lip numbness as well as 2 negative responses to electric pulp testing. The HP-VAS was used to grade the patient's pain during caries removal (CR), AOP, and working length measurement (WLM). Successful anesthesia was identified either by the absence of pain or slight pain through CR, AOP, and WLM, with no requirement of a further anesthetic dose. A statistical analysis was done using the Shapiro-Wilk and Mann-Whitney U tests.
-
Results
The piroxicam group presented a significantly lower (p < 0.05) mean pain score than the saline group during AOP.
-
Conclusions
Buccal infiltration with piroxicam enhanced the efficacy of anesthesia with IANB and buccal infiltration with lignocaine in patients with irreversible pulpitis.
-
Keywords: Anesthesia; Buccal infiltration; Inferior alveolar nerve block; Irreversible pulpitis; Lignocaine; Piroxicam
INTRODUCTION
Irreversible pulpitis is a pulpal pathosis characterized by an exaggerated painful thermal response, particularly to cold stimuli, wherein the inflamed pulp is incapable of healing and returning to a normal state. Pain can either be intensified by a stimulus or be spontaneous [
1]. The principal treatment for this condition is root canal therapy [
2].
Complete pulpal anesthesia, particularly inferior alveolar nerve block (IANB), may be difficult to attain in mandibular molars with irreversible pulpitis, and the presence of irreversible pulpitis therefore reduces the success rate of IANB [
3,
4,
5,
6]. Studies have reported that achieving successful anesthesia in inflamed pulp is 8 times more difficult than in normal pulp, with the failure rate of IANB ranging from 44% to 81% [
4,
6,
7]. Explanations for this difficulty include the tachyphylaxis of the anesthetic solution, decreased pH, and stimulation and modifications of the response of nociceptors (including tetrodotoxin-sensitive and capsaicin-sensitive transient receptor potential vanilloid type 1 [TRPV1] nociceptors) in the presence of inflammatory mediators such as prostaglandins (PGs) [
4,
8].
Nonsteroidal anti-inflammatory drugs (NSAIDs), which deactivate the cyclooxygenase (COX) pathway, have an inhibitory impact on PG production [
9]. Thus, it has been hypothesized that in irreversible pulpitis, premedication with NSAIDs helps to achieve anesthesia. Some studies have reported that supplemental infiltration with ketorolac tromethamine and dexamethasone increased the effectiveness of IANB in irreversible pulpitis [
4,
10].
Piroxicam, an oxicam derivative with pain-relieving and anti-inflammatory effects, is a non-selective COX inhibitor that acts by inhibiting PG synthesis [
11,
12]. A study reported significant improvement in pain within 1 hour of the initial dose that improved over a 12-hour period and lasted for 24 hours after parenteral administration of piroxicam [
13]. To date, no study in the literature has evaluated the efficacy of buccal piroxicam infiltration on the success of IANB. Hence, this prospective, double-blind, randomized trial was conducted to gauge the influence of buccal infiltration with piroxicam on the success of IANB and buccal infiltration using 2% lignocaine with adrenaline (1:80,000) in patients with irreversible pulpitis.
MATERIALS AND METHODS
After receiving approval from the Ethics Committee of the institution and registering the trial with the Clinical Trial Registry-India (CTRI Reg. No.-CTRI/2018/10/016010), the study was commenced. This trial included 56 patients, aged 15 to 65 years, who visited the Department of Endodontics at Rama Dental College, Hospital and Research Centre, Kanpur, India.
The effect size was computed using G*Power 3.1 software (Heinrich-Heine-Universität Düsseldorf, Dusseldorf, Germany) as the least difference expected between groups, using the mean visual analogue scale (VAS) scores of patients from a previous study [
4]. The sample size was then calculated using an effect size of 0.82 at α = 0.05 and a power of 0.95, resulting in a total sample size for the 2 groups being 84. For a power of 0.8, the sample size would be 52, thus, the calculated sample size for our study was 52 (26 in each group). To control for the possibility of manual errors, we rounded the sample size to 56 (28 in each group). Each patient completed a written informed consent form, as well as an oral and written questionnaire to assess his or her health and preoperative pain. Before commencing the procedure, each patient was taught how to score his or her pain on the 170-mm Heft-Parker VAS (HP-VAS), with 0 indicating no pain, 1–54 indicating slight pain, 55–114 indicating moderate pain, and > 114 indicating extreme pain.
Patients with pain in the mandibular molars (> 54 on the HP-VAS), vital teeth with no distinct periapical pathologies, an exaggerated response to cold testing (Endo-Frost, Coltene Whaledent, Langenau, Germany) with pain lasting > 45 seconds, an initial response recorded as 0–40 with an electric pulp tester (EPT) (COXO SOCO C-Pulse pulp tester, Foshan COXO Medical Instrument Co. Ltd, Guangdong, China) were included in this study if they were categorized as having either a class I or II medical history (American Society of Anesthesiologists) and could comprehend the consent form and the HP-VAS. Individuals with more than 1 infected tooth; an inability to achieve lip numbness with a single infusion of IANB; known hypersensitivity or allergy to piroxicam or other anti-inflammatory drugs; or a history of peptic ulcers, renal, liver or bleeding disorders were excluded from the study, as were pregnant or lactating patients.
Each patient was handed a packet containing a consent form, questionnaire, a bottle of 2% lignocaine with adrenaline (1:80,000; Lignox 2%, Indoco Remedies, Gandhinagar, Gujarat, India) and 25-gauge syringes (Hi-Tech, Mumbai, India). Normal saline (Pentagon Labs, Dewas, Madhya Pradesh, India) and piroxicam (Dolonex, Pfizer Ltd., Mumbai, India) were loaded into disposable syringes and coded as A and B, respectively.
A standard IANB injection using 1.8 mL of 2% lignocaine with 1:80,000 epinephrine, immediately followed by buccal infiltration of 0.9 mL lignocaine, was administered to every patient. After 5 minutes, randomized allocation ensuring blinding of the subjects was done in 2 groups (n = 28) by a skilled postgraduate student who was ignorant of the study design and purpose and was requested to provide supplemental buccal infiltration with syringe A and syringe B for the patients in groups 1 and 2, respectively. Thus, group 1 was the control group (buccal infiltration with normal saline, syringe A), and group 2 was the test group (buccal infiltration with 20 mg/mL of piroxicam, syringe B).
Pulpal anesthesia was evaluated every 5 minutes by using an analogue EPT and assessing lip numbness. An access opening procedure (AOP) was performed 15 minutes post-IANB once the patient showed signs of lip numbness and gave 2 successive negative responses to the EPT. If not, the patient was not included in the study.
The 2 groups were compared in terms of patients' age and sex (
Table 1). The HP-VAS was used to grade the patient's initial pain score (IPS) and pain during caries removal (CR), AOP, and working length measurement (WLM) (
Table 2). Successful anesthesia was identified by either the absence of pain or mild/slight pain during CR, AOP, and WLM, with no requirement for a further anesthetic dose. Any additional anesthetic dose, if required due to severe pain, led to the exclusion of that patient from the trial.
Table 1 Demographic details of the study population
|
Variables |
Control (n = 28) |
Test (n = 28) |
p value |
|
Age |
29.57 ± 13.05 |
30.42 ± 9.02 |
0.072 |
|
Sex |
|
|
0.632 |
|
Male |
12 (42.8) |
12 (42.8) |
|
Female |
16 (57.2) |
16 (57.2) |
Table 2 Comparison of mean pain scores during various procedures between the control and test groups
|
Times |
Groups |
No. |
Mean ± SD |
p value*
|
|
IPS |
Control |
28 |
112.35 ± 31.62 |
0.762 |
|
Test |
28 |
115.75 ± 31.87 |
|
CR |
Control |
28 |
43.44 ± 43.62 |
0.623 |
|
Test |
28 |
36.08 ± 39.95 |
|
AOP |
Control |
28 |
76.57 ± 47.33 |
0.008†
|
|
Test |
28 |
44.66 ± 50.56 |
|
WLM |
Control |
28 |
65.16 ± 52.18 |
0.141 |
|
Test |
28 |
47.33 ± 56.71 |
Descriptive and analytical statistics were calculated using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). A p value < 0.05 was considered to indicate statistical significance. The Shapiro-Wilk test was applied to analyze the normality of the data. As the data did not follow a normal distribution, the analysis was done using non-parametric tests. The mean differences were checked using the Mann-Whitney U test wherever appropriate.
RESULTS
The mean age of the selected subjects was 29.57 ± 13.05 years and 30.42 ± 9.02 years in the control and test groups, respectively. There were 12 males (42.8%) and 16 females (57.2%) in each group. The difference in the sex and age distribution between the groups was not statistically significant (
Table 1).
The mean pain score during various steps was compared between the control and test groups (
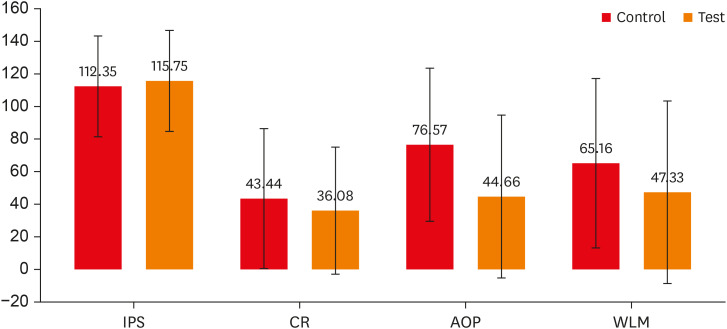
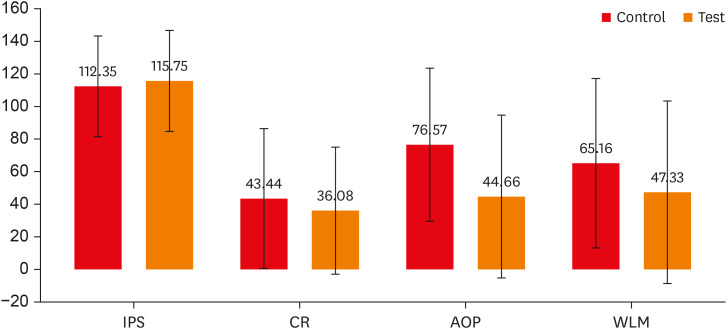
Table 2,
Figure 1). The IPS for both groups was nearly the same (control group, 112.35 ± 31.62; test group, 115.75 ± 31.87). During CR and WLM, although the control group exhibited a higher pain level than the test group, a significant difference was not observed (
p > 0.05) in their pain scores. However, during AOP, the mean pain score of the test group (44.66 ± 50.56) was significantly lower (
p = 0.008) than that of the control group (76.57 ± 47.33).
Figure 1
Comparison of mean pain scores during various procedures between the 2 groups. Error bars represent standard deviations.
IPS, initial pain score; CR, caries removal; AOP, access opening procedure; WLM, working length measurement.
The number of pain-free patients during CR, AOP, and WLM for the saline group was 8, 5, and 6, respectively. In the piroxicam group, the number of pain-free patients during CR, AOP, and WLM was 10, 10, and 9, respectively (
Table 3). However, no statistically significant differences between the 2 groups were found in the number of pain-free patients or those with slight, moderate, or extreme pain (
p > 0.05) (
Table 3).
Table 3 Distribution and comparison of the number of patients according to their pain categories between the control and test groups
|
Variables |
Control (saline) group |
Test (piroxicam) group |
p value |
|
IPS |
|
|
1.000 |
|
No pain |
0 (0) |
0 (0) |
|
Slight pain |
0 (0) |
0 (0) |
|
Moderate pain |
16 (57.1) |
16 (57.1) |
|
Extreme pain |
12 (42.9) |
12 (42.9) |
|
CR |
|
|
0.673 |
|
No pain |
8 (28.6) |
10 (35.7) |
|
Slight pain |
11 (39.3) |
13 (46.4) |
|
Moderate pain |
7 (25.0) |
4 (14.3) |
|
Extreme pain |
2 (7.1) |
1 (3.6) |
|
AOP |
|
|
0.263 |
|
No pain |
5 (17.9) |
10 (35.7) |
|
Slight pain |
7 (25.0) |
9 (32.1) |
|
Moderate pain |
10 (35.7) |
5 (17.9) |
|
Extreme pain |
6 (21.4) |
4 (14.3) |
|
WLM |
|
|
0.086 |
|
No pain |
6 (21.4) |
9 (32.1) |
|
Slight pain |
6 (21.4) |
11 (39.3) |
|
Moderate pain |
9 (32.1) |
2 (7.1) |
|
Extreme pain |
7 (25.0) |
6 (21.4) |
DISCUSSION
Several studies have found that supplementary anesthesia is often required with IANB injections for attaining complete anesthesia, particularly for molars with irreversible pulpitis [
14]. Parirokh
et al. [
15] concluded that a higher rate of achieving complete anesthesia (65.4%) was attainable with a combination of IANB and buccal infiltration [
15,
16].
Some of the factors that contribute to incomplete anesthesia include cross-innervation, central sensitization, tissue pH, movement of blood, and acute tachyphylaxis [
8]. Inflammation stimulates TRPV1 nociceptors, which produce calcitonin gene-related peptide (CGRP) and modify the pulpal pH and temperature [
10]. The constant afferent flow leads to extended production of CGRP and substance P, triggering N-methyl-D-aspartate receptors and stimulating inflammatory mediators such as PGs, which are arachidonic acid derivatives produced through the COX pathway [
8,
12]. Pulpal inflammation also leads to an upsurge in the Nav-1.8 and 1.9 subtypes of tetrodotoxin-resistant sodium channels in C-fibers, which are resistant to anesthetic agents such as lignocaine and therefore inhibit the achievement of complete anesthesia [
17].
NSAIDs block the COX pathway, thereby influencing the hypothalamic PG system and neural pathways involved in nociception [
12,
18]. The inflammatory mediators in inflamed pulp aggravate pain through nerve sensitization. Numerous studies have evaluated the role of NSAIDs in IANB and reported diverse outcomes depending on the drug administration method and the local anesthetic solution used [
3,
4,
6,
10].
In our study, we used piroxicam, an N-heterocyclic carboxamide (specifically, 1,2-benzothiazine-1,1 dioxide). With a half-life of nearly 40 hours, piroxicam can be administered as a single daily dose of 20 mg in patients with moderate to severe pain [
11]. Administration through the parenteral route is preferred since it provides immediate release and enhances the start of action, absorption, and bioavailability [
6,
19]. Piroxicam acts by blocking C-fibers, which are highly resistant to local anesthetics. Furthermore, it opens the K
+ channels located in the afferent fibers, triggering the analgesic and antinociceptive action of NSAIDs [
6]. These could be plausible reasons for why buccal piroxicam infiltration augments the success rate of IANB.
Prasanna
et al. [
6] found that pre-procedural lornoxicam administration led to significant improvements in the success rate of IANB in patients with irreversible pulpitis. Another study concluded that intraligamentary administration of piroxicam was useful for the management of post-procedural discomfort in molars with irreversible pulpitis [
20,
21].
Some harmful effects of piroxicam include peptic ulcers, skin rashes, edema, dizziness, headache, and potential for renal damage depending on the quantity and duration of use [
11]. Hence, piroxicam must be administered cautiously to patients with impaired hepatic, renal, and gastrointestinal systems and to pregnant patients. However, a single dose is likely to generate minimal or no adverse consequences in healthy individuals if the previously mentioned inclusion and exclusion criteria are followed.
We chose buccal infiltration since it has been suggested that administering a drug via the buccal mucosa can bypass the hepatic clearance and plasma binding. Furthermore, buccal infiltration aids in the direct delivery of the drug to the site of inflammation [
10]. Other supplemental techniques include intraligamentary and intraosseous injections. The complications associated with intraligamentary injections include swelling, discoloration, and prolonged ischemia of the interdental papilla followed by sloughing and exposure of crestal bone. However, intraosseous injections require a specific apparatus for drilling the cortical bone; thus, they are not a favored technique [
19,
22].
In patients with irreversible pulpitis, numb lips alone do not imply pulpal anesthesia or demonstrate that the IANB injection was successful. A more reliable indicator is a negative response to EPT [
4,
6]. Therefore, in this study, lip numbness in conjunction with 2 negative responses to EPT was considered as an appropriate indication of pulpal anesthesia.
The VAS is a simple, quick, and extensively utilized tool for quantifying pain. The patient shows the magnitude of a pain stimulus by marking a point equivalent to the intensity of the discomfort. However, reports have suggested that some individuals find it difficult to use the VAS since they are likely to overestimate their initial pain. Another way of gauging pain is to use a simple and clear 4-point categorical oral scale: no pain, slight pain, moderate pain, and excessive pain. The only disadvantage of this scale is its constrained groups in comparison to the VAS. Using this scale, individuals are directed towards selecting a category that may not precisely depict the intensity of their pain [
23,
24]. Hence, we chose the integrated, 170-mm HP-VAS for our trial; using this tool, the patient marks a point equivalent of his/her discomfort based on hints provided by the various categorical points. An equivalent 170-mm scale with millimeter marks was printed behind the HP-VAS, such that the markings were not visible to the patient.
The proportion of patients who were pain-free or who had slight pain was compared between the saline and piroxicam groups (
Table 3). Piroxicam administration was effective in reducing pain during treatment procedures to the slight pain level or below. We also evaluated the mean pain scores during various procedures in the test (piroxicam) and control (saline) groups (
Table 2). The findings showed that the IPS of both groups was almost the same. During CR, the pain score of the saline group remained greater than that of the piroxicam group, although the difference was not statistically significant (
p = 0.623). Jena
et al. [
25] suggested that endodontic access enables a clinically appropriate assessment of pulpal anesthesia and used endodontic access in their study to analyze anesthetic efficacy. They recorded a decrease in pain during endodontic access preparation. Similarly, in our study, anesthetic efficiency was evaluated by pain scores during the AOP in both the piroxicam and control groups. During AOP, a statistically significant (
p = 0.008) difference in the mean pain score between the groups was observed. The finding that the pain score was significantly lower in the piroxicam group during AOP was considered to be a good indicator of pulpal anesthesia.
During WLM, the pain score of the saline group was higher than that of the piroxicam group, although the difference was not statistically significant (
p = 0.141). Our results are in accordance with the study conducted by Akhlaghi
et al. [
4], in which the researchers observed a significant decrease in pain during access cavity preparation, but no significant decrease in pain during WLM, in the ketorolac group compared to the control group.
Therefore, based on the findings of this study, it can be assumed that piroxicam delivery near inflamed teeth significantly improves the success of IANB. Further clinical studies should compare the effectiveness of piroxicam with that of other pharmacological agents.
CONCLUSIONS
Within the constraints of the present trial, we conclude that piroxicam buccal infiltration significantly enhances the successful achievement of IANB accompanied with buccal infiltration of lignocaine in individuals with irreversible pulpitis.
ACKNOWLEDGEMENTS
The authors are grateful to every patient involved in this research for providing his/her consent, thereby allowing the completion of the trial.
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Paul S, Nandamuri S.
Data curation: Paul S, Nandamuri S, Bansal M.
Formal analysis: Paul S, Raina A.
Funding acquisition: Paul S, Nandamuri S, Raina A, Bansal M.
Investigation: Paul S, Raina A, Bansal M.
Methodology: Paul S, Nandamuri S, Raina A, Bansal M.
Project administration: Paul S, Nandamuri S, Raina A, Bansal M.
Resources: Paul S, Nandamuri S, Raina A.
Software: Paul S, Bansal M.
Supervision: Raina A.
Validation: Paul S, Nandamuri S.
Visualization: Paul S, Raina A, Bansal M.
Writing - original draft: Paul S, Nandamuri S, Raina A, Bansal M.
Writing - review & editing: Paul S, Nandamuri S.
REFERENCES
- 1. Handysides RA, Jaramillo DE, Ingle JI. Examination, evaluation, diagnosis and treatment planning. In: Ingle JI, Bakland LK, Baumgartner JC, editors. Ingle's endodontics. 6th ed. Hamilton, ON: BC Decker Inc.; 2008. p. Chapter 14.
- 2. Ali SG, Mulay S. Pulpitis: a review. IOSR J Dent Med Sci 2015;14:92-97.
- 3. Aggarwal V, Singla M, Kabi D. Comparative evaluation of effect of preoperative oral medication of ibuprofen and ketorolac on anesthetic efficacy of inferior alveolar nerve block with lidocaine in patients with irreversible pulpitis: a prospective, double-blind, randomized clinical trial. J Endod 2010;36:375-378.ArticlePubMed
- 4. Akhlaghi NM, Hormozi B, Abbott PV, Khalilak Z. Efficacy of ketorolac buccal infiltrations and inferior alveolar nerve blocks in patients with irreversible pulpitis: a prospective, double-blind, randomized clinical trial. J Endod 2016;42:691-695.ArticlePubMed
- 5. Rogers BS, Botero TM, McDonald NJ, Gardner RJ, Peters MC. Efficacy of articaine versus lidocaine as a supplemental buccal infiltration in mandibular molars with irreversible pulpitis: a prospective, randomized, double-blind study. J Endod 2014;40:753-758.ArticlePubMed
- 6. Prasanna N, Subbarao CV, Gutmann JL. The efficacy of pre-operative oral medication of lornoxicam and diclofenac potassium on the success of inferior alveolar nerve block in patients with irreversible pulpitis: a double-blind, randomised controlled clinical trial. Int Endod J 2011;44:330-336.ArticlePubMed
- 7. Verma PK, Srivastava R, Ramesh KM. Anesthetic efficacy of X-tip intraosseous injection using 2% lidocaine with 1:80,000 epinephrine in patients with irreversible pulpitis after inferior alveolar nerve block: a clinical study. J Conserv Dent 2013;16:162-166.ArticlePubMedPMC
- 8. Hargreaves KM, Keiser K. Local anesthetic failure in endodontics: mechanisms and management. Endod Topics 2002;1:26-39.
- 9. Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 2000;69:145-182.ArticlePubMed
- 10. Aggarwal V, Singla M, Rizvi A, Miglani S. Comparative evaluation of local infiltration of articaine, articaine plus ketorolac, and dexamethasone on anesthetic efficacy of inferior alveolar nerve block with lidocaine in patients with irreversible pulpitis. J Endod 2011;37:445-449.ArticlePubMed
- 11. Brogden RN, Heel RC, Speight TM, Avery GS. Piroxicam: a review of its pharmacological properties and therapeutic efficacy. Drugs 1981;22:165-187.ArticlePubMed
- 12. Shanbagh TV, Shenoy S, Nayak V. Autacoids and respiratory system. In: Shanbagh TV, Shenoy S, Nayak V, editors. Pharmacology for dental students. 2nd ed. Taramani, Chennai: Elsevier; 2010. p. Chapter 7.
- 13. Wiseman RL, Kilgour M. Intramuscular piroxicam, a new dosage form, in the treatment of acute musculoskeletal disorders. J Int Med Res 1985;13:255-262.ArticlePubMedPDF
- 14. Cohen HP, Cha BY, Spångberg LS. Endodontic anesthesia in mandibular molars: a clinical study. J Endod 1993;19:370-373.ArticlePubMed
- 15. Parirokh M, Satvati SA, Sharifi R, Rekabi AR, Gorjestani H, Nakhaee N, Abbott PV. Efficacy of combining a buccal infiltration with an inferior alveolar nerve block for mandibular molars with irreversible pulpitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:468-473.ArticlePubMed
- 16. Parirokh M, Yosefi MH, Nakhaee N, Abbott PV, Manochehrifar H. The success rate of bupivacaine and lidocaine as anesthetic agents in inferior alveolar nerve block in teeth with irreversible pulpitis without spontaneous pain. Restor Dent Endod 2015;40:155-160.ArticlePubMedPMC
- 17. Kaviani N, Khademi A, Ebtehaj I, Mohammadi Z. The effect of orally administered ketamine on requirement for anesthetics and postoperative pain in mandibular molar teeth with irreversible pulpitis. J Oral Sci 2011;53:461-465.ArticlePubMed
- 18. Vadivelu N, Gowda AM, Urman RD, Jolly S, Kodumudi V, Maria M, Taylor R Jr, Pergolizzi JV Jr. Ketorolac tromethamine - routes and clinical implications. Pain Pract 2015;15:175-193.ArticlePubMed
- 19. Joshi N, Mathew S, George JV, Hegde S, Bhandi S, Madhu KS. Comparative evaluation of the efficacy of two modes of delivery of Piroxicam (Dolonex®) for the management of postendodontic pain: a randomized control trial. J Conserv Dent 2016;19:301-305.ArticlePubMedPMC
- 20. Atbaei A, Mortazavi N. Prophylactic intraligamentary injection of piroxicam (feldene) for the management of post-endodontic pain in molar teeth with irreversible pulpitis. Aust Endod J 2012;38:31-35.ArticlePubMed
- 21. Subhan Z, Shami S, Ahmed Rana MJ. Comparison of prophylactic intraligamentary injection of piroxicam and lignocaine for management of postoperative endodontic pain. Pak Oral Dent J 2016;36:480-483.
- 22. Yadav S. Anesthetic success of supplemental infiltration in mandibular molars with irreversible pulpitis: a systematic review. J Conserv Dent 2015;18:182-186.ArticlePubMedPMC
- 23. Goddard G, Karibe H, McNeill C. Reproducibility of visual analog scale (VAS) pain scores to mechanical pressure. Cranio 2004;22:250-256.ArticlePubMed
- 24. Averbuch M, Katzper M. Assessment of visual analog versus categorical scale for measurement of osteoarthritis pain. J Clin Pharmacol 2004;44:368-372.ArticlePubMed
- 25. Jena A, Shashirekha G. Effect of preoperative medications on the efficacy of inferior alveolar nerve block in patients with irreversible pulpitis: a placebo-controlled clinical study. J Conserv Dent 2013;16:171-174.ArticlePubMedPMC
 , Sridevi Nandamuri
, Sridevi Nandamuri , Aakrati Raina
, Aakrati Raina , Mukta Bansal
, Mukta Bansal



 KACD
KACD
 ePub Link
ePub Link Cite
Cite

