Search
- Page Path
- HOME > Search
- Effect of phytic acid as an endodontic chelator on resin adhesion to sodium hypochlorite-treated dentin
- Mohannad Nassar, Noriko Hiraishi, Md. Sofiqul Islam, Maria JRH. Romero, Masayuki Otsuki, Junji Tagami
- Restor Dent Endod 2020;45(4):e44. Published online August 24, 2020
- DOI: https://doi.org/10.5395/rde.2020.45.e44
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Objectives Phytic acid (IP6), a naturally occurring agent, has been previously reported as a potential alternative to ethylenediaminetetraacetic acid (EDTA). However, its effect on adhesion to sodium hypochlorite (NaOCl)-treated dentin and its interactions with NaOCl have not been previously reported. Thus, in this study, the effects of IP6 on resin adhesion to NaOCl-treated dentin and the failure mode were investigated and the interactions between the used agents were analyzed.
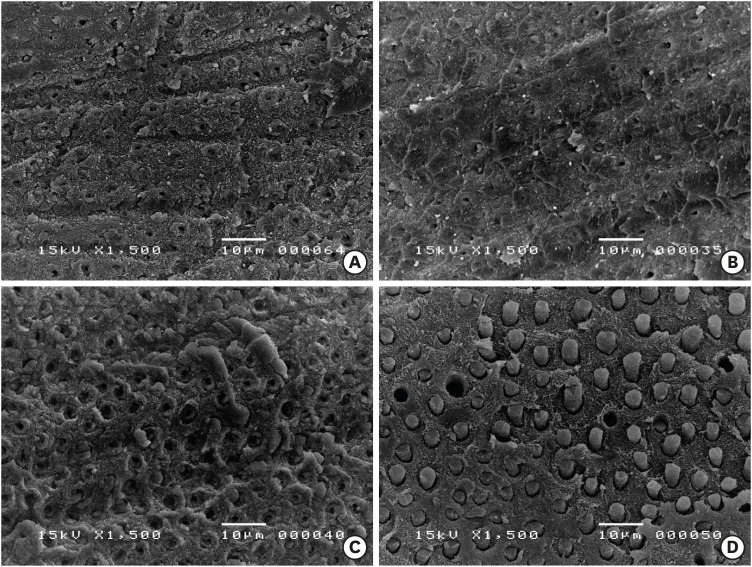
Materials and Methods Micro-tensile bond strength (µTBS) testing was performed until failure on dentin treated with either distilled water (control), 5% NaOCl, or 5% NaOCl followed with chelators: 17% EDTA for 1 minute or 1% IP6 for 30 seconds or 1 minute. The failed specimens were assessed under a scanning electron microscope. The reaction of NaOCl with EDTA or IP6 was analyzed in terms of temperature, pH, effervescence, and chlorine odor, and the effects of the resulting mixtures on the color of a stained paper were recorded.
Results The µTBS values of the control and NaOCl with chelator groups were not significantly different, but were all significantly higher than that of the group treated with NaOCl only. In the failure analysis, a distinctive feature was the presence of resin tags in samples conditioned with IP6 after treatment with NaOCl. The reaction of 1% IP6 with 5% NaOCl was less aggressive than the reaction of the latter with 17% EDTA.
Conclusions IP6 reversed the adverse effects of NaOCl on resin-dentin adhesion without the chlorine-depleting effect of EDTA.
-
Citations
Citations to this article as recorded by- The Effect of Chemical Surface Modification on the Repair Bond Strength of Resin Composite: An In Vitro Study
Md Sofiqul Islam, Shadi El Bahra, Smriti Aryal A C, Vivek Padmanabhan, Abdulaziz Al Tawil, Ihab Saleh, Muhammed Mustahsen Rahman, Upoma Guha
Polymers.2025; 17(4): 513. CrossRef - Advancing Adhesive Strategies for Endodontically Treated Teeth—Part I: Impact of Endodontic Irrigation Protocols on the Chemical Composition and Structural Integrity of Coronal Dentin
Joana A. Marques, Rui I. Falacho, Sara Fateixa, Francisco Caramelo, João Miguel Santos, João Rocha, Markus B. Blatz, João Carlos Ramos, Paulo J. Palma
Journal of Esthetic and Restorative Dentistry.2025; 37(7): 1848. CrossRef - Effect of collagen crosslinkers on sodium hypochlorite treated dentin bond strength: a systematic review and meta-analysis
Weiqing Zhou, Shuting Feng, Xiaojun Chu, Shuaimei Xu, Xiongqun Zeng
Frontiers in Bioengineering and Biotechnology.2025;[Epub] CrossRef - Advancing Adhesive Strategies for Endodontically Treated Teeth—Part II: Dentin Sealing Before Irrigation Increases Long‐Term Microtensile Bond Strength to Coronal Dentin
Joana A. Marques, Rui I. Falacho, Gabriela Almeida, Francisco Caramelo, João Miguel Santos, João Rocha, Markus B. Blatz, João Carlos Ramos, Paulo J. Palma
Journal of Esthetic and Restorative Dentistry.2025; 37(7): 1865. CrossRef - Effects of phytic acid and etidronic acid using continuous and sequential chelation on the removal of smear layer, dentin microhardness, and push-out bond strength of calcium silicate-based cement
Ecehan Hazar, Ahmet Hazar
BMC Oral Health.2025;[Epub] CrossRef - Comparative evaluation of free available chlorine in sodium hypochlorite solutions admixed with novel chelating agents
Somya Tyagi, Sonali Taneja, Kandasamy Nagarajan, Divya Chowdhary
Endodontology.2025; 37(2): 188. CrossRef - Effect of different chelating agents, with and without activation, including XP-endo Finisher, on root dentin microhardness: An in vitro study
Mahmoud Mohamed A. Sherif, Mai Hamdy Ragab, Marwa ElSayed Sharaan
Saudi Endodontic Journal.2025; 15(3): 282. CrossRef - Oracle of phytic acid in dental panacea – Insight into properties, therapeutic effect, regeneration, materials interaction and oral physiology
Ummey Salma, C. Pushpalatha, SV. Sowmya, Dominic Augustine, Ahmed Alamoudi, Bassam Zidane, Nassreen Hassan Mohammad Albar, Shilpa Bhandi
The Saudi Dental Journal.2024; 36(8): 1093. CrossRef - In Vitro Bond Strength of Dentin Treated with Sodium Hypochlorite: Effects of Antioxidant Solutions
Guillermo Grazioli, Elisa de León Cáceres, Romina Tessore, Rafael Lund, Ana Monjarás-Ávila, Monika Lukomska-Szymanska, Louis Hardan, Rim Bourgi, Carlos Cuevas-Suárez
Antioxidants.2024; 13(9): 1116. CrossRef - Is a mix – A fix? “A microscopic analysis of depth of penetration of three combinations of irrigants”
Yantrapragada Lakshmi Sunanda, Krishna Prasad Parvathaneni, T. B. V. G. Raju, Abitha Seshadri, Nadimpalli Mahendra Varma, Gowtam Dev Dondapati
Journal of Conservative Dentistry and Endodontics.2024; 27(2): 186. CrossRef - Effect of phytic acid on dentinal collagen solubilization and its binding and debinding potentials to dentin
Diletta Forgione, Mohannad Nassar, Roda Seseogullari-Dirihan, Ahmed Jamleh, Arzu Tezvergil-Mutluay
Journal of Dentistry.2023; 128: 104361. CrossRef - Application of Inositol Hexaphosphate and Inositol in Dental Medicine: An Overview
Ana Druzijanic, Mare Kovic, Marija Roguljic, Livia Cigic, Martina Majstorovic, Ivana Vucenik
Biomolecules.2023; 13(6): 913. CrossRef - Ex-vivo study about antimicrobial effectiveness of phytic acid against Enterococcus faecalis into root canals
Giulia BOSCHI, Giorgio PICCINELLI, Carlo BONFANTI, Stefano A. SALGARELLO
Minerva Dental and Oral Science.2023;[Epub] CrossRef - Effect of phytic acid on bond strength and interfacial integrity of universal adhesive to deep dentin
Ahmed Mostafa Attia, Ahmed Fawzy Abo-Elezz, Rehab Khalil Safy
Brazilian Dental Journal.2022; 33(5): 116. CrossRef - Resin-Based Cement Applied to Enamel and Dentin Pre-Treated with Phytic Acid: An In Vitro Study
Mohannad Nassar, Md. Sofiqul Islam, Smriti Aryal A C, Hatem Mostafa El-Damanhoury, Salvatore Sauro, Noriko Hiraishi
Applied Sciences.2021; 11(24): 11976. CrossRef - Postspace pretreatment with 17% ethylenediamine tetraacetic acid, 7% maleic acid, and 1% phytic acid on bond strength of fiber posts luted with a self-adhesive resin cement
PriyaC Yadav, Ramya Raghu, Ashish Shetty, Subhashini Rajasekhara
Journal of Conservative Dentistry.2021; 24(6): 558. CrossRef - Phytic Acid: Properties and Potential Applications in Dentistry
Mohannad Nassar, Rania Nassar, Husain Maki, Abdullah Al-Yagoob, Mahmood Hachim, Abiola Senok, David Williams, Noriko Hiraishi
Frontiers in Materials.2021;[Epub] CrossRef
- The Effect of Chemical Surface Modification on the Repair Bond Strength of Resin Composite: An In Vitro Study
- 2,159 View
- 17 Download
- 17 Crossref


 KACD
KACD

 First
First Prev
Prev


