Search
- Page Path
- HOME > Search
-
Comparative evaluation of
Emblica officinalis as an etchant and an MMP inhibitor with orthophosphoric acid and chlorhexidine on the microshear bond strength of composite resin: anex vivo study - Divya Sangeetha Rajkumar, Annapoorna Ballagere Mariswamy
- Restor Dent Endod 2021;46(3):e36. Published online June 8, 2021
- DOI: https://doi.org/10.5395/rde.2021.46.e36
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Objectives This study aimed to evaluate
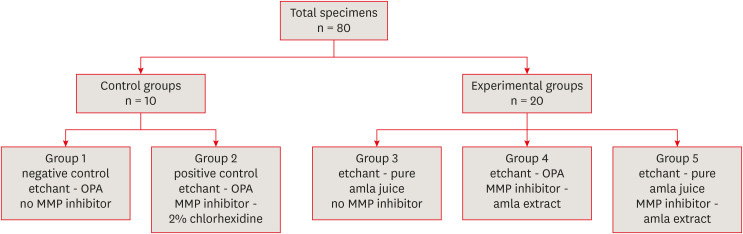
Emblica officinalis (Indian gooseberry or amla) as an acid etchant and matrix metalloproteinase (MMP) inhibitor, and to compare its effect on the microshear bond strength of composite resin with orthophosphoric acid (OPA) and 2% chlorhexidine (CHX) as an acid etchant and MMP inhibitor, respectively.Materials and Methods The etching effect and MMP-inhibiting action of amla on dentin samples were confirmed by scanning electron microscopy (SEM) and gelatin zymography, respectively. Dentinal slabs (3 mm thick) from 80 extracted human molars were divided into 10 and 20 samples to form 2 control groups and 3 experimental groups. Groups 1, 2, and 4 were etched with OPA and groups 3 and 5 with amla juice. An MMP inhibitor was then applied: CHX for group 2 and amla extract for groups 4 and 5. Groups 1 and 3 received no MMP inhibitor. All specimens received a standardized bonding protocol and composite resin build-up, and were subjected to microshear bond strength testing. The force at which the fracture occurred was recorded and statistically analyzed.
Results Amla juice had a similar etching effect as a self-etch adhesive in SEM and 100% amla extract was found to inhibit MMP-9 by gelatin zymography. The microshear bond strength values of amla were lower than those obtained for OPA and CHX, but the difference was not statistically significant.
Conclusions Amla has a promising role as an acid etchant and MMP inhibitor, but further studies are necessary to substantiate its efficacy.
-
Citations
Citations to this article as recorded by- In vitro assessment of anti-glioblastoma potential of Emblica officinalis methanolic fruit extract and green nanoparticles in U87-MG cells
Kokkonda Jackson Sugunakara Chary, Anuradha Sharma, Amrita Singh
Medical Oncology.2025;[Epub] CrossRef - Eco-conscious synthesis of novel 1,2,4-triazolo[1,5-a]pyrimidine derivatives as potent Anti-microbial agent and comparative study of cell viability and cytotoxicity in HEK-293 cell line utilizing Indian gooseberry (Phyllanthus emblica) fruit extract
Bhaktiben R. Bhatt, Kamalkishor Pandey, Tarosh Patel, Anupama Modi, Chandani Halpani, Vaibhav D. Bhatt, Bharat C. Dixit
Bioorganic Chemistry.2024; 153: 107936. CrossRef - Cell mediated ECM-degradation as an emerging tool for anti-fibrotic strategy
Peng Zhao, Tian Sun, Cheng Lyu, Kaini Liang, Yanan Du
Cell Regeneration.2023;[Epub] CrossRef - Insight into the development of versatile dentin bonding agents to increase the durability of the bonding interface
Isabel Cristina Celerino de Moraes Porto, Teresa de Lisieux Guedes Ferreira Lôbo, Raphaela Farias Rodrigues, Rodrigo Barros Esteves Lins, Marcos Aurélio Bomfim da Silva
Frontiers in Dental Medicine.2023;[Epub] CrossRef
- In vitro assessment of anti-glioblastoma potential of Emblica officinalis methanolic fruit extract and green nanoparticles in U87-MG cells
- 1,667 View
- 20 Download
- 4 Web of Science
- 4 Crossref

- Inhibition of matrix metalloproteinases: a troubleshooting for dentin adhesion
- Izadora Quintela Souza de Moraes, Ticiano Gomes do Nascimento, Antonio Thomás da Silva, Lilian Maria Santos Silva de Lira, Abhishek Parolia, Isabel Cristina Celerino de Moraes Porto
- Restor Dent Endod 2020;45(3):e31. Published online May 22, 2020
- DOI: https://doi.org/10.5395/rde.2020.45.e31
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Matrix metalloproteinases (MMPs) are enzymes that can degrade collagen in hybrid layer and reduce the longevity of adhesive restorations. As scientific understanding of the MMPs has advanced, useful strategies focusing on preventing these enzymes' actions by MMP inhibitors have quickly developed in many medical fields. However, in restorative dentistry, it is still not well established. This paper is an overview of the strategies to inhibit MMPs that can achieve a long-lasting material-tooth adhesion. Literature search was performed comprehensively using the electronic databases: PubMed, ScienceDirect and Scopus including articles from May 2007 to December 2019 and the main search terms were “matrix metalloproteinases”, “collagen”, and “dentin” and “hybrid layer”. MMPs typical structure consists of several distinct domains. MMP inhibitors can be divided into 2 main groups: synthetic (synthetic-peptides, non-peptide molecules and compounds, tetracyclines, metallic ions, and others) and natural bioactive inhibitors mainly flavonoids. Selective inhibitors of MMPs promise to be the future for specific targeting of preventing dentin proteolysis. The knowledge about MMPs functionality should be considered to synthesize drugs capable to efficiently and selectively block MMPs chemical routes targeting their inactivation in order to overcome the current limitations of the therapeutic use of MMPs inhibitors,
i.e ., easy clinical application and long-lasting effect.-
Citations
Citations to this article as recorded by- Exploring the effectiveness of doxycycline in restorative dentistry: a systematic review of in vitro studies
Bruna Tavares Carneiro, Marina Minici Dumont Prado, Iara de Oliveira Nogueira, Allyson Nogueira Moreira, Carolina Bosso André
Odontology.2025; 113(1): 15. CrossRef - Multifunctional Dual Enzyme-Responsive Nanostructured Lipid Carriers for Targeting and Enhancing the Treatment of Bacterial Infections
Kerisha Chetty, Xylia Q. Peters, Calvin A. Omolo, Eman A. Ismail, Mohammed A. Gafar, Eman Elhassan, Sania Z. F. Kassam, Jasoda Govender, Sbongumusa Dlamini, Thirumala Govender
ACS Applied Bio Materials.2025; 8(1): 548. CrossRef - Impact of silver diamine fluoride on composite resin bond strength: An In vitro study with various adhesive systems
Farzaneh Shirani, Shirin Ravanbod, Mohammad Soroush Sehat
Heliyon.2025; 11(2): e41731. CrossRef - Riboflavin-Mediated Photodynamic Therapy in Periodontology: A Systematic Review of Applications and Outcomes
Jakub Fiegler-Rudol, Maciej Łopaciński, Artur Los, Dariusz Skaba, Rafał Wiench
Pharmaceutics.2025; 17(2): 217. CrossRef - Evolution of Dental Resin Adhesives—A Comprehensive Review
Waad Khalid Alomran, Mohammed Zahedul Islam Nizami, Hockin H. K. Xu, Jirun Sun
Journal of Functional Biomaterials.2025; 16(3): 104. CrossRef - Dentin biomodification with Er, Cr: YSGG laser prior to conditioning with grape seed, green tea and Salvadora persica extracts
Abdullah Alshehri, Ali A. Elkaffas, Abdullah Ali Alqahtani, Mubashir Baig Mirza, Yasser F. Alfawaz, Laila Taher Kashkosh, Basil Almutairi, Abdulellah F. Almudahi, Rania Bayoumi
Lasers in Dental Science.2025;[Epub] CrossRef - A Novel Approach to Strengthening the Microtensile Bond Between Lithium Disilicate Ceramics Manufactured by CAD/CAM and Dentin Using Coatings of Natural and Synthetic Bio-Modifiers
Abdulellah Almudahi, Abdullah Alshehri, Ali R. Alqahtani, Basil Almutairi, Ali A. Elkaffas, Refal Saad Albaijan, Mohammed Ali Abuelqomsan
Ceramics.2025; 8(2): 34. CrossRef - Bioinspired Dentin Biomodification: Current Evidence and Emerging Approaches
Priyanka S R, Sharath Pare
International Journal of Innovative Science and Research Technology.2025; : 219. CrossRef - Development and characterization of self-etch adhesive system containing curcumin anchored to nanosilica on the bond strength to sound and caries-affected dentin
Júlia Correa Raffaini, Rocio Geng Vivanco, Viviane de Cássia Oliveira, Eduardo José Soares, Mario Alexandre Coelho Sinhoreti, Fernanda de Carvalho Panzeri
International Journal of Adhesion and Adhesives.2025; 142: 104070. CrossRef - Inhibitory effect of natural polyphenols on recombinant and endogenous dentinal proteases
Yingheng Liu, Hongye Yang, Kaiyu Qiu, Kang Li, Jian Yu, Chenmin Yao, Cui Huang
Journal of Dentistry.2025; 160: 105895. CrossRef - Marginal integrity produced by quaternary ammonium methacrylate-based dental adhesive tested under physiologically relevant models
Fernanda de Lucena, Peter Nguyen, Tiana Pham, Samuel Weber, Matthew Logan, Steven Lewis, Carmem Pfeifer
Dental Materials.2025; 41(12): 1521. CrossRef - Effect of Laser Conditioning on Shear Bond Strength and Durability of Universal Adhesive to Dentin: An In Vitro Study
Dina Kamal, Doaa Abdou
International Journal of Prosthodontics and Restorative Dentistry.2025; 15(3): 121. CrossRef - Effect of Matrix Metalloproteinase Inhibitors on the Bonding Durability of Nanocomposite Resin to Caries-affected Dentin: An In Vitro Study
Faeiza D Abdel-Salam, Noha Sheta, El-Sayed Gad Eid
The Journal of Contemporary Dental Practice.2025; 26(11): 1096. CrossRef - Long-term hybrid stability and matrix metalloproteinase inhibition by fucosterol in resin-dentin bonding biomechanics
Hyeryeong Kim, Yu-Jung Jung, Yeon Kim, Moon-Kyoung Bae, Kyung-Hyeon Yoo, Seog-Young Yoon, Hae Ryoun Park, In-Ryoung Kim, Yong-Il Kim
Scientific Reports.2024;[Epub] CrossRef - Multifunctional Dental Adhesives Formulated with Silane-Coated Magnetic Fe3O4@m-SiO2 Core–Shell Particles to Counteract Adhesive Interfacial Breakdown
Lamia Sami Mokeem, Isadora Martini Garcia, Abdulrahman A. Balhaddad, Yucheng Lan, Dereje Seifu, Michael D. Weir, Mary Anne Melo
ACS Applied Materials & Interfaces.2024; 16(2): 2120. CrossRef - Improving the Push-Out Bond Strength of Fiber Posts in Diabetic Dentin: The Role of Chlorexidine Irrigation and Resin Cements
Beyza Arslandaş Dinçtürk, Arzu Şahin Mantı, Cemile Kedici Alp, Ayşenur Altuğ Yıldırım, Arzu Kaya Mumcu
Journal of Functional Biomaterials.2024; 16(1): 4. CrossRef - Serum levels of matrix metalloproteinases 1, 2, and 7, and their tissue inhibitors 1, 2, 3, and 4 in polytraumatized patients: Time trajectories, correlations, and their ability to predict mortality
Lukas L. Negrin, Greta L. Carlin, Robin Ristl, Stefan Hajdu, Andre van Wijnen
PLOS ONE.2024; 19(3): e0300258. CrossRef - Current approaches to produce durable biomaterials: Trends in polymeric materials for restorative dentistry applications
Carmem S. Pfeifer, Fernanda S. Lucena, Matthew G. Logan, Devatha Nair, Steven H. Lewis
Dental Materials.2024; 40(12): 2122. CrossRef - Effect of Different MMP Inhibitors on the Bond Strength and Durability of an Etch-and-rinse and a Self-etch Adhesive
Ahmet Hazar, Mine Betül Üçtaşlı
ADO Klinik Bilimler Dergisi.2024; 13(3): 453. CrossRef - Matrix metalloproteinases in dentin: Assessing their presence, activity, and inhibitors – a review of current trends
Lavanya Anumula, Sindhu Ramesh, Venkata Suneel Kumar Kolaparthi
Dental Materials.2024; 40(11): 2051. CrossRef - Effect of nonthermal atmospheric plasma application at different time intervals on the dentinal shear bond strength pretreated with 2% chlorhexidine as cavity disinfectant: An in vitro study
Roopadevi Garlapati, Nagesh Bolla, Gali Praveen Kumar, Mayana Aameena Banu, Bandlapally Sreenivasa Guptha Anila, Shaik Afreen Kamal
Journal of Conservative Dentistry and Endodontics.2024; 27(7): 769. CrossRef - Effect of MMP Inhibitors on Shear Bond Strength of Adhesive to Dentin after Different Drying Techniques: An In-Vitro Study
Sheethal K. Narayanan, Krishnan Hari, Joy Mathew, Anila Sukumaran, Basil Joy, Joel Mathew
Journal of Pharmacy and Bioallied Sciences.2024; 16(Suppl 5): S4639. CrossRef - Effect of Selective Dentine Pre-Treatment with Butane Tetracarboxylic Acid on Composite-Dentine Bond
Nikita Sharma, Anand Susila, Aruna Kumari Veronica, R. J. Fiona, Shamini Sai, Sriganesh Anguswamy
Indian Journal of Dental Research.2024; 35(4): 443. CrossRef - In situ detection of endogenous proteolytic activity and the effect of inhibitors on tooth root surface
Izumi Sakurai, Gen Mayanagi, Satoru Yamada, Nobuhiro Takahashi
Journal of Dentistry.2023; 131: 104471. CrossRef - Effects of Cathepsin K Inhibitors on Dentin Erosion: An in vitro Study
Yi-ying Chen, Xiu-jiao Lin, Zhi-cen Lu, Annette Wiegand, Hao Yu
Caries Research.2023; 57(2): 159. CrossRef - The Role of Duration of Chlorhexidine Gluconate 2% Application on the Shear Bond Strength of a Total Etch Bonding Agent: A Comparative Study
Azmi Fasya, Yolanda Yolanda, Ayu Trisna Hayati
Clinical, Cosmetic and Investigational Dentistry.2023; Volume 15: 281. CrossRef - The effect of kaempferol on the dentin bonding stability through matrix metalloproteinases inhibition and collagen crosslink in dentin biomodification
Jeonghwa Cho, Hyeryeong Kim, Kyung-Hyeon Yoo, Youna Paik, In-Ryoung Kim, Seog-Young Yoon, Yong-Il Kim
Journal of Dental Sciences.2023; 18(3): 1023. CrossRef - EFFECT OF TiO2 DECORATED CELLULOSIC MATERIALS ADDITION ON MECHANICAL AND BIOLOGICAL PROPERTIES OF DENTAL ADHESIVE COMPOSITES
HUMAIRA JABEEN, NAWSHAD MUHAMMAD, USAMA SIDDIQUI, MUHAMMAD SABIR, NAVEED AHMAD, SAAD LIAQAT
Cellulose Chemistry and Technology.2023; 57(5-6): 541. CrossRef - Insight into the development of versatile dentin bonding agents to increase the durability of the bonding interface
Isabel Cristina Celerino de Moraes Porto, Teresa de Lisieux Guedes Ferreira Lôbo, Raphaela Farias Rodrigues, Rodrigo Barros Esteves Lins, Marcos Aurélio Bomfim da Silva
Frontiers in Dental Medicine.2023;[Epub] CrossRef - Current Strategies to Control Recurrent and Residual Caries with Resin Composite Restorations: Operator- and Material-Related Factors
Moataz Elgezawi, Rasha Haridy, Moamen A. Abdalla, Katrin Heck, Miriam Draenert, Dalia Kaisarly
Journal of Clinical Medicine.2022; 11(21): 6591. CrossRef - Adhesive Strength in Dentin Conditioned with 18% Ethylenediaminetetraacetic Acid versus 35% Phosphoric Acid: In Vitro Study with 1-Year Artificial Aging
Esther Alcántara-Obispo, Flor Santander-Rengifo, Marysela Ladera-Castañeda, Carlos López-Gurreonero, Antonieta Castro Pérez-Vargas, Alberto Cornejo-Pinto, Luis Cervantes-Ganoza, César Cayo-Rojas
Polymers.2022; 14(20): 4291. CrossRef - Effects of the Combined Application of Trimethylated Chitosan and Carbodiimide on the Biostability and Antibacterial Activity of Dentin Collagen Matrix
Xiangyao Wang, Qilin Li, Haibo Lu, Zhuo Liu, Yaxin Wu, Jing Mao, Shiqiang Gong
Polymers.2022; 14(15): 3166. CrossRef - Matrix Metalloproteinases in Dental and Periodontal Tissues and Their Current Inhibitors: Developmental, Degradational and Pathological Aspects
Moataz Elgezawi, Rasha Haridy, Khalid Almas, Moamen A. Abdalla, Omar Omar, Hatem Abuohashish, Abeer Elembaby, Uta Christine Wölfle, Yasir Siddiqui, Dalia Kaisarly
International Journal of Molecular Sciences.2022; 23(16): 8929. CrossRef - Mussel-inspired monomer – A new selective protease inhibitor against dentine collagen degradation
Kang Li, Fung Man Ngo, Angela Yat Laam Yau, Winnie Wai Ling Tam, Edmund Chun Ming Tse, James Kit Hon Tsoi, Cynthia Kar Yung Yiu
Dental Materials.2022; 38(7): 1149. CrossRef - Effect of Collagen Crosslinkers on Dentin Bond Strength of Adhesive Systems: A Systematic Review and Meta-Analysis
Louis Hardan, Umer Daood, Rim Bourgi, Carlos Enrique Cuevas-Suárez, Walter Devoto, Maciej Zarow, Natalia Jakubowicz, Juan Eliezer Zamarripa-Calderón, Mateusz Radwanski, Giovana Orsini, Monika Lukomska-Szymanska
Cells.2022; 11(15): 2417. CrossRef - Marginal Integrity of Composite Restoration with and without Surface Pretreatment by Gold and Silver Nanoparticles vs Chlorhexidine: A Randomized Controlled Trial
Aya AEM Nemt-Allah, Shereen H Ibrahim, Amira F El-Zoghby
The Journal of Contemporary Dental Practice.2022; 22(10): 1087. CrossRef - Use of the adhesive layer as a controlled release platform for doxycycline, as promising advancement for longer durability of dentin adhesion
Lívia Rodrigues de Menezes, Dayane Carvalho Ramos Salles de Oliveira, Cássia Almeida Brito, Emerson Oliveira da Silva
International Journal of Adhesion and Adhesives.2021; 108: 102889. CrossRef - Understanding collagen interactions and their targeted regulation by novel drugs
Marialucia Gallorini, Simone Carradori
Expert Opinion on Drug Discovery.2021; 16(11): 1239. CrossRef - Effect of Curcumin Suspension and Vitamin C on Dentin Shear Bond Strength and Durability. A Pilot Study
Dalia A. Abuelenain, Ensanya A. Abou Neel, Tariq S. Abuhaimed, Amal M. Alamri, Hanan S. Ammar, Sahar M. N. Bukhary
The Open Dentistry Journal.2021; 15(1): 540. CrossRef - Reinforced Universal Adhesive by Ribose Crosslinker: A Novel Strategy in Adhesive Dentistry
Rim Bourgi, Umer Daood, Mohammed Nadeem Bijle, Amr Fawzy, Maroun Ghaleb, Louis Hardan
Polymers.2021; 13(5): 704. CrossRef
- Exploring the effectiveness of doxycycline in restorative dentistry: a systematic review of in vitro studies
- 6,949 View
- 155 Download
- 40 Crossref

- The effect of tumor necrosis factor (TNF)-α to induce matrix metalloproteinase (MMPs) from the human dental pulp, gingival, and periodontal ligament cells
- Eun-Mi Rhim, Sang-Hyuk Park, Duck-Su Kim, Sun-Young Kim, Kyoung-Kyu Choi, Gi-Woon Choi
- J Korean Acad Conserv Dent 2011;36(1):26-36. Published online January 31, 2011
- DOI: https://doi.org/10.5395/JKACD.2011.36.1.26
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
ePub Objectives In the present study, three kinds of tissues cells (pulp, gingiva, and periodontal ligament) were investigated if those cells express MMP and TIMP when they were stimulated with neuropeptides (substance P, CGRP) or proinflammatory cytokine, TNF-α.
Materials and Methods The cells cultured from human dental pulp (PF), gingiva (GF) and periodontal ligament were (PDLF) stimulated with Mock, SP, TNF-α, and CGRP for 24 hrs and 48 hrs. for an RNase protection assay and Enzyme Linked Immunosorbent Assay.
Cells (PF, GF and PDLF) seeded in 100 mm culture dish were stimulated with SP (10-5, 10-8 M) or only with medium (Mock stimulation) for 4hrs and for 24 hrs for RNase Protection Assay, and they were stimulated with CGRP (10-5 M) and TNF-α (2 ng/mL) for 24 hrs and with various concentraion of TNF-α (2, 10, and 100 ng/mL) for Rnase Protection Assay with a human MMP-1 probe set including MMP 1, 2, 8, 7, 8, 9, 12, and TIMP 2, 3.
In addition, cells (PF, GF and PDLF) were stimulated with Mock and various concentraion of TNF-α (2, 10, and 100 ng/mL) for 24 hrs and with TNF-α (10 ng/mL) for 48 hrs, and the supernatents from the cells were collected for Enzyme Linked Immunosorbent Assay (ELISA) for MMP-1 and MMP-13.
Results The expression of MMPs in PF, GF, PDLF after stimulation with SP and CGRP were not changed compared with Mock stimulation for 4 hrs and 24 hrs. The expression of MMP-1, -12, -13 24 hrs after stimulation with TNF-α were upregulated, however the expression of TIMP-3 in PF, GF, PDLF after stimulation with TNF-α were downregulated. TNF-α (2 ng/mL, 10 ng/mL, 100 ng/mL) increased MMP-1 and MMP-12 expression in PF dose dependently for 24 hrs.
Conclusions TNF-α in the area of inflammation may play an important role in regulating the remodeling of dentin, cementum, and alveolar bone.
-
Citations
Citations to this article as recorded by- Decoding the Ultimate Effects of Dipeptidyl Peptidase‐4 Inhibitors on Angiogenesis: An Updated Comprehensive Review of the Cellular and Molecular Mechanisms
Andrew Z. Zaka, Safwat A. Mangoura, Marwa A. Ahmed, Beshoy Allam
ChemistrySelect.2025;[Epub] CrossRef - Anti‐Inflammatory Effects of Melatonin and 5‐Methoxytryptophol on Lipopolysaccharide‐Induced Acute Pulpitis in Rats
Fatma Kermeoğlu, Umut Aksoy, Abdullah Sebai, Gökçe Savtekin, Hanife Özkayalar, Serkan Sayıner, Ahmet Özer Şehirli, Shuai CHEN
BioMed Research International.2021;[Epub] CrossRef - Cross-Talk between Ciliary Epithelium and Trabecular Meshwork Cells In-Vitro: A New Insight into Glaucoma
Natalie Lerner, Elie Beit-Yannai, Wayne Iwan Lee Davies
PLoS ONE.2014; 9(11): e112259. CrossRef
- Decoding the Ultimate Effects of Dipeptidyl Peptidase‐4 Inhibitors on Angiogenesis: An Updated Comprehensive Review of the Cellular and Molecular Mechanisms
- 1,363 View
- 4 Download
- 3 Crossref


 KACD
KACD

 First
First Prev
Prev


