Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(2); 2021 > Article
- Research Article Cryopreservation of mesenchymal stem cells derived from dental pulp: a systematic review
-
Sabrina Moreira Paes1
 , Yasmine Mendes Pupo1
, Yasmine Mendes Pupo1 , Bruno Cavalini Cavenago1
, Bruno Cavalini Cavenago1 , Thiago Fonseca-Silva2
, Thiago Fonseca-Silva2 , Carolina Carvalho de Oliveira Santos1,2
, Carolina Carvalho de Oliveira Santos1,2
-
Restor Dent Endod 2021;46(2):e26.
DOI: https://doi.org/10.5395/rde.2021.46.e26
Published online: April 29, 2021
1Department of Restorative Dentistry, Universidade Federal do Paraná, Curitiba/PR, Brazil.
2Department of Dentistry, Universidade Federal dos Vales do Jequitinhonha e Mucuri, Diamantina/MG, Brazil.
- Correspondence to Carolina Carvalho de Oliveira Santos, DDS, MSc, PhD. Professor, Department of Dentistry Universidade Federal dos Vales do Jequitinhonha e Mucuri Rua da Glória, 181 Diamantina/MG 39100-000, Brazil. carolinaccos@gmail.com
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives The aim of the present systematic review was to investigate the cryopreservation process of dental pulp mesenchymal stromal cells and whether cryopreservation is effective in promoting cell viability and recovery.
-
Materials and Methods This systematic review was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the research question was determined using the population, exposure, comparison, and outcomes strategy. Electronic searches were conducted in the PubMed, Cochrane Library, Science Direct, LILACS, and SciELO databases and in the gray literature (dissertations and thesis databases and Google Scholar) for relevant articles published up to March 2019. Clinical trial studies performed with dental pulp of human permanent or primary teeth, containing concrete information regarding the cryopreservation stages, and with cryopreservation performed for a period of at least 1 week were included in this study.
-
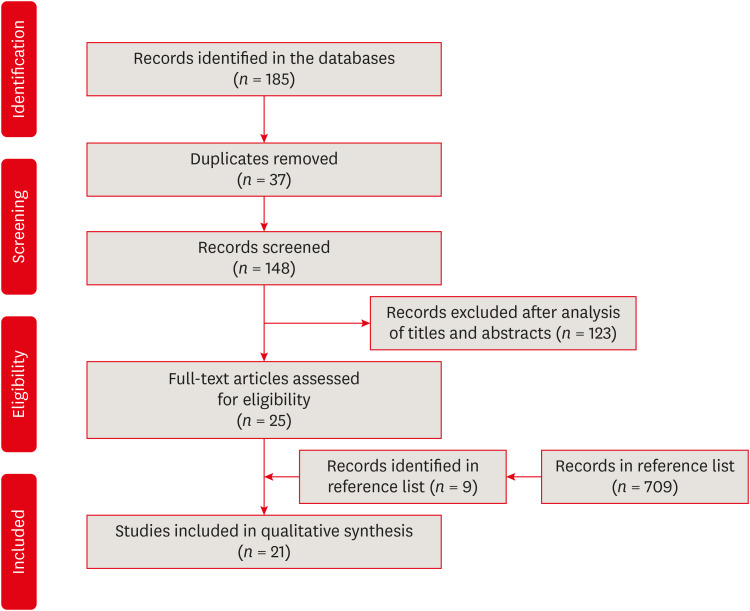
Results The search strategy resulted in the retrieval of 185 publications. After the application of the eligibility criteria, 21 articles were selected for a qualitative analysis.
-
Conclusions The cryopreservation process must be carried out in 6 stages: tooth disinfection, pulp extraction, cell isolation, cell proliferation, cryopreservation, and thawing. In addition, it can be inferred that the use of dimethyl sulfoxide, programmable freezing, and storage in liquid nitrogen are associated with a high rate of cell viability after thawing and a high rate of cell proliferation in both primary and permanent teeth.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
Flowchart of the bibliographic search and selection process adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol.
Characteristics of studies included
| Authors | Year | Country | Study design | Sample | Stages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tooth type | Size | Storage | Disinfection | Pulp extraction | Cell isolation | Cell proliferation | Cryopreservation | Time | Thawing | ||||
| Papaccio et al. [33] | 2006 | Italy | RCT | P | Teeth of healthy patients aged between 21 and 45 years (unspecified number of teeth) | Immediate disinfection | Immersion in chlorhexidine gel and PBS medium containing penicillin, streptomycin, and clarithromycin | Gracey's dentin digger and curette | Enzymatic digestion with collagenase I and dispase | Immersion in alpha-MEM medium supplemented with 20% FCS, 2-phosphate ascorbic acid, glutamine, penicillin, streptomycin. Incubation at 37°C in a humid atmosphere with 5% CO2 | Transfer of cells to medium containing 10% FCS-supplemented DMSO with immediate storage in liquid nitrogen | More than 2 yr | Water bath at 37°C |
| Zhang et al. [37] | 2006 | China | RCT | P | Third impacted molars in patients aged between 18 and 24 yr | Immersion in alpha-MEM | Alpha-MEM containing 0.5 mg/mL gentamicin and 3 g/mL amphotericin B | High-speed drill for crown cutting and pulping | Explantation technique associated with enzymatic digestion with collagenase type I for 1 hr | Immersion in alpha-MEM medium containing 20% FCS. Incubation at 37°C in a humid atmosphere with 5% CO2 | Liquid nitrogen storage immediately after handling | Less than 1 mon | Water bath at 37°C |
| Perry et al. [34] | 2008 | India | RCT | P | 31 third molars from patients aged between 18 and 30 yr | HypoThermosol, Mesencult basal medium, or PBS, the medium being chosen randomly | PBS, sodium iodopovidone, and sodium thiosulfate baths | High-speed drill for crown and endodontic cutting | Enzyme digestion with dispase and type I collagenase | Cells were transferred to Mesencult medium supplemented with Pen-Strep and amphotericin B. | Cell transfer to Mesencult medium containing DMSO. Gradual freezing at −1°C/min to −85°C and subsequent transfer to liquid nitrogen | 1 mon | Water bath at 37°C |
| Woods et al. [36] | 2009 | United States of America | RCT | P | Teeth of patients aged between 18 and 30 yr | PBS solution | PBS baths, iodopovidone at 1%, and sodium thiosulfate (0.1%) | High-speed drill for crown cutting and pulping | Enzyme digestion with collagenase type I, II and thermolysin | Immersion in Mesencult. Incubation at 37°C in a humid atmosphere with 5% CO2 | This solution was supplemented with DMSO. Gradual freezing at −1°C/min to −85°C and subsequent transfer to liquid nitrogen | 1 mon | Water bath at 37°C |
| Lee et al. [27] | 2010 | Japan | RCT | P | Premolar dental pulp of adults aged 18–30 yr | PBS solution | Immersion in PBS | High-speed drill for crown and endodontic cutting | Scalpel explantation technique and enzymatic digestion with collagenase type I and dispase | Incubation at 37°C in a humid atmosphere with 5% CO2 | Prior storage in 10% DMSO, maintenance at 4°C during transport. Gradual freezing at −5°C for 15 min with cooling rate at −0.5°C/min to −32°C. Transfer after temperature of −152°C | 1 wk | Water bath at 37°C |
| Temmerman et al. [35] | 2010 | Belgium | RCT | P | Third molars of patients aged 15–25 yr | Immersion in medium containing DMEM, FCS, Raid solution, fungizone, and gentamicin | Immersion in medium containing DMEM, FCS, penicillin, fungizone and gentamicin | Carborundum disc for crown and endodontic pulp cutting | Explantation technique with scalpel | Immersion in Optimem I medium with Pen-Strep, glutamine and FCS. Incubation at 37°C in a humid atmosphere with 10% CO2 | Medium cell transfer with FCS and DMSO. Gradual freezing at −1°C/min to −80°C with subsequent transfer to liquid nitrogen | 1 mon | Water bath at 37°C |
| Abedini et al. [21] | 2011 | Japan | RCT | P | Third molar dental pulp of 10 patients aged 18–30 yr | Not specified | Successive baths in PBS | Vertical cut of tooth and pulp removal with curette | Explantation technique with scalpel | Immersion in alpha-MEM medium supplemented with FBS, penicillin, amphotericin B and kanamycin. Incubation at 37°C in a humid atmosphere with 5% and CO2 | Prior immersion in 10% DMSO associated with FBS. Freezing in a magnetic freezer with initial maintenance at −5°C for 15 min and subsequent cooling at a rate of −0.5°C/min to −30°C. Subsequent culture transfer at −150°C. | 3 mon | Water bath at 37°C |
| Chen et al. [22] | 2011 | Taiwan | RCT | P | 50 teeth of patients with an average age of 25.5 yr | Not specified | PBS immersion | High rotation drill for cutting the crown at the dentin junction and endodontic file | Enzymatic digestion with collagenase type I and dispase | Immersion in alpha-MEM solution containing FBS, Pen-Strep, and ascorbic acid. Incubation at 37°C in a humid atmosphere of 5% CO2 up to 80% confluence | Transfer of cells to medium containing DMSO and FBS. Gradual freezing to 4°C for 2 hr, up to −80°C for 8 hr and transfer to liquid nitrogen. Cooling rate −1°C/min. | 1 mon | Water bath at 37°C |
| Gioventu et al. [23] | 2012 | Italy | RCT | P | 10 non-exfoliated teeth obtained from children aged 7–11-yr-old | Sterile RPMI 1640 | Immersion in sterile RPMI 1640 medium | Making a cavity at the cementoenamel junction height with Nd:YAG laser | Enzymatic digestion with collagenase type A | Immersion in alpha-MEM Glutamax 1% medium supplemented with 20% FBS and 1% Pen-Strep. Incubation at 37°C in a humid atmosphere containing 5% CO2 until 80% confluence. | Immersion in sterile RPMI 1640 medium containing 10% DMSO and 10% human albumin. −80°C culture storage in programmable freezer | 10 days | Water bath at 37°C |
| Lee et al. [28] | 2012 | Taiwan | CCT | P | Orthodontically exposed incisors of a 28-yr-old woman and a 25-yr-old man | Immediately proceeded to cleaning, no storage | Immersion in Dulbecco's phosphate-buffered saline solution | High speed drill for crown and endodontic file cutting | Scalpel explantation technique and enzymatic digestion with collagenase type I and dispase | Immersion in alpha-MEM medium supplemented with 15% FBS, 2-phosphate ascorbic acid, antibiotics and antimicrobials. Incubation at 37°C in a humid atmosphere with 5% CO2 | Non-magnetic freezing group: freezes for 1 day at −80°C and −150°C for storage. Magnetic freezing: Immersed in 10% DMSO, performed in a programmable freezer, cooling rate from −0.5°C to −32°C and storage at −150°C | 1 wk | Water bath at 37°C |
| Antunes [17] | 2013 | Brazil | RCT | D | 3 teeth of children aged 6 to 12 yr | Immersion in alpha-MEM medium and transport on ice | Immersion in medium containing alpha-MEM, penicillin, streptomycin, gentamicin, and amphotericin B | Diamond blade for crown cutting and pulp tissue curettage | Enzymatic digestion with collagenase type I and dispase | Immersion in solution with alpha-MEM and FBS. Incubation at 37°C in a humid atmosphere of 5% and CO2 up to 70%–90% confluence | Transfer cells to medium containing DMSO and FBS. Freeze gradually at 4°C for 2 hr, −20°C for 18 hr and up to −80°C with transfer to liquid nitrogen | 1 mon | Water bath at 37°C |
| Ji et al. [16] | 2014 | South Korea | RCT | D | 122 teeth obtained from 105 healthy patients aged 3–16 yr | FBS immersion | Immersion in alpha-MEM medium containing FBS, Pen-Strep, ascorbic acid, and glutamine | Made by a device called “Barbed Broach” (Mani, Utsunomiya Toshi-ken, Japan) | Scalpel explantation technique | Same composition of medium used for decontamination. Incubation at 37°C in a humid atmosphere with 5% CO2 | Transfer of cells to medium containing DMSO and FBS. Gradual freezing to 4°C for 1 hr and to −80°C with transfer to liquid nitrogen. Cooling rate −1°C/min | 1–9 mon | Water bath at 37°C |
| Lindemann et al. [29] | 2014 | Brazil | RCT | D | 26 teeth of children aged 9–11 yr | Direct immersion in disinfection medium | Immersion in DMEM medium supplemented with FBS, penicillin, streptomycin, and gentamicin | Endodontic file for pulp collection | Enzyme digestion with collagenase type I | FBS (20%) added to the enzyme digestion solution. Incubation at 37°C in a humid atmosphere with 5% CO2 | Immersion in 10% DMSO medium associated with FBS with initial temperature maintenance at 4°C for 1 hr. The temperature was gradually cooled at a rate of −1°C/min to −80°C and maintained for 24 hr, with subsequent transfer to liquid nitrogen at −196°C. | 1 wk | Water bath at 37°C |
| Kumar et al. [25] | 2015 | India | RCT | P | Impacted teeth pulp of 16-yr-old patients | Hank's balanced solution | PBS baths | High-rotation drill for crown cutting and curettage | Scalpel explantation technique | Immersion in alpha-MEM medium containing glutamine, FBS, and Pen-StrepIncubation at 37°C in a humid atmosphere with 5% CO2 | Transfer of cells to medium containing DMSO and FBS. The best protocol was gradual freezing at 0°C for 15 min, −20°C for 1 hr, and up to −80°C with transfer to liquid nitrogen. Freezing at −1°C/min | 1 yr | Water bath at 37°C |
| Lee et al. [26] | 2015 | South Korea | RCT | D | 20 teeth of children aged 5–14 yr | Not specified | Immersion in PBS | Endodontic pulp collection file | Scalpel explantation technique | Immersion in alpha-MEM medium supplemented with 10% FBS, ascorbic acid, glutamine, penicillin, and streptomycin. Incubation at 37°C in a humid atmosphere with 5% CO2 | Pre-storage in FBS medium supplemented with 10% DMSO. Gradual freezing starting at 4°C with cooling rate at −1°C/min to −80°C and then storage in liquid nitrogen at −196°C | 1–8 mon | Water bath at 37°C |
| Munevar et al. [32] | 2015 | Colombia | RCT | P | Teeth of patients aged 18–31 yr | Immersion in PBS and transported in ice | Immersion in 1% sodium hypochlorite and PBS baths | High-speed drill for crown and endodontic cutting | Enzyme digestion with dispase and collagenase type I | Immersion in DMEM medium supplemented with Pen-Strep and amphotericin B. Incubation at 37°C in humidified atmosphere with 5% CO2 until 70% confluence | Transfer of cells to medium containing FCS and DMSO. The samples were stored in liquid nitrogen | 2 yr | Water bath at 37°C |
| Alsulaimani et al. [15] | 2016 | Saudi Arabia | RCT | P | 17 teeth of 30-yr-old | Not specified | Chlorhexidine gluconate for 30 sec, immersion in saline and lysine | Diamond blade for crown and file cutting K-file | Explant and enzymatic digestion with collagenase type I and dispase | Immersion in solution with DMEM, FBS, penicillin, streptomycin and alpha-MEM medium. Incubation at 37°C in a humid atmosphere with 5% CO2 | Transfer of cells to medium containing DMEM, FBS, Pen-strep and DMSO. Gradual freezing to −20°C for 20 min and −80°C for 4 days and transfer to liquid nitrogen | 2 yr | DMEM added to the environment and gentle aspiration |
| Malekfar et al. [30] | 2016 | India | RCT | P | 20 teeth pulp samples from patients aged 15–30 yr | Not specified | Pulp tissue was washed with Dulbecco and PBS solution | High-speed drill for crown and endodontic cutting | Scalpel explantation technique and type I collagenase enzymatic digestion | Immersion in DMEM supplemented with alpha-MEM, glutamine, FBS, and Pen-Strep. Incubation at 37°C in a humid atmosphere with 5% CO2 | Transfer cells to medium containing FBS and DMSO maintained at 4°C for osmotic balance. Freezing gradually at −1°C/min to −80°C. Subsequent transfer to liquid nitrogen | 3 mon | Water bath at 37°C |
| Han et al. [24] | 2017 | South Korea | RCT | P | 12 teeth of patients with an average age of 19 yr | Not specified | Immersion in PBS medium containing Pen-Strep | High-rotation drill for crown cutting and curettage | Scalpel explantation technique, type I collagenase enzyme digestion | Immersion in Dulbecco medium supplemented with 10% FBS, Pen-Strep. Incubation at 37°C in humid atmosphere with 5% CO2 | Immersion in cryo-protective solution containing glucose, sucrose and ethylene glycol. Culture maintained at 1°C for 30 min, cooled to −2°C/min to −9°C, maintained for 5 min and cooled again to −0.3°C/min to −40°C and to −10°C/min to −140°C with liquid nitrogen storage | 1 yr | Water bath at 37°C |
| Huynh et al. [14] | 2017 | Vietnam | RCT | P | Third molars of patients aged 18–25 yr | Immersion in DMEM medium containing FBS and Pen-Strep or gentamicin saline | Immersion in DMEM medium containing glutamine and Pep-Strep with subsequent immersion in PBS | High-rotation drill for cutting the crown at the dentin-junction and endodontic file | Scalpel explantation technique | Immersion in DMEM medium with glutamine, FBS, and antibiotics. Incubation at 37°C in a humid atmosphere with 5% CO2 to 80% confluence | Medium cell transfer with different percentages of DMSO and FBS. Dual freezing to −80°C and transfer to liquid nitrogen. Cooling rate −1°C/min | 6 mon | Water bath at 37°C |
| Mochizuki and Nakahara [31] | 2018 | Japan | RCT | P | Dental pulp of 8 healthy young adults aged 20–37 yr | Not specified | DMEM/F12 medium supplemented with FBS-free, M-MS, penicillin, streptomycin, and fungizone | High-rotation drill for crown cutting and curettage | Scalpel explantation technique and enzymatic digestion with collagenase type I and dispase | Immersion of cells in serum-free xenogenic medium. Incubation at 37°C in a humid atmosphere with 4.7% CO2 up to 80% confluence | Transfer cells to medium containing DMSO-free medium and store at −80°C | 1–3 mon | Water bath at 37°C |
Cryopreservation methods
| Method 1 | Method 2 | Method 3 |
|---|---|---|
| Direct immersion in liquid nitrogen | Programmable freezing with the cooling rate at −1°C/min to −80°C or −85°C and subsequent transfer to liquid nitrogen for storage | Programmable freezing with the addition of breaks at set temperatures. A fixed cooling rate of −0.5°C/min starting at −5°C, maintained for 15 minutes, further cooled to −30°C or −35°C and then transferred to storage medium at −150°C or −152°C |
| Papaccio et al. [33], Zhang et al. [37], and Munévar et al. [32] | Perry et al. [34], Woods et al. [36], Temmerman et al. [35], Gioventu et al. [23], Ji et al. [16], Lindemann et al. [29], Lee et al. [26], Malekfar et al. [30], Huynh et al. [14], and Mochizuki and Nakahara [31] | Lee et al. [27], Abedini et al. [21], Chen et al. [22], Lee et al. [28], Antunes [17], Kumar et al. [25], Alsulaimani et al. [15], and Han et al. [24] |
Risk of bias assessed through the Joanna Briggs Institute Critical Appraisal Checklist for Quasi-Experimental Studies
| Studies | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | % Yes | Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mochizuki and Nakahara [31] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Huynh et al. [14] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Han et al. [24] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Malekfar et al. [30] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Alsulaimani et al. [15] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Munevar et al. [32] | Y | Y | Y | N | Y | Y | Y | Y | NA | 77.77 | Low |
| Lee et al. [26] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Kumar et al. [25] | Y | Y | Y | N | Y | Y | Y | Y | NA | 77.77 | Low |
| Lindemann et al. [29] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Ji et al. [16] | Y | Y | Y | N | UC | Y | Y | Y | NA | 66.66 | Moderate |
| Antunes et al. [17] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Lee et al. [28] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Gioventu et al. [23] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Chen et al. [22] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Abedini et al. [21] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Temmerman et al. [35] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Lee et al. [27] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Woods et al. [36] | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Perry et al. [34] | Y | Y | Y | N | Y | Y | Y | Y | NA | 77.77 | Low |
| Zhang et al. [37] | Y | Y | UC | Y | Y | Y | Y | Y | NA | 77.77 | Low |
| Papaccio et al. [33] | Y | Y | Y | N | Y | Y | Y | Y | NA | 77.77 | Low |
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
- 1. Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu GT, Liang A, Liu S. Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015;33:627-638.ArticlePubMedPDF
- 2. Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med 2015;9:1205-1216.ArticlePubMed
- 3. Kawashima N, Noda S, Yamamoto M, Okiji T. Properties of dental pulp-derived mesenchymal stem cells and the effects of culture conditions. J Endod 2017;43:S31-S34.ArticlePubMed
- 4. Mayo V, Sawatari Y, Huang CY, Garcia-Godoy F. Neural crest-derived dental stem cells--where we are and where we are going. J Dent 2014;42:1043-1051.ArticlePubMed
- 5. Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res 2014;78:16-20.ArticlePubMed
- 6. Wang J, Ma H, Jin X, Hu J, Liu X, Ni L, Ma PX. The effect of scaffold architecture on odontogenic differentiation of human dental pulp stem cells. Biomaterials 2011;32:7822-7830.ArticlePubMedPMC
- 7. Nakashima M, Iohara K. Mobilized dental pulp stem cells for pulp regeneration: initiation of clinical trial. J Endod 2014;40:S26-S32.ArticlePubMed
- 8. Rosa V, Zhang Z, Grande RH, Nör JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res 2013;92:970-975.ArticlePubMedPMCPDF
- 9. Saghiri MA, Asatourian A, Sorenson CM, Sheibani N. Role of angiogenesis in endodontics: contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J Endod 2015;41:797-803.ArticlePubMedPMC
- 10. Chamieh F, Collignon AM, Coyac BR, Lesieur J, Ribes S, Sadoine J, Llorens A, Nicoletti A, Letourneur D, Colombier ML, Nazhat SN, Bouchard P, Chaussain C, Rochefort GY. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep 2016;6:38814.ArticlePubMedPMCPDF
- 11. Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E. Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J 2011;22:91-98.ArticlePubMed
- 12. Martin-Piedra MA, Garzon I, Oliveira AC, Alfonso-Rodriguez CA, Carriel V, Scionti G, Alaminos M. Cell viability and proliferation capability of long-term human dental pulp stem cell cultures. Cytotherapy 2014;16:266-277.ArticlePubMed
- 13. Pegg DE. Principles of cryopreservation. Methods Mol Biol 2007;368:39-57.ArticlePubMed
- 14. Huynh NC, Le SH, Doan VN, Ngo LT, Tran HL. Simplified conditions for storing and cryopreservation of dental pulp stem cells. Arch Oral Biol 2017;84:74-81.ArticlePubMed
- 15. Alsulaimani RS, Ajlan SA, Aldahmash AM, Alnabaheen MS, Ashri NY. Isolation of dental pulp stem cells from a single donor and characterization of their ability to differentiate after 2 years of cryopreservation. Saudi Med J 2016;37:551-560.ArticlePubMedPMC
- 16. Ji EH, Song JS, Kim SO, Jeon M, Choi BJ, Lee JH. Viability of pulp stromal cells in cryopreserved deciduous teeth. Cell Tissue Bank 2014;15:67-74.ArticlePubMedPDF
- 17. Antunes FG. Atividade biológica de células-tronco da polpa de dentes decíduos humanos submetidas à criopreservação. Natal: Universidade Federal do Rio Grande do Norte; 2013.
- 18. Jesus AA, Soares MB, Soares AP, Nogueira RC, Guimarães ET, Araújo TM, Santos RR. Coleta e cultura de células-tronco obtidas da polpa de dentes decíduos: técnica e relato de caso clínico. Dental Press J Orthod 2011;16:111-118.Article
- 19. Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute reviewer's manual. Adelaide: JBI; 2017.
- 20. Lima IF, de Andrade Vieira W, de Macedo Bernardino Í, Costa PA, Lima AP, Pithon MM, Paranhos LR. Influence of reminder therapy for controlling bacterial plaque in patients undergoing orthodontic treatment: A systematic review and meta-analysis. Angle Orthod 2018;88:483-493.ArticlePubMedPMCPDF
- 21. Abedini S, Kaku M, Kawata T, Koseki H, Kojima S, Sumi H, Motokawa M, Fujita T, Ohtani J, Ohwada N, Tanne K. Effects of cryopreservation with a newly-developed magnetic field programmed freezer on periodontal ligament cells and pulp tissues. Cryobiology 2011;62:181-187.ArticlePubMed
- 22. Chen YK, Huang AH, Chan AW, Shieh TY, Lin LM. Human dental pulp stem cells derived from different cryopreservation methods of human dental pulp tissues of diseased teeth. J Oral Pathol Med 2011;40:793-800.ArticlePubMedPMC
- 23. Gioventù S, Andriolo G, Bonino F, Frasca S, Lazzari L, Montelatici E, Santoro F, Rebulla P. A novel method for banking dental pulp stem cells. Transfus Apheresis Sci 2012;47:199-206.Article
- 24. Han YJ, Kang YH, Shivakumar SB, Bharti D, Son YB, Choi YH, Park WU, Byun JH, Rho GJ, Park BW. Stem cells from cryopreserved human dental pulp tissues sequentially differentiate into definitive endoderm and hepatocyte-like cells in vitro . Int J Med Sci 2017;14:1418-1429.ArticlePubMedPMC
- 25. Kumar A, Bhattacharyya S, Rattan V. Effect of uncontrolled freezing on biological characteristics of human dental pulp stem cells. Cell Tissue Bank 2015;16:513-522.ArticlePubMedPDF
- 26. Lee HS, Jeon M, Kim SO, Kim SH, Lee JH, Ahn SJ, Shin Y, Song JS. Characteristics of stem cells from human exfoliated deciduous teeth (SHED) from intact cryopreserved deciduous teeth. Cryobiology 2015;71:374-383.ArticlePubMed
- 27. Lee SY, Chiang PC, Tsai YH, Tsai SY, Jeng JH, Kawata T, Huang HM. Effects of cryopreservation of intact teeth on the isolated dental pulp stem cells. J Endod 2010;36:1336-1340.ArticlePubMed
- 28. Lee SY, Huang GW, Shiung JN, Huang YH, Jeng JH, Kuo TF, Yang JC, Yang WC. Magnetic cryopreservation for dental pulp stem cells. Cells Tissues Organs 2012;196:23-33.ArticlePubMedPDF
- 29. Lindemann D, Werle SB, Steffens D, Garcia-Godoy F, Pranke P, Casagrande L. Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch Oral Biol 2014;59:970-976.ArticlePubMed
- 30. Malekfar A, Valli KS, Kanafi MM, Bhonde RR. Isolation and characterization of human dental pulp stem cells from cryopreserved pulp tissues obtained from teeth with irreversible pulpitis. J Endod 2016;42:76-81.ArticlePubMed
- 31. Mochizuki M, Nakahara T. Establishment of xenogeneic serum-free culture methods for handling human dental pulp stem cells using clinically oriented in-vitro and in-vivo conditions. Stem Cell Res Ther 2018;9:25.ArticlePubMedPMCPDF
- 32. Munévar JC, Gutiérrez N, Jiménez NT, Lafaurie GI. Evaluation of two human dental pulp stem cell cryopreservation methods. Acta Odontol Latinoam 2015;28:114-121.PubMed
- 33. Papaccio G, Graziano A, d'Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol 2006;208:319-325.ArticlePubMed
- 34. Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods 2008;14:149-156.ArticlePubMedPMC
- 35. Temmerman L, Beele H, Dermaut LR, Van Maele G, De Pauw GA. Influence of cryopreservation on the pulpal tissue of immature third molars in vitro. Cell Tissue Bank 2010;11:281-289.ArticlePubMedPDF
- 36. Woods EJ, Perry BC, Hockema JJ, Larson L, Zhou D, Goebel WS. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology 2009;59:150-157.ArticlePubMedPMC
- 37. Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng 2006;12:2813-2823.ArticlePubMed
- 38. Hilkens P, Driesen RB, Wolfs E, Gervois P, Vangansewinkel T, Ratajczak J, Dillen Y, Bronckaers A, Lambrichts I. Cryopreservation and Banking of Dental Stem Cells. Adv Exp Med Biol 2016;951:199-235.ArticlePubMed
- 39. Oh YH, Che ZM, Hong JC, Lee EJ, Lee SJ, Kim J. Cryopreservation of human teeth for future organization of a tooth bank--a preliminary study. Cryobiology 2005;51:322-329.ArticlePubMed
- 40. Khademi AA, Saei S, Mohajeri MR, Mirkheshti N, Ghassami F, Torabi nia N, Alavi SA. A new storage medium for an avulsed tooth. J Contemp Dent Pract 2008;9:25-32.Article
- 41. Eubanks EJ, Tarle SA, Kaigler D. Tooth storage, dental pulp stem cell isolation, and clinical scale expansion without animal serum. J Endod 2014;40:652-657.ArticlePubMed
- 42. Salehinejad P, Alitheen NB, Ali AM, Omar AR, Mohit M, Janzamin E, Samani FS, Torshizi Z, Nematollahi-Mahani SN. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton's jelly. In Vitro Cell Dev Biol Anim 2012;48:75-83.ArticlePubMedPDF
REFERENCES
Tables & Figures
REFERENCES
Citations

- Comparative study on biological characteristics of dental mesenchymal stem cells isolated from gingiva, periodontal ligament, and dental follicle and their derived conditioned medium
Xianyi He, Yichen Gao, Haiyin Wan, Xia Wang, Jie Shen, Yun He, Junliang Chen
Annals of Anatomy - Anatomischer Anzeiger.2026; 263: 152751. CrossRef - Effect of the pulp harvesting method on the viability of human dental pulp stem cells and their odontogenic differentiation potential
Justine De Visscher, Lore Vermeir, Natasja Van den Vreken, Liesbeth Temmerman, Noëmi De Roo, Jolanda van Hengel, Guy De Pauw
Cell and Tissue Banking.2025;[Epub] CrossRef - The Antimicrobial Effect of the Incorporation of Inorganic Substances into Heat-Cured Denture Base Resins—A Systematic Review
Mariana Lima, Helena Salgado, André Correia, Patrícia Fonseca
Prosthesis.2024; 6(5): 1189. CrossRef - Sphingosine-1-phosphate Treatment Improves Cryopreservation Efficiency in Human Mesenchymal Stem Cells
Seong-Ju Oh, Chan-Hee Jo, Tae-Seok Kim, Chae-Yeon Hong, Sung-Lim Lee, Young-Hoon Kang, Gyu-Jin Rho
Life.2023; 13(6): 1286. CrossRef - Time- and Concentration-Dependent Effects of the Stem Cells Derived from Human Exfoliated Deciduous Teeth on Osteosarcoma Cells
Razieh Alipour, Batool Hashemibeni, Vajihe Asgari, Hamid Bahramian
Advanced Biomedical Research.2023;[Epub] CrossRef
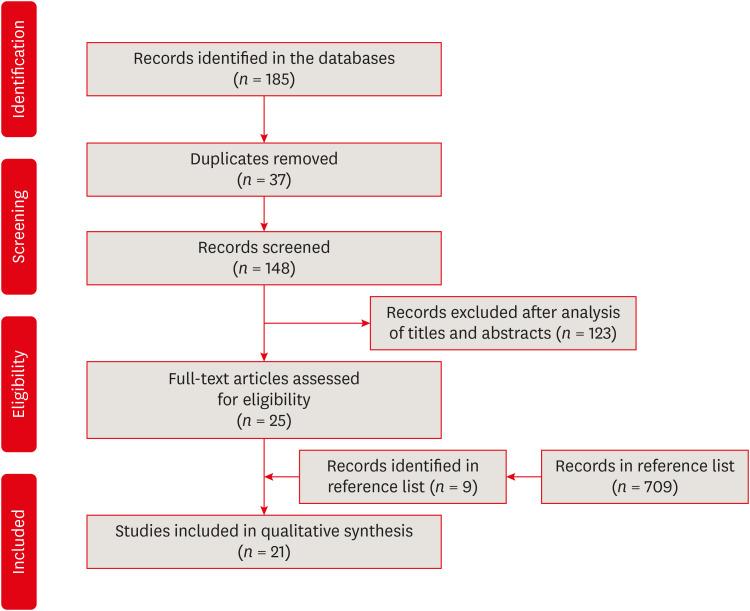
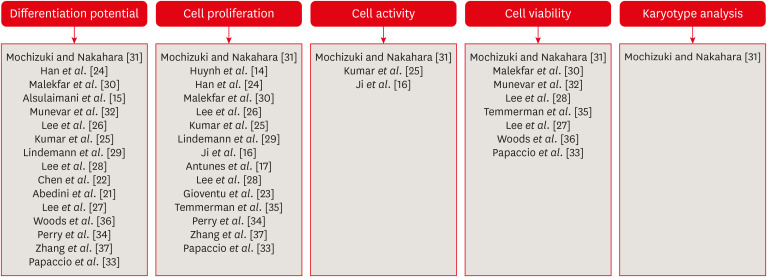
Figure 1
Figure 2
Characteristics of studies included
| Authors | Year | Country | Study design | Sample | Stages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tooth type | Size | Storage | Disinfection | Pulp extraction | Cell isolation | Cell proliferation | Cryopreservation | Time | Thawing | ||||
| Papaccio et al. [ | 2006 | Italy | RCT | P | Teeth of healthy patients aged between 21 and 45 years (unspecified number of teeth) | Immediate disinfection | Immersion in chlorhexidine gel and PBS medium containing penicillin, streptomycin, and clarithromycin | Gracey's dentin digger and curette | Enzymatic digestion with collagenase I and dispase | Immersion in alpha-MEM medium supplemented with 20% FCS, 2-phosphate ascorbic acid, glutamine, penicillin, streptomycin. Incubation at 37°C in a humid atmosphere with 5% CO2 | Transfer of cells to medium containing 10% FCS-supplemented DMSO with immediate storage in liquid nitrogen | More than 2 yr | Water bath at 37°C |
| Zhang et al. [ | 2006 | China | RCT | P | Third impacted molars in patients aged between 18 and 24 yr | Immersion in alpha-MEM | Alpha-MEM containing 0.5 mg/mL gentamicin and 3 g/mL amphotericin B | High-speed drill for crown cutting and pulping | Explantation technique associated with enzymatic digestion with collagenase type I for 1 hr | Immersion in alpha-MEM medium containing 20% FCS. Incubation at 37°C in a humid atmosphere with 5% CO2 | Liquid nitrogen storage immediately after handling | Less than 1 mon | Water bath at 37°C |
| Perry et al. [ | 2008 | India | RCT | P | 31 third molars from patients aged between 18 and 30 yr | HypoThermosol, Mesencult basal medium, or PBS, the medium being chosen randomly | PBS, sodium iodopovidone, and sodium thiosulfate baths | High-speed drill for crown and endodontic cutting | Enzyme digestion with dispase and type I collagenase | Cells were transferred to Mesencult medium supplemented with Pen-Strep and amphotericin B. | Cell transfer to Mesencult medium containing DMSO. Gradual freezing at −1°C/min to −85°C and subsequent transfer to liquid nitrogen | 1 mon | Water bath at 37°C |
| Woods et al. [ | 2009 | United States of America | RCT | P | Teeth of patients aged between 18 and 30 yr | PBS solution | PBS baths, iodopovidone at 1%, and sodium thiosulfate (0.1%) | High-speed drill for crown cutting and pulping | Enzyme digestion with collagenase type I, II and thermolysin | Immersion in Mesencult. Incubation at 37°C in a humid atmosphere with 5% CO2 | This solution was supplemented with DMSO. Gradual freezing at −1°C/min to −85°C and subsequent transfer to liquid nitrogen | 1 mon | Water bath at 37°C |
| Lee et al. [ | 2010 | Japan | RCT | P | Premolar dental pulp of adults aged 18–30 yr | PBS solution | Immersion in PBS | High-speed drill for crown and endodontic cutting | Scalpel explantation technique and enzymatic digestion with collagenase type I and dispase | Incubation at 37°C in a humid atmosphere with 5% CO2 | Prior storage in 10% DMSO, maintenance at 4°C during transport. Gradual freezing at −5°C for 15 min with cooling rate at −0.5°C/min to −32°C. Transfer after temperature of −152°C | 1 wk | Water bath at 37°C |
| Temmerman et al. [ | 2010 | Belgium | RCT | P | Third molars of patients aged 15–25 yr | Immersion in medium containing DMEM, FCS, Raid solution, fungizone, and gentamicin | Immersion in medium containing DMEM, FCS, penicillin, fungizone and gentamicin | Carborundum disc for crown and endodontic pulp cutting | Explantation technique with scalpel | Immersion in Optimem I medium with Pen-Strep, glutamine and FCS. Incubation at 37°C in a humid atmosphere with 10% CO2 | Medium cell transfer with FCS and DMSO. Gradual freezing at −1°C/min to −80°C with subsequent transfer to liquid nitrogen | 1 mon | Water bath at 37°C |
| Abedini et al. [ | 2011 | Japan | RCT | P | Third molar dental pulp of 10 patients aged 18–30 yr | Not specified | Successive baths in PBS | Vertical cut of tooth and pulp removal with curette | Explantation technique with scalpel | Immersion in alpha-MEM medium supplemented with FBS, penicillin, amphotericin B and kanamycin. Incubation at 37°C in a humid atmosphere with 5% and CO2 | Prior immersion in 10% DMSO associated with FBS. Freezing in a magnetic freezer with initial maintenance at −5°C for 15 min and subsequent cooling at a rate of −0.5°C/min to −30°C. Subsequent culture transfer at −150°C. | 3 mon | Water bath at 37°C |
| Chen et al. [ | 2011 | Taiwan | RCT | P | 50 teeth of patients with an average age of 25.5 yr | Not specified | PBS immersion | High rotation drill for cutting the crown at the dentin junction and endodontic file | Enzymatic digestion with collagenase type I and dispase | Immersion in alpha-MEM solution containing FBS, Pen-Strep, and ascorbic acid. Incubation at 37°C in a humid atmosphere of 5% CO2 up to 80% confluence | Transfer of cells to medium containing DMSO and FBS. Gradual freezing to 4°C for 2 hr, up to −80°C for 8 hr and transfer to liquid nitrogen. Cooling rate −1°C/min. | 1 mon | Water bath at 37°C |
| Gioventu et al. [ | 2012 | Italy | RCT | P | 10 non-exfoliated teeth obtained from children aged 7–11-yr-old | Sterile RPMI 1640 | Immersion in sterile RPMI 1640 medium | Making a cavity at the cementoenamel junction height with Nd:YAG laser | Enzymatic digestion with collagenase type A | Immersion in alpha-MEM Glutamax 1% medium supplemented with 20% FBS and 1% Pen-Strep. Incubation at 37°C in a humid atmosphere containing 5% CO2 until 80% confluence. | Immersion in sterile RPMI 1640 medium containing 10% DMSO and 10% human albumin. −80°C culture storage in programmable freezer | 10 days | Water bath at 37°C |
| Lee et al. [ | 2012 | Taiwan | CCT | P | Orthodontically exposed incisors of a 28-yr-old woman and a 25-yr-old man | Immediately proceeded to cleaning, no storage | Immersion in Dulbecco's phosphate-buffered saline solution | High speed drill for crown and endodontic file cutting | Scalpel explantation technique and enzymatic digestion with collagenase type I and dispase | Immersion in alpha-MEM medium supplemented with 15% FBS, 2-phosphate ascorbic acid, antibiotics and antimicrobials. Incubation at 37°C in a humid atmosphere with 5% CO2 | Non-magnetic freezing group: freezes for 1 day at −80°C and −150°C for storage. Magnetic freezing: Immersed in 10% DMSO, performed in a programmable freezer, cooling rate from −0.5°C to −32°C and storage at −150°C | 1 wk | Water bath at 37°C |
| Antunes [ | 2013 | Brazil | RCT | D | 3 teeth of children aged 6 to 12 yr | Immersion in alpha-MEM medium and transport on ice | Immersion in medium containing alpha-MEM, penicillin, streptomycin, gentamicin, and amphotericin B | Diamond blade for crown cutting and pulp tissue curettage | Enzymatic digestion with collagenase type I and dispase | Immersion in solution with alpha-MEM and FBS. Incubation at 37°C in a humid atmosphere of 5% and CO2 up to 70%–90% confluence | Transfer cells to medium containing DMSO and FBS. Freeze gradually at 4°C for 2 hr, −20°C for 18 hr and up to −80°C with transfer to liquid nitrogen | 1 mon | Water bath at 37°C |
| Ji et al. [ | 2014 | South Korea | RCT | D | 122 teeth obtained from 105 healthy patients aged 3–16 yr | FBS immersion | Immersion in alpha-MEM medium containing FBS, Pen-Strep, ascorbic acid, and glutamine | Made by a device called “Barbed Broach” (Mani, Utsunomiya Toshi-ken, Japan) | Scalpel explantation technique | Same composition of medium used for decontamination. Incubation at 37°C in a humid atmosphere with 5% CO2 | Transfer of cells to medium containing DMSO and FBS. Gradual freezing to 4°C for 1 hr and to −80°C with transfer to liquid nitrogen. Cooling rate −1°C/min | 1–9 mon | Water bath at 37°C |
| Lindemann et al. [ | 2014 | Brazil | RCT | D | 26 teeth of children aged 9–11 yr | Direct immersion in disinfection medium | Immersion in DMEM medium supplemented with FBS, penicillin, streptomycin, and gentamicin | Endodontic file for pulp collection | Enzyme digestion with collagenase type I | FBS (20%) added to the enzyme digestion solution. Incubation at 37°C in a humid atmosphere with 5% CO2 | Immersion in 10% DMSO medium associated with FBS with initial temperature maintenance at 4°C for 1 hr. The temperature was gradually cooled at a rate of −1°C/min to −80°C and maintained for 24 hr, with subsequent transfer to liquid nitrogen at −196°C. | 1 wk | Water bath at 37°C |
| Kumar et al. [ | 2015 | India | RCT | P | Impacted teeth pulp of 16-yr-old patients | Hank's balanced solution | PBS baths | High-rotation drill for crown cutting and curettage | Scalpel explantation technique | Immersion in alpha-MEM medium containing glutamine, FBS, and Pen-StrepIncubation at 37°C in a humid atmosphere with 5% CO2 | Transfer of cells to medium containing DMSO and FBS. The best protocol was gradual freezing at 0°C for 15 min, −20°C for 1 hr, and up to −80°C with transfer to liquid nitrogen. Freezing at −1°C/min | 1 yr | Water bath at 37°C |
| Lee et al. [ | 2015 | South Korea | RCT | D | 20 teeth of children aged 5–14 yr | Not specified | Immersion in PBS | Endodontic pulp collection file | Scalpel explantation technique | Immersion in alpha-MEM medium supplemented with 10% FBS, ascorbic acid, glutamine, penicillin, and streptomycin. Incubation at 37°C in a humid atmosphere with 5% CO2 | Pre-storage in FBS medium supplemented with 10% DMSO. Gradual freezing starting at 4°C with cooling rate at −1°C/min to −80°C and then storage in liquid nitrogen at −196°C | 1–8 mon | Water bath at 37°C |
| Munevar et al. [ | 2015 | Colombia | RCT | P | Teeth of patients aged 18–31 yr | Immersion in PBS and transported in ice | Immersion in 1% sodium hypochlorite and PBS baths | High-speed drill for crown and endodontic cutting | Enzyme digestion with dispase and collagenase type I | Immersion in DMEM medium supplemented with Pen-Strep and amphotericin B. Incubation at 37°C in humidified atmosphere with 5% CO2 until 70% confluence | Transfer of cells to medium containing FCS and DMSO. The samples were stored in liquid nitrogen | 2 yr | Water bath at 37°C |
| Alsulaimani et al. [ | 2016 | Saudi Arabia | RCT | P | 17 teeth of 30-yr-old | Not specified | Chlorhexidine gluconate for 30 sec, immersion in saline and lysine | Diamond blade for crown and file cutting K-file | Explant and enzymatic digestion with collagenase type I and dispase | Immersion in solution with DMEM, FBS, penicillin, streptomycin and alpha-MEM medium. Incubation at 37°C in a humid atmosphere with 5% CO2 | Transfer of cells to medium containing DMEM, FBS, Pen-strep and DMSO. Gradual freezing to −20°C for 20 min and −80°C for 4 days and transfer to liquid nitrogen | 2 yr | DMEM added to the environment and gentle aspiration |
| Malekfar et al. [ | 2016 | India | RCT | P | 20 teeth pulp samples from patients aged 15–30 yr | Not specified | Pulp tissue was washed with Dulbecco and PBS solution | High-speed drill for crown and endodontic cutting | Scalpel explantation technique and type I collagenase enzymatic digestion | Immersion in DMEM supplemented with alpha-MEM, glutamine, FBS, and Pen-Strep. Incubation at 37°C in a humid atmosphere with 5% CO2 | Transfer cells to medium containing FBS and DMSO maintained at 4°C for osmotic balance. Freezing gradually at −1°C/min to −80°C. Subsequent transfer to liquid nitrogen | 3 mon | Water bath at 37°C |
| Han et al. [ | 2017 | South Korea | RCT | P | 12 teeth of patients with an average age of 19 yr | Not specified | Immersion in PBS medium containing Pen-Strep | High-rotation drill for crown cutting and curettage | Scalpel explantation technique, type I collagenase enzyme digestion | Immersion in Dulbecco medium supplemented with 10% FBS, Pen-Strep. Incubation at 37°C in humid atmosphere with 5% CO2 | Immersion in cryo-protective solution containing glucose, sucrose and ethylene glycol. Culture maintained at 1°C for 30 min, cooled to −2°C/min to −9°C, maintained for 5 min and cooled again to −0.3°C/min to −40°C and to −10°C/min to −140°C with liquid nitrogen storage | 1 yr | Water bath at 37°C |
| Huynh et al. [ | 2017 | Vietnam | RCT | P | Third molars of patients aged 18–25 yr | Immersion in DMEM medium containing FBS and Pen-Strep or gentamicin saline | Immersion in DMEM medium containing glutamine and Pep-Strep with subsequent immersion in PBS | High-rotation drill for cutting the crown at the dentin-junction and endodontic file | Scalpel explantation technique | Immersion in DMEM medium with glutamine, FBS, and antibiotics. Incubation at 37°C in a humid atmosphere with 5% CO2 to 80% confluence | Medium cell transfer with different percentages of DMSO and FBS. Dual freezing to −80°C and transfer to liquid nitrogen. Cooling rate −1°C/min | 6 mon | Water bath at 37°C |
| Mochizuki and Nakahara [ | 2018 | Japan | RCT | P | Dental pulp of 8 healthy young adults aged 20–37 yr | Not specified | DMEM/F12 medium supplemented with FBS-free, M-MS, penicillin, streptomycin, and fungizone | High-rotation drill for crown cutting and curettage | Scalpel explantation technique and enzymatic digestion with collagenase type I and dispase | Immersion of cells in serum-free xenogenic medium. Incubation at 37°C in a humid atmosphere with 4.7% CO2 up to 80% confluence | Transfer cells to medium containing DMSO-free medium and store at −80°C | 1–3 mon | Water bath at 37°C |
RCT, randomized clinical trial; CCT, controlled clinical trial; P, permanent; D, deciduous. MEM, modified Eagle's essential medium; FCS, fetal calf serum; FBS, fetal bovine serum; DMSO, dimethyl sulfoxide; PBS, phosphate-buffered saline; DMEM, Dulbecco's modified Eagle's essential medium.
Cryopreservation methods
| Method 1 | Method 2 | Method 3 |
|---|---|---|
| Direct immersion in liquid nitrogen | Programmable freezing with the cooling rate at −1°C/min to −80°C or −85°C and subsequent transfer to liquid nitrogen for storage | Programmable freezing with the addition of breaks at set temperatures. A fixed cooling rate of −0.5°C/min starting at −5°C, maintained for 15 minutes, further cooled to −30°C or −35°C and then transferred to storage medium at −150°C or −152°C |
| Papaccio et al. [ | Perry et al. [ | Lee et al. [ |
Risk of bias assessed through the Joanna Briggs Institute Critical Appraisal Checklist for Quasi-Experimental Studies
| Studies | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | % Yes | Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mochizuki and Nakahara [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Huynh et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Han et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Malekfar et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Alsulaimani et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Munevar et al. [ | Y | Y | Y | N | Y | Y | Y | Y | NA | 77.77 | Low |
| Lee et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Kumar et al. [ | Y | Y | Y | N | Y | Y | Y | Y | NA | 77.77 | Low |
| Lindemann et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Ji et al. [ | Y | Y | Y | N | UC | Y | Y | Y | NA | 66.66 | Moderate |
| Antunes et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Lee et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Gioventu et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Chen et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Abedini et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Temmerman et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Lee et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Woods et al. [ | Y | Y | Y | Y | Y | Y | Y | Y | NA | 88.88 | Low |
| Perry et al. [ | Y | Y | Y | N | Y | Y | Y | Y | NA | 77.77 | Low |
| Zhang et al. [ | Y | Y | UC | Y | Y | Y | Y | Y | NA | 77.77 | Low |
| Papaccio et al. [ | Y | Y | Y | N | Y | Y | Y | Y | NA | 77.77 | Low |
Y, yes; N, no; UC, unclear; NA, not applicable.
RCT, randomized clinical trial; CCT, controlled clinical trial; P, permanent; D, deciduous. MEM, modified Eagle's essential medium; FCS, fetal calf serum; FBS, fetal bovine serum; DMSO, dimethyl sulfoxide; PBS, phosphate-buffered saline; DMEM, Dulbecco's modified Eagle's essential medium.
Y, yes; N, no; UC, unclear; NA, not applicable.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

