Articles
- Page Path
- HOME > Restor Dent Endod > Volume 46(1); 2021 > Article
- Research Article Smear layer removal by passive ultrasonic irrigation and 2 new mechanical methods for activation of the chelating solution
-
Ricardo Machado1
 , Isadora da Silva2
, Isadora da Silva2 , Daniel Comparin2
, Daniel Comparin2 , Bianca Araujo Marques de Mattos1
, Bianca Araujo Marques de Mattos1 , Luiz Rômulo Alberton3
, Luiz Rômulo Alberton3 , Ulisses Xavier da Silva Neto1
, Ulisses Xavier da Silva Neto1
-
Restor Dent Endod 2021;46(1):e11.
DOI: https://doi.org/10.5395/rde.2021.46.e11
Published online: January 26, 2021
1Department of Endodontics, Pontifical Catholic University of Paraná – PUCPR, Curitiba, Paraná, Brazil.
2Department of Endodontics, Paranaense University – UNIPAR, Francisco Beltrão, Paraná, Brazil.
3Department of Veterinary Medicine, Paranaense University– UNIPAR, Umuarama, Paraná, Brazil.
- Correspondence to Ricardo Machado, DDS, MSc, PhD. Postdoctoral student (Endodontics), Pontifical Catholic University of Paraná – PUCPR, Rua Imaculada Conceição, n. 1155, Prado Velho, Curitiba, Paraná, 80.215-901, Brazil. ricardo.machado.endo@gmail.com
Copyright © 2021. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives The aim of this study was to compare smear layer removal by conventional application (CA), passive ultrasonic irrigation (PUI), EasyClean (EC), and XP-Endo Finisher (XPF), using 17% ethylenediaminetetraacetic acid (EDTA) after chemomechanical preparation, as evaluated with scanning electron microscopy (SEM).
-
Materials and Methods Forty-five single-rooted human mandibular premolars were selected for this study. After chemomechanical preparation, the teeth were randomly divided into 5 groups according to the protocol for smear layer removal, as follows: G1 (control): CA of distilled water; G2 (CA): CA of 17% EDTA; G3 (PUI): 17% EDTA activated by PUI; G4 (EC): 17% EDTA activated by EC; and G5 (XPF): 17% EDTA activated by XPF. SEM images (×1,000) were obtained from each root third and scored by 3 examiners. Data were evaluated using the Kruskal-Wallis and Dunn tests (p < 0.05).
-
Results In the apical third, there were no statistically significant differences among the groups (p > 0.05). In the cervical and middle thirds, the experimental groups performed better than the control group (p < 0.05); however, G2 presented better results than G3, G4, and G5 (p < 0.05), which showed no differences among one another (p > 0.05).
-
Conclusions No irrigation method was able to completely remove the smear layer, especially in the apical third. Using CA for the chelating solution performed better than any form of activation.
INTRODUCTION
MATERIALS AND METHODS
• G1 (control): The root canals were filled with 2.5 mL of distilled water using a 31-gauge NaviTip double sideport needle (Ultradent Products) calibrated to reach 1 mm short of the WL.
• G2 (CA): The root canals were filled with 2.5 mL of 17% EDTA using a 31-gauge NaviTip double sideport needle (Ultradent Products) calibrated to reach 1 mm short of the WL.
• G3 (PUI): The root canals were filled with 2.5 mL of 17% EDTA using a 31-gauge NaviTip double sideport needle (Ultradent Products) calibrated to reach 1 mm short of the WL. PUI was performed with a special tip having no cutting power, with a #20 and 0.01 apical diameter and taper, respectively (Irrisonic E1; Helse, Santa Rosa de Viterbo, Brazil), calibrated to 1 mm short of the WL, activated by ultrasound (Profi Neo – US, Dabi Atlante, Ribeirão Preto, SP, Brazil) at a power of 40%, as indicated by the manufacturer. Care was taken to avoid contact with the walls of the root canal for more than 20 seconds.
• G4 (EC): The root canals were filled with 2.5 mL of 17% EDTA using a 31-gauge NaviTip double sideport needle (Ultradent Products) calibrated to reach 1 mm short of the WL. EC was introduced 1 mm short of the WL, and operated at low rotary speed for 20 seconds.
• G5 (XPF): Root canals were filled with 2.5 mL of 17% EDTA using a NaviTip 31-gauge double sideport needle (Ultradent Products) calibrated to reach 1 mm short of the WL. XPF was placed 1 mm short of the WL and activated for 20 seconds.
Scanning electron microscope images representative of the root canal walls according to groups and thirds.
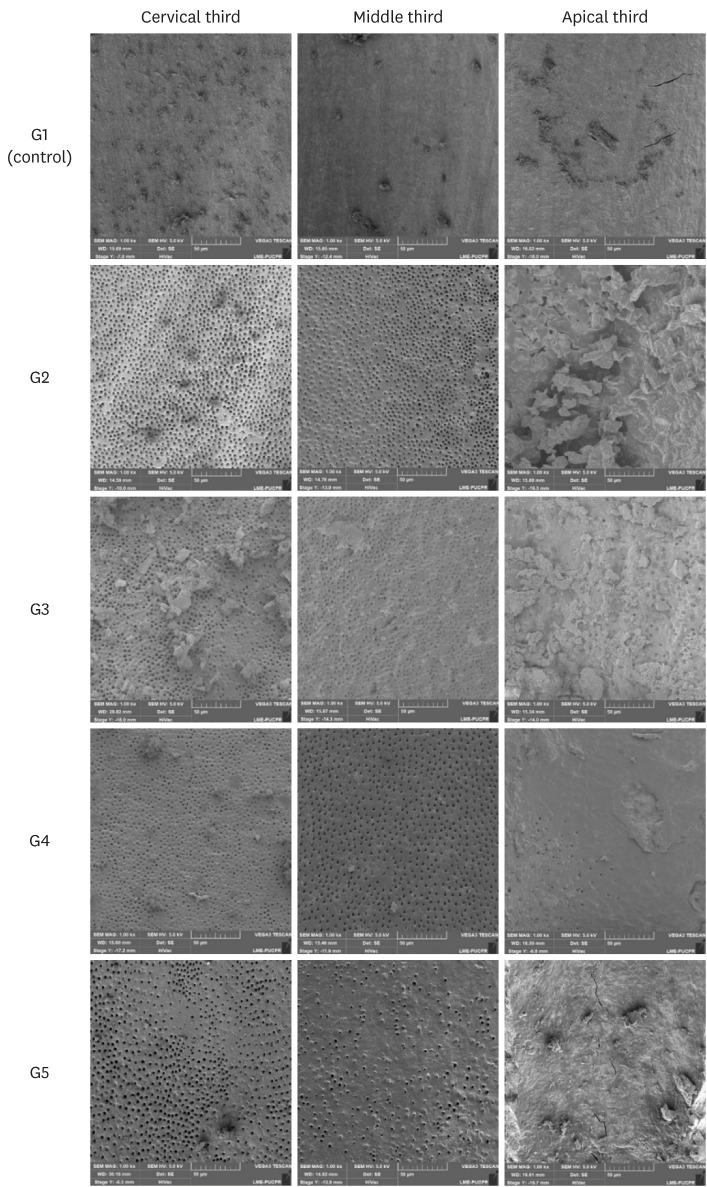
RESULTS
General analysis of the root thirds, regardless of the group
| Third | n | Median | Quartile deviation | p value |
|---|---|---|---|---|
| Cervical | 45 | 2.00a | 0.50 | p < 0.05 |
| Middle | 45 | 2.00a | 0.50 | |
| Apical | 45 | 3.00b | 1.00 | |
| Total | 135 | - | - |
Results obtained for groups according to the root thirds
DISCUSSION
CONCLUSIONS
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Machado R.
Data curation: Silva I, Comparin D.
Formal analysis: Machado R, Comparin D.
Funding acquisition: Silva I, Comparin D.
Investigation: Comparin D, Mattos BM.
Methodology: Machado R.
Project administration: Machado R.
Resources: Silva I, Comparin D.
Software: Machado R, Comparin D, Mattos BM.
Supervision: Machado R, Silva Neto UX.
Validation: Alberton LR.
Visualization: Machado R.
Writing - original draft: Machado R.
Writing - review & editing: Silva Neto UX.
- 1. Siqueira JF Jr, Rocas IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod 2008;34:1291-1301.e3.ArticlePubMed
- 2. Haapasalo M, Shen Y, Wang Z, Gao Y. Irrigation in endodontics. Br Dent J 2014;216:299-303.ArticlePubMedPDF
- 3. Violich DR, Chandler NP. The smear layer in endodontics - a review. Int Endod J 2010;43:2-15.ArticlePubMed
- 4. Machado R, Garcia LD, da Silva Neto UX, Cruz Filho AM, Silva RG, Vansan LP. Evaluation of 17% EDTA and 10% citric acid in smear layer removal and tubular dentin sealer penetration. Microsc Res Tech 2018;81:275-282.ArticlePubMedPDF
- 5. Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. J Endod 2007;33:96-105.ArticlePubMed
- 6. Mozo S, Llena C, Forner L. Review of ultrasonic irrigation in endodontics: increasing action of irrigating solutions. Med Oral Patol Oral Cir Bucal 2012;17:e512-e516.ArticlePubMed
- 7. Weller RN, Brady JM, Bernier WE. Efficacy of ultrasonic cleaning. J Endod 1980;6:740-743.ArticlePubMed
- 8. Leoni GB, Versiani MA, Silva-Sousa YT, Bruniera JF, Pécora JD, Sousa-Neto MD. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int Endod J 2017;50:398-406.ArticlePubMedPDF
- 9. Mancini M, Cerroni L, Iorio L, Armellin E, Conte G, Cianconi L. Smear layer removal and canal cleanliness using different irrigation systems (EndoActivator, EndoVac, and passive ultrasonic irrigation): field emission scanning electron microscopic evaluation in an in vitro study. J Endod 2013;39:1456-1460.ArticlePubMed
- 10. Schmidt TF, Teixeira CS, Felippe MC, Felippe WT, Pashley DH, Bortoluzzi EA. Effect of ultrasonic activation of irrigants on smear layer removal. J Endod 2015;41:1359-1363.ArticlePubMed
- 11. Saber SD, Hashem AA. Efficacy of different final irrigation activation techniques on smear layer removal. J Endod 2011;37:1272-1275.ArticlePubMed
- 12. De-Deus G, Belladonna FG, de Siqueira Zuolo A, Perez R, Carvalho MS, Souza EM, Lopes RT, Silva EJ. Micro-CT comparison of XP-endo Finisher and passive ultrasonic irrigation as final irrigation protocols on the removal of accumulated hard-tissue debris from oval shaped-canals. Clin Oral Investig 2019;23:3087-3093.ArticlePubMedPDF
- 13. Kolli S, Balasubramanian SK, Kittappa K, Mahalaxmi S. Efficacy of XP-endo Finisher files in endodontics. Aust Endod J 2018;44:71-72.PubMed
- 14. Kato AS, Cunha RS, da Silveira Bueno CE, Pelegrine RA, Fontana CE, de Martin AS. Investigation of the efficacy of passive ultrasonic irrigation versus irrigation with reciprocating activation: an environmental scanning electron microscopic study. J Endod 2016;42:659-663.ArticlePubMed
- 15. Silva EJ, Carvalho CR, Belladonna FG, Prado MC, Lopes RT, De-Deus G, Moreira EJ. Micro-CT evaluation of different final irrigation protocols on the removal of hard-tissue debris from isthmus-containing mesial root of mandibular molars. Clin Oral Investig 2019;23:681-687.ArticlePubMedPDF
- 16. Bueno CR, Cury MT, Vasques AM, Sarmiento JL, Trizzi JQ, Jacinto RC, Sivieri-Araujo G, Dezan Júnior E. Cleaning effectiveness of a nickel-titanium ultrasonic tip in ultrasonically activated irrigation: a SEM study. Braz Oral Res 2019;33:e017.ArticlePubMed
- 17. Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, Kim J, Shabahang S. A new solution for the removal of the smear layer. J Endod 2003;29:170-175.ArticlePubMed
- 18. Xin Y, Yang J, Song KY. In vitro evaluation of the effectiveness of XP-endo Finisher file on smear layer removal after root canal instrumentation. Hua Xi Kou Qiang Yi Xue Za Zhi 2019;37:48-52.PubMedPMC
- 19. Haupt F, Meinel M, Gunawardana A, Hulsmann M. Effectiveness of different activated irrigation techniques on debris and smear layer removal from curved root canals: a SEM evaluation. Aust Endod J 2020;46:40-46.PubMed
- 20. Ballal V, Rao S, Al-Haj Husain N, Özcan M. Evaluation of smear layer removal using different irrigation methods in root canals. Eur J Prosthodont Restor Dent 2019;27:97-102.PubMed
- 21. Mancini M, Cerroni L, Iorio L, Dall’Asta L, Cianconi L. FESEM evaluation of smear layer removal using different irrigant activation methods (EndoActivator, EndoVac, PUI and LAI). An in vitro study. Clin Oral Investig 2018;22:993-999.ArticlePubMedPDF
- 22. Caron G, Nham K, Bronnec F, Machtou P. Effectiveness of different final irrigant activation protocols on smear layer removal in curved canals. J Endod 2010;36:1361-1366.ArticlePubMed
- 23. Singh N, Chandra A, Tikku AP, Verma P. A comparative evaluation of different irrigation activation systems on smear layer removal from root canal: an in-vitro scanning electron microscope study. J Conserv Dent 2014;17:159-163.ArticlePubMedPMC
- 24. Kuah HG, Lui JN, Tseng PS, Chen NN. The effect of EDTA with and without ultrasonics on removal of the smear layer. J Endod 2009;35:393-396.ArticlePubMed
- 25. Perez F, Rouqueyrol-Pourcel N. Effect of a low-concentration EDTA solution on root canal walls: a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:383-387.ArticlePubMed
- 26. Mancini M, Armellin E, Casaglia A, Cerroni L, Cianconi L. A comparative study of smear layer removal and erosion in apical intraradicular dentine with three irrigating solutions: a scanning electron microscopy evaluation. J Endod 2009;35:900-903.ArticlePubMed
- 27. Shahriari S, Kasraei S, Roshanaei G, Karkeabadi H, Davanloo H. Efficacy of sodium hypochlorite activated with laser in intracanal smear layer removal: an SEM study. J Lasers Med Sci 2017;8:36-41.ArticlePubMedPMC
- 28. Machado R, Comparin D, Back ED, Garcia LD, Alberton LR. Residual smear layer after root canal instrumentation by using Niti, M-Wire and CM-Wire instruments: a scanning electron microscopy analysis. Eur J Dent 2018;12:403-409.ArticlePubMedPMC
- 29. Susin L, Liu Y, Yoon JC, Parente JM, Loushine RJ, Ricucci D, Bryan T, Weller RN, Pashley DH, Tay FR. Canal and isthmus debridement efficacies of two irrigant agitation techniques in a closed system. Int Endod J 2010;43:1077-1090.ArticlePubMedPMC
- 30. Prado MC, Leal F, Simão RA, Gusman H, do Prado M. The use of auxiliary devices during irrigation to increase the cleaning ability of a chelating agent. Restor Dent Endod 2017;42:105-110.ArticlePubMedPMCPDF
- 31. Wu L, Mu Y, Deng X, Zhang S, Zhou D. Comparison of the effect of four decalcifying agents combined with 60°C 3% sodium hypochlorite on smear layer removal. J Endod 2012;38:381-384.ArticlePubMed
- 32. Goel S, Tewari S. Smear layer removal with passive ultrasonic irrigation and the NaviTip FX: a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:465-470.ArticlePubMed
- 33. Gulabivala K, Ng YL, Gilbertson M, Eames I. The fluid mechanics of root canal irrigation. Physiol Meas 2010;31:R49-R84.ArticlePubMed
- 34. De-Deus G, Marins J, Silva EJ, Souza E, Belladonna FG, Reis C, Machado AS, Lopes RT, Versiani MA, Paciornik S, Neves AA. Accumulated hard tissue debris produced during reciprocating and rotary nickel-titanium canal preparation. J Endod 2015;41:676-681.ArticlePubMed
- 35. Marques AC, Aguiar BA, Frota LM, Guimarães BM, Vivacqua-Gomes N, Vivan RR, Duarte MA, de Vasconcelos BC. Evaluation of influence of widening apical preparation of root canals on efficiency of ethylenediaminetetraacetic acid agitation protocols: study by scanning electron microscopy. J Contemp Dent Pract 2018;19:1087-1094.ArticlePubMed
- 36. Simezo AP, da Silveira Bueno CE, Cunha RS, Pelegrine RA, Rocha DG, de Martin AS, Kato AS. Comparative analysis of dentinal erosion after passive ultrasonic irrigation versus irrigation with reciprocating activation: an environmental scanning electron study. J Endod 2017;43:141-146.ArticlePubMed
- 37. Kanaan CG, Pelegrine RA, da Silveira Bueno CE, Shimabuko DM, Valamatos Pinto NM, Kato AS. Can irrigant agitation lead to the formation of a smear layer? J Endod 2020;46:1120-1124.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Smear layer removal comparing conventional irrigation, passive ultrasonic irrigation, EndoActivator System, and a new sonic device (Perfect Clean System) by scanning electron microscopy: An ex vivo study
Bruna Fernanda Alionço Gonçalves, Divya Reddy, Ricardo Machado, Paulo César Soares Júunior, Sérgio Aparecido Ignácio, Douglas Augusto Fernandes Couto, Karine Santos Frasquetti, Vânia Portela Ditzel Westphalen, Everdan Carneiro, Ulisses Xavier da Silva Net
PLOS ONE.2024; 19(12): e0314940. CrossRef - Impact of different agitation methods on smear layer cleaning of mesial canals with accentuated curvature
Abel Teves Cordova, Murilo Priori Alcalde, Michel Espinosa Klymus, Leonardo Rigoldi Bonjardim, Rodrigo Ricci Vivan, Marco Antonio Hungaro Duarte
Restorative Dentistry & Endodontics.2024;[Epub] CrossRef - Advances in hybridized nanoarchitectures for improved oro-dental health
Jun Guo, Pei Wang, Yuyao Li, Yifan Liu, Yingtong Ye, Yi Chen, Ranjith Kumar Kankala, Fei Tong
Journal of Nanobiotechnology.2024;[Epub] CrossRef - Cleaning and disinfection of the root canal system provided by four active supplementary irrigation methods
Alessandra Timponi Goes Cruz, Adriane Antoniw Klemz, Edvaldo Antônio Ribeiro Rosa, Fabiana Soares Grecca, Bianca Mattos, Lucila Piasecki, Ricardo Machado, Sérgio Aparecido Ignácio, Ulisses Xavier da Silva Neto
Scientific Reports.2024;[Epub] CrossRef - Scanning electron microscopic study of smear layer changes following ultrasonic endoactivator irrigation system during root canal treatment of primary teeth
Mohamed Ghaly, Aya Alsherif, Arafa Khatab
Tanta Dental Journal.2023; 20(2): 137. CrossRef - Influence of agitation methods of irrigants after methylene blue-mediated PDT on the bonding interface of a fiber post cementation system
Lucas David Galvani, Joatan Lucas de Sousa Gomes Costa, João Felipe Besegato, Joissi Ferrari Zaniboni, Wilfredo Gustavo Escalante-Otárola, Milton Carlos Kuga
Photodiagnosis and Photodynamic Therapy.2022; 37: 102708. CrossRef
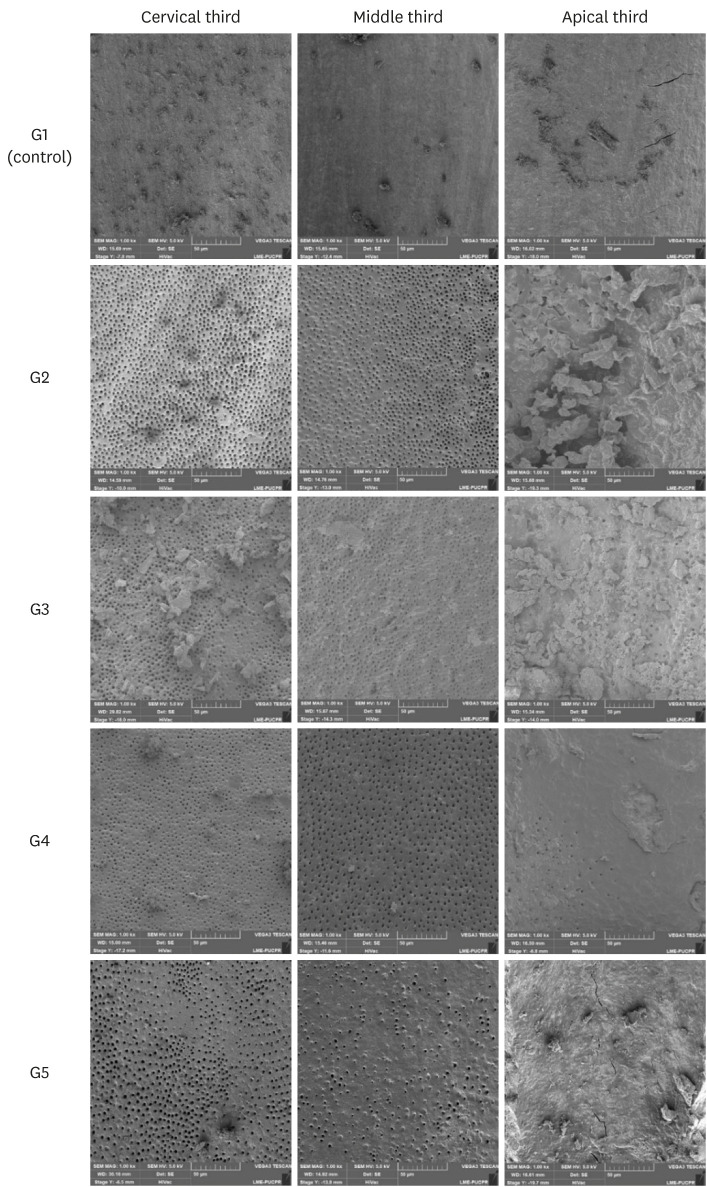
Figure 1
General analysis of the root thirds, regardless of the group
| Third | n | Median | Quartile deviation | p value |
|---|---|---|---|---|
| Cervical | 45 | 2.00a | 0.50 | p < 0.05 |
| Middle | 45 | 2.00a | 0.50 | |
| Apical | 45 | 3.00b | 1.00 | |
| Total | 135 | - | - |
Different superscript letters indicate statistically significant differences (p < 0.05).
Results obtained for groups according to the root thirds
| Group | Root third | p value | ||
|---|---|---|---|---|
| Cervical (n = 45) | Middle (n = 45) | Apical (n = 45) | ||
| G1: control group (n = 5) | 3.00 ± 0.00A,a | 3.00 ± 0.0A,a | 3.00 ± 0.00A,a | 1.0000 |
| G2: CA (n = 10) | 1.00 ± 0.00B,b | 1.00 ± 0.37B,b | 3.00 ± 0.37A,a | 0.0002 |
| G3: PUI (n = 10) | 2.00 ± 0.00A,ab | 2.00 ± 0.37A,ab | 2.50 ± 0.50A,a | 0.1220 |
| G4 EC (n = 10) | 1.50 ± 0.50B,ab | 2.00 ± 0.50AB,ab | 3.00 ± 0.37A,a | 0.0104 |
| G5: XPF (n = 10) | 2.50 ± 1.00A,ab | 2.00 ± 0.37A,ab | 3.00 ± 0.37A,a | 0.1433 |
| p value | 0.002 | 0.005 | 0.807 | |
Different superscript letters indicate statistically significant differences (p < 0.05). Considering thirds (columns): uppercase letters; considering rows: lowercase letters.
CA, conventional application; PUI, passive ultrasonic irrigation; EC, EasyClean; XPF, XP-Endo Finisher.
Different superscript letters indicate statistically significant differences (
Different superscript letters indicate statistically significant differences (
CA, conventional application; PUI, passive ultrasonic irrigation; EC, EasyClean; XPF, XP-Endo Finisher.

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

