Articles
- Page Path
- HOME > Restor Dent Endod > Volume 44(4); 2019 > Article
- Research Article Influence of 10-MDP concentration on the adhesion and physical properties of self-adhesive resin cements
-
Kazuhiko Shibuya
 , Naoko Ohara
, Naoko Ohara , Serina Ono
, Serina Ono , Kumiko Matsuzaki
, Kumiko Matsuzaki , Masahiro Yoshiyama
, Masahiro Yoshiyama
-
Restor Dent Endod 2019;44(4):e45.
DOI: https://doi.org/10.5395/rde.2019.44.e45
Published online: November 12, 2019
Department of Operative Dentistry, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan.
- Correspondence to Kazuhiko Shibuya, DDS, PhD. Assistant Professor, Department of Operative Dentistry, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama 700-8525, Japan. shibuya-k@okayama-u.ac.jp
Copyright © 2019. The Korean Academy of Conservative Dentistry
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,799 Views
- 17 Download
- 10 Crossref
Abstract
-
Objectives Self-adhesive resin cements contain functional monomers that enable them to adhere to the tooth structure without a separate adhesive or etchant. One of the most stable functional monomers used for chemical bonding to calcium in hydroxyapatite is 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP). The aim of this study was to evaluate the influence of the10-MDP concentration on the bond strength and physical properties of self-adhesive resin cements.
-
Materials and Methods We used experimental resin cements containing 3 different concentrations of 10-MDP: 3.3 wt% (RC1), 6.6 wt% (RC2), or 9.9 wt% (RC3). The micro-tensile bond strength of each resin cement to dentin and a hybrid resin block (Estenia C&B, Kuraray Noritake Dental) was measured, and the fractured surface morphology was analyzed. Further, the flexural strength of the resin cements was measured using the three-point bending test. The water sorption and solubility of the cements following 30 days of immersion in water were measured.
-
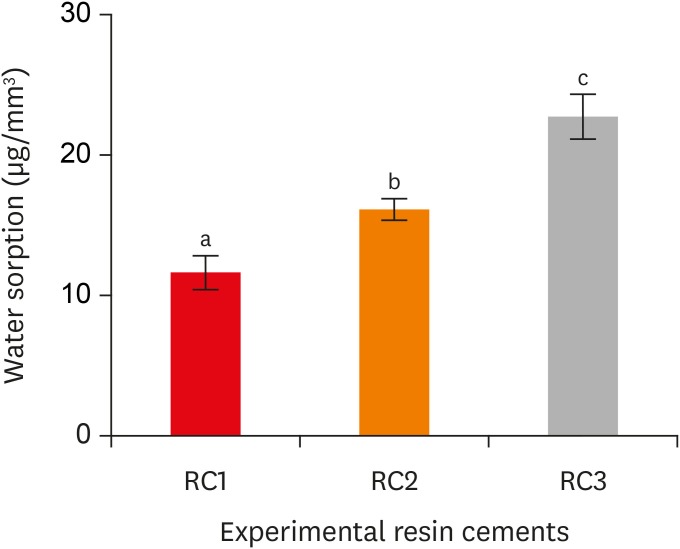
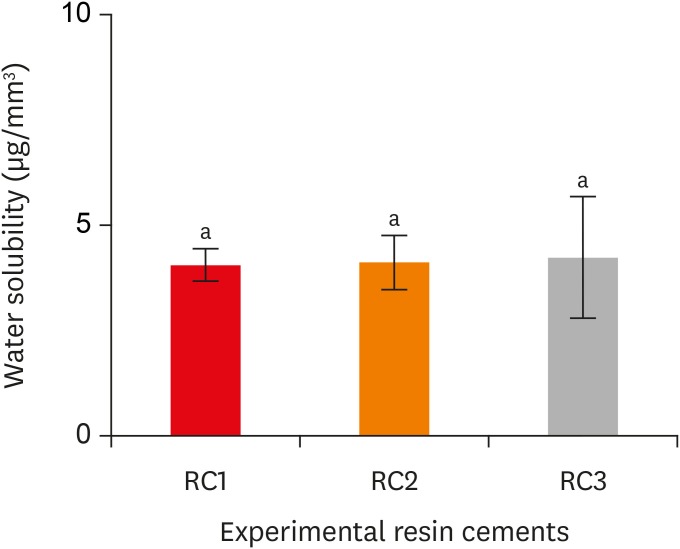
Results The bond strength of RC2 was significantly higher than that of RC1. There was no significant difference between the bond strength of RC2 and that of RC3. The water sorption of RC3 was higher than that of any other cement. There were no significant differences in the three-point bending strength or water solubility among all three types of cements.
-
Conclusions Within the limitations of this study, it is suggested that 6.6 wt% 10-MDP showed superior properties than 3.3 wt% or 9.9 wt% 10-MDP in self-adhesive resin cement.
INTRODUCTION
MATERIALS AND METHODS
Chemical composition of the experimental resin cements
RESULTS
Micro-tensile bond strength of the experimental resin cements. RC1, RC2, and RC3 are the experimental resin cements containing 3.3 wt%, 6.6 wt%, and 9.9 wt% concentrations of 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) monomer, respectively.
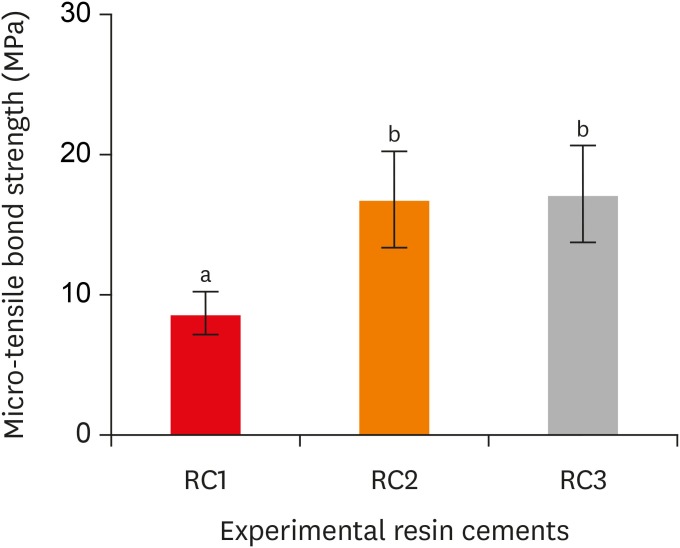
Fracture mode analysis of the specimens in each group after micro-tensile bond strength testing. RC1, RC2, and RC3 are the experimental resin cements containing 3.3 wt%, 6.6 wt%, and 9.9 wt% concentrations of 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) monomer, respectively.
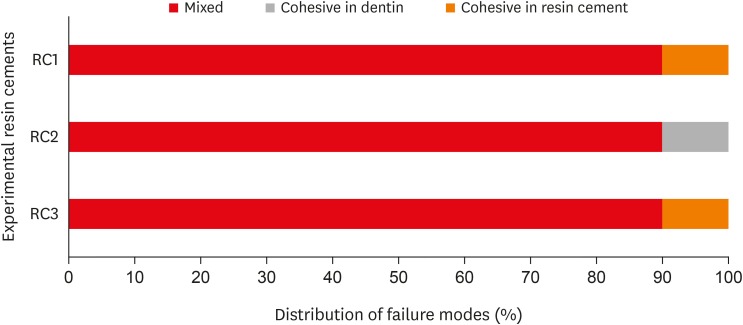
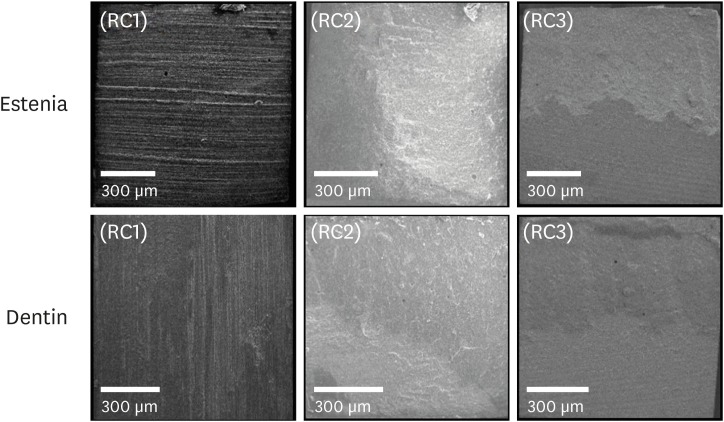
Scanning electron microscopic observations of the fractured dentin and Estenia C&B surfaces. RC1, RC2, and RC3 are the experimental resin cements containing 3.3 wt%, 6.6 wt%, and 9.9 wt% concentrations of 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) monomer, respectively.
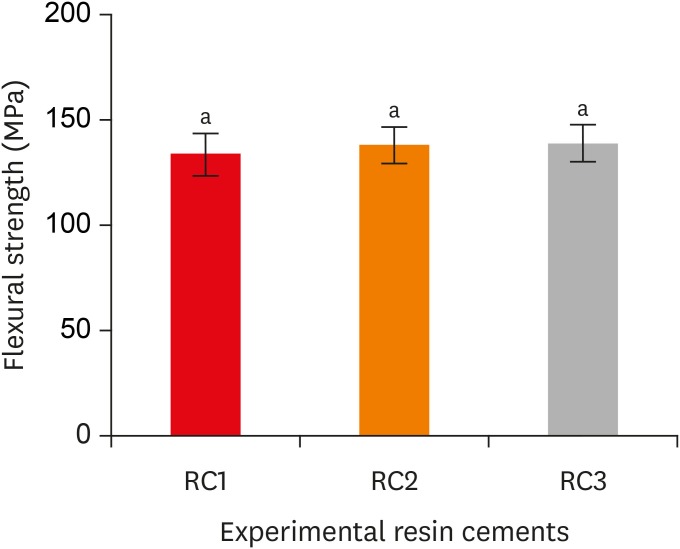
Flexural strength of the experimental resin cements. RC1, RC2, and RC3 are the experimental resin cements containing 3.3 wt%, 6.6 wt%, and 9.9 wt% concentrations of 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) monomer, respectively.
Water sorption of the experimental resin cements. RC1, RC2, and RC3 are the experimental resin cements containing 3.3 wt%, 6.6 wt%, and 9.9 wt% concentrations of 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) monomer, respectively.
Water solubility of the experimental resin cements. RC1, RC2, and RC3 are the experimental resin cements containing 3.3 wt%, 6.6 wt%, and 9.9 wt% concentrations of 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) monomer, respectively.
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
-
Funding: This work was supported by the grant-in-aid for Scientific Research of Japan Society of the Promotion of Science (No 17K17134).
-
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
-
Author Contributions:
Conceptualization: Shibuya K, Yoshiyama M.
Data curation: Shibuya K, Ohara N.
Formal analysis: Shibuya K, Ono S.
Funding acquisition: Yoshiyama M.
Investigation: Shibuya K, Ohara N.
Methodology: Shibuya K, Ohara N.
Project administration: Shibuya K, Yoshiyama M.
Resources: Shibuya K.
Software: Ono S, Matsuzaki K.
Supervision: Yoshiyama M.
Validation: Matsuzaki K, Yoshiyama M.
Visualization: Ohara N.
Writing - original draft: Shibuya K, Ohara N.
Writing - review & editing: Shibuya K, Ohara N, Yoshiyama M.
- 1. Van Meerbeek B, Yoshida Y, Snauwaert J, Hellemans L, Lambrechts P, Vanherle G, Wakasa K, Pashley DH. Hybridization effectiveness of a two-step versus a three-step smear layer removing adhesive system examined correlatively by TEM and AFM. J Adhes Dent 1999;1:7-23.PubMed
- 2. Asmussen E, Peutzfeldt A. Short- and long-term bonding efficacy of a self-etching, one-step adhesive. J Adhes Dent 2003;5:41-45.PubMed
- 3. Beier US, Kapferer I, Dumfahrt H. Clinical long-term evaluation and failure characteristics of 1,335 all-ceramic restorations. Int J Prosthodont 2012;25:70-78.PubMed
- 4. van Dijken JW, Hasselrot L. A prospective 15-year evaluation of extensive dentin-enamel-bonded pressed ceramic coverages. Dent Mater 2010;26:929-939.ArticlePubMed
- 5. Spencer P, Swafford JR. Unprotected protein at the dentin-adhesive interface. Quintessence Int 1999;30:501-507.PubMed
- 6. Walshaw PR, McComb D. Clinical considerations for optimal dentinal bonding. Quintessence Int 1996;27:619-625.PubMed
- 7. Frankenberger R, Krämer N, Petschelt A. Technique sensitivity of dentin bonding: effect of application mistakes on bond strength and marginal adaptation. Oper Dent 2000;25:324-330.PubMed
- 8. De Munck J, Vargas M, Van Landuyt K, Hikita K, Lambrechts P, Van Meerbeek B. Bonding of an auto-adhesive luting material to enamel and dentin. Dent Mater 2004;20:963-971.ArticlePubMed
- 9. Ibarra G, Johnson GH, Geurtsen W, Vargas MA. Microleakage of porcelain veneer restorations bonded to enamel and dentin with a new self-adhesive resin-based dental cement. Dent Mater 2007;23:218-225.PubMed
- 10. Hosaka K, Tagami J, Nishitani Y, Yoshiyama M, Carrilho M, Tay FR, Agee KA, Pashley DH. Effect of wet vs. dry testing on the mechanical properties of hydrophilic self-etching primer polymers. Eur J Oral Sci 2007;115:239-245.ArticlePubMed
- 11. Ikemura K, Tay FR, Nishiyama N, Pashley DH, Endo T. Design of new phosphonic acid monomers for dental adhesives--synthesis of (meth)acryloxyalkyl 3-phosphonopropionates and evaluation of their adhesion-promoting functions. Dent Mater J 2006;25:566-575.ArticlePubMed
- 12. Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, Inoue S, Tagawa Y, Suzuki K, De Munck J, Van Meerbeek B. Comparative study on adhesive performance of functional monomers. J Dent Res 2004;83:454-458.ArticlePubMedPDF
- 13. Braem M, Finger W, Van Doren VE, Lambrechts P, Vanherle G. Mechanical properties and filler fraction of dental composites. Dent Mater 1989;5:346-348.ArticlePubMed
- 14. Söderholm KJ. Influence of silane treatment and filler fraction on thermal expansion of composite resins. J Dent Res 1984;63:1321-1326.ArticlePubMedPDF
- 15. Asmussen E. Factors affecting the color stability of restorative resins. Acta Odontol Scand 1983;41:11-18.ArticlePubMed
- 16. Asmussen E. Softening of BISGMA-based polymers by ethanol and by organic acids of plaque. Scand J Dent Res 1984;92:257-261.ArticlePubMed
- 17. Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg FA, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 2005;26:6449-6459.ArticlePubMed
- 18. Vanlandingham MR, Eduljee RF, Gillespie JW. Moisture diffusion in epoxy systems. J Appl Polym Sci 1999;71:787-798.Article
- 19. Ping ZH, Nguyen QT, Chen SM, Zhou JQ, Ding YD. States of water in different hydrophilic polymers–DSC and FTIR studies. Polymer (Guildf) 2001;42:8461-8467.Article
- 20. Liu M, Wu P, Dinga Y, Li S. Study on diffusion behavior of water in epoxy resins cured by active ester. Phys Chem Chem Phys 2003;5:1848-1852.Article
- 21. Azevedo CG, De Goes MF, Ambrosano GM, Chan DC. 1-Year clinical study of indirect resin composite restorations luted with a self-adhesive resin cement: effect of enamel etching. Braz Dent J 2012;23:97-103.ArticlePubMed
- 22. Aschenbrenner CM, Lang R, Handel G, Behr M. Analysis of marginal adaptation and sealing to enamel and dentin of four self-adhesive resin cements. Clin Oral Investig 2012;16:191-200.ArticlePubMedPDF
- 23. Pan Y, Xu X, Sun F, Meng X. Surface morphology and mechanical properties of conventional and self-adhesive resin cements after aqueous aging. J Appl Oral Sci 2018;27:e20170449.ArticlePubMedPMC
- 24. Sokolowski G, Szczesio A, Bociong K, Kaluzinska K, Lapinska B, Sokolowski J, Domarecka M, Lukomska-Szymanska M. Dental resin cements-the influence of water sorption on contraction stress changes and hydroscopic expansion. Materials (Basel) 2018;11:E973.ArticlePubMedPMC
- 25. Tay FR, Pashley DH. Aggressiveness of contemporary self-etching systems. I: Depth of penetration beyond dentin smear layers. Dent Mater 2001;17:296-308.PubMed
- 26. Hiraishi N, Nishiyama N, Ikemura K, Yau JY, King NM, Tagami J, Pashley DH, Tay FR. Water concentration in self-etching primers affects their aggressiveness and bonding efficacy to dentin. J Dent Res 2005;84:653-658.ArticlePubMedPDF
- 27. Bowen RL, Bennett PS, Groh RJ, Farahani M, Eichmiller FC. New surface-active comonomer for adhesive bonding. J Dent Res 1996;75:606-610.ArticlePubMedPDF
- 28. Watanabe I, Nakabayashi N, Pashley DH. Bonding to ground dentin by a phenyl-P self-etching primer. J Dent Res 1994;73:1212-1220.ArticlePubMedPDF
- 29. Tay FR, Pashley DH, Suh BI, Hiraishi N, Yiu CK. Water treeing in simplified dentin adhesives--déjà vu? Oper Dent 2005;30:561-579.PubMed
- 30. Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc 2003;69:726-731.PubMed
- 31. Tay FR, Pashley DH, Suh BI, Carvalho RM, Itthagarun A. Single-step adhesives are permeable membranes. J Dent 2002;30:371-382.ArticlePubMed
- 32. Lin J, Shinya A, Gomi H, Shinya A. Bonding of self-adhesive resin cements to enamel using different surface treatments: bond strength and etching pattern evaluations. Dent Mater J 2010;29:425-432.ArticlePubMed
- 33. Wu J, Zhang Q, Weir MD, Oates TW, Zhou C, Chang X, Xu HH. Novel self-healing dental luting cements with microcapsules for indirect restorations. J Dent 2017;66:76-82.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations

- Bonding effectiveness of 10-MDP containing resin composite cements: a systematic review with meta-analysis
Sofia Bignotto de Carvalho, Lívia Maiumi Uehara, João Marcos Carvalho-Silva, Andréa Cândido dos Reis
International Journal of Adhesion and Adhesives.2026; 146: 104260. CrossRef - Comparative Evaluation of Color Stability in Bioactive and Conventional Resin Cements Under Thermal Stress Conditions
Alaa Turkistani, Hanin E. Yeslam
Biomimetics.2025; 10(7): 432. CrossRef - Influence of temperature and curing modes on polymerization of self-adhesive resin cements
Hae-In Kim, Jin-Woo Kim, Se-Hee Park, Kyung-Mo Cho
Korean Journal of Dental Materials.2025; 52(3): 143. CrossRef - Clinical Performance and Retention of Partial Implant Restorations Cemented with Fuji Plus® and DentoTemp™: A Retrospective Clinical Study with Mechanical Validation
Sergiu-Manuel Antonie, Laura-Cristina Rusu, Ioan-Achim Borsanu, Remus Christian Bratu, Emanuel-Adrian Bratu
Medicina.2025; 61(12): 2183. CrossRef - A thorough assessment of 10-MDP primers in modern dental adhesive systems
Ahmed A Abduljawad, Harraa SM Salih, Omar F Tawfiq
Journal of Baghdad College of Dentistry.2024; 36(3): 79. CrossRef - Material properties and finite element analysis of adhesive cements used for zirconia crowns on dental implants
Megha Satpathy, Hai Pham, Shreya Shah
Computer Methods in Biomechanics and Biomedical Engineering.2024; : 1. CrossRef - Clinical reliability of self-adhesive luting resins compared to other adhesive procedures: A systematic review and meta-analysis
Mohammed Ahmed Alghauli, Ahmed Yaseen Alqutaibi, Sebastian Wille, Matthias Kern
Journal of Dentistry.2023; 129: 104394. CrossRef - Influence of autoclave sterilization on bond strength between zirconia frameworks and Ti-base abutments using different resin cements
Reinhold Lang, Karl-Anton Hiller, Lena Kienböck, Katrin Friedl, Karl-Heinz Friedl
The Journal of Prosthetic Dentistry.2022; 127(4): 617.e1. CrossRef - Varying 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) level improves polymerisation kinetics and flexural strength in self-adhesive, remineralising composites
António H.S. Delgado, Nazanin Owji, Paul Ashley, Anne M. Young
Dental Materials.2021; 37(9): 1366. CrossRef - Investigating a Commercial Functional Adhesive with 12-MDPB and Reactive Filler to Strengthen the Adhesive Interface in Eroded Dentin
Madalena Belmar da Costa, António HS Delgado, Tomás Amorim Afonso, Luís Proença, Ana Sofia Ramos, Ana Mano Azul
Polymers.2021; 13(20): 3562. CrossRef
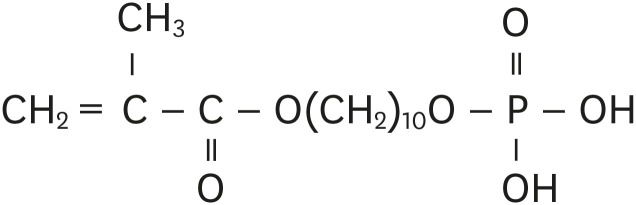
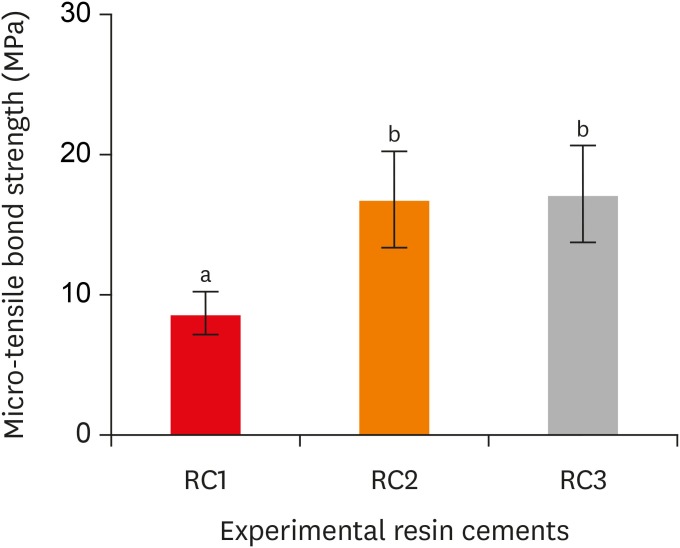
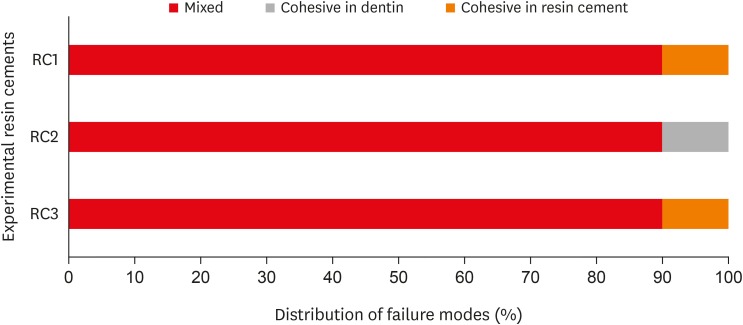
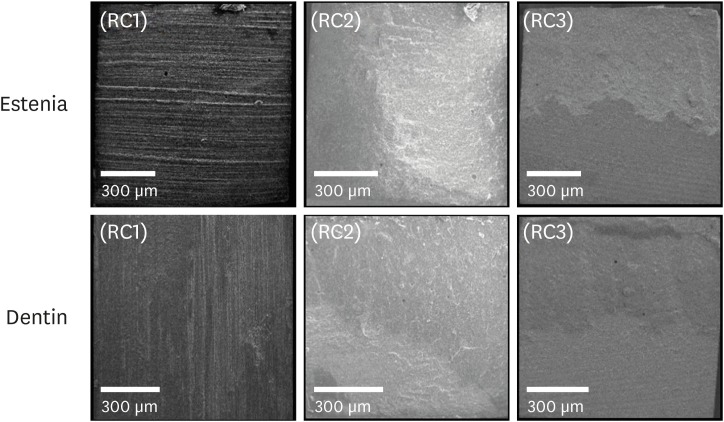
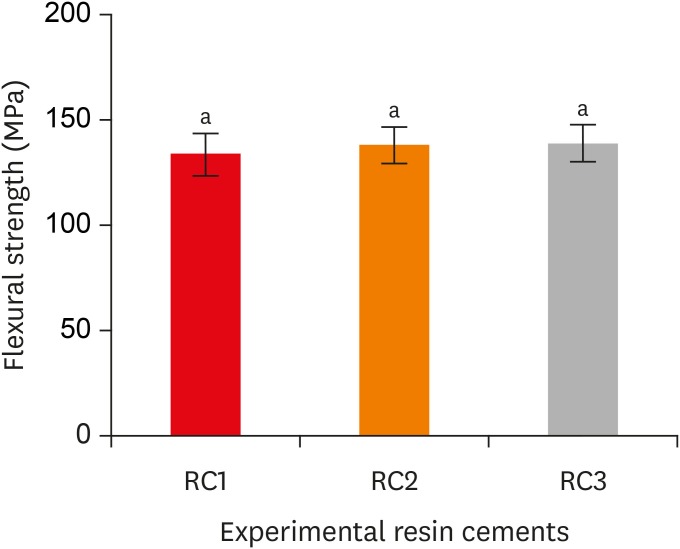
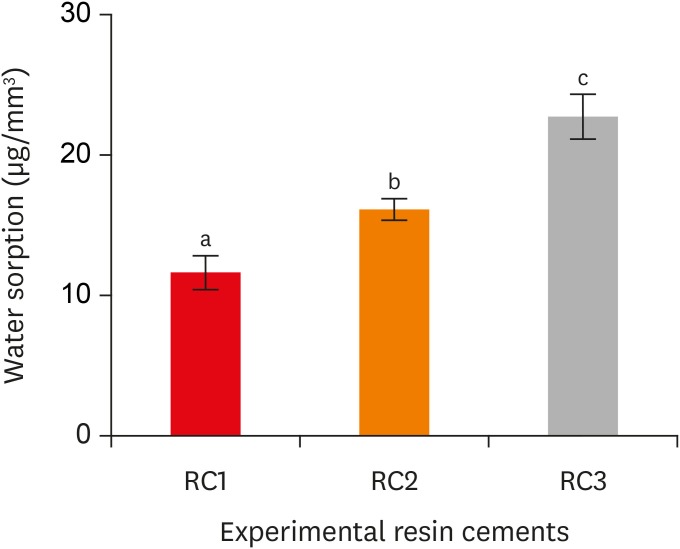
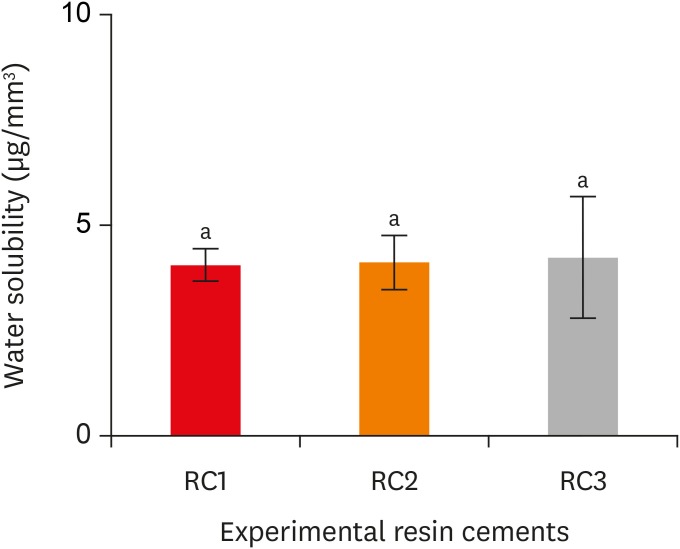
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Chemical composition of the experimental resin cements
| Paste | Chemical composition |
|---|---|
| Paste A | • Methacrylate monomers |
| • 10-Methacryloyloxydecyl dihydrogen phosphate | |
| • Silanated glass filler | |
| • Photo initiator | |
| • Chemical initiator | |
| Paste B | • Methacrylate monomers |
| • Silanated glass filler | |
| • Surface-treated sodium fluoride | |
| • Accelerators |

 KACD
KACD
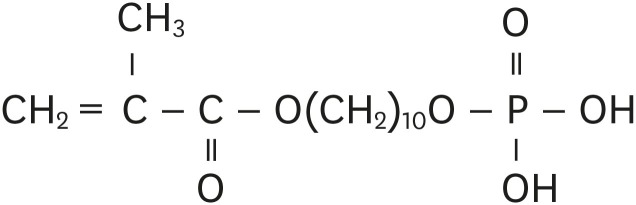
 ePub Link
ePub Link Cite
Cite

