Articles
- Page Path
- HOME > Restor Dent Endod > Volume 33(6); 2008 > Article
-
Original Article
Reconsideration of treatment protocol on the reduction of
Enterococcus faecalis associated with failed root canal treatment - Woo Cheol Lee, Seong-Tae Hong, WonJun Shon
-
2008;33(6):-569.
DOI: https://doi.org/10.5395/JKACD.2008.33.6.560
Published online: November 30, 2008
Department of Conservative Dentistry, School of Dentistry, Seoul National University, Seoul, Korea.
- Corresponding Author: Woo Cheol Lee. Department of Conservative Dentistry, School of Dentistry, Seoul National University & Dental Research Institute, 28 YunGun-Dong, JongNo-Gu, Seoul, Korea. 110-749, Tel: 82-2-2072-1634, Fax: 82-2-2072-3859, jimin525@snu.ac.kr
• Received: September 23, 2008 • Revised: October 21, 2008 • Accepted: October 23, 2008
Copyright © 2008 The Korean Academy of Conservative Dentistry
- 1,000 Views
- 4 Download
Abstract
- Microorganism survived in the root canal after root canal cleaning and shaping procedure is a main cause of root canal treatment failure. There are several mechanisms for the bacteria to survive in the root canal after chemomechanical preparation and root canal irrigation. Bacteria organized as biofilm has been suggested as an etiology of persistent periapical lesion. Recent studies were focus on removal of Enterococcus faecalis biofilm due to the report that the persistence of this bacteria after root canal treatment may be associated with its ability to form biofilm. Several investigations demonstrated that current root canal treatment protocol including use of NaOCl, EDTA and Chlorhexidine as irrigants is quite effective in eliminating E. faecalis biofilm. However, this microorganism still can survive in inaccessible areas of root canal system and evade host immune response, suppress immune activity and produce biofilm. Up to date, there is no possible clinical method to completely get rid of bacteria from the root canal. Once the root canal treatment failure occurred, and conventional treatment incorporating current therapeutic protocol has failed, periapical surgery or extraction should be considered rather than prolong the ineffected retreatment procedure.
- 1. Imura N, Pinheiro ET, Gomes BPFA, Zaia AA, Ferraz CCR, Sauza-Filho FJ. The outcome of endodontic treatment : A retrospective study of 2000 cases performed by a specialist. J Endod. 2007;33: 1278-1282.ArticlePubMed
- 2. Swartz DB, Skidmore AE, Griffin JA Jr. Twenty years of endodontic success and failure. J Endod. 1983;9: 198-202.ArticlePubMed
- 3. Benenati FW, Khajotia SS. A Radiographic Recall Evaluation of 894 Endodontic Cases Treated in a Dental School Setting. J Endod. 2002;28: 391-395.ArticlePubMed
- 4. Gomes BP, Vianna ME, Sena NT, Zaia AA, Ferraz CCR, Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102: 544-550.ArticlePubMed
- 5. Siqueira JF, Araujo MCP, Garcia PF, Fraga RC, Dantas CJS. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod. 1997;23: 499-502.ArticlePubMed
- 6. Friedman S, Abitbol S, Lawrence HP. Treatment outcome in endodontics : The Toronto study. Phase 1: initial treatment. J Endod. 2003;29: 787-793.ArticlePubMed
- 7. Farzaneh M, Abitbol S, Lawrence HP, Friedman S. Treatment outcome in endodontics : The Toronto study. Phase II: initial treatment. J Endod. 2004;30: 302-309.ArticlePubMed
- 8. Marquis VL, Dao T, Farzaneh M, Abitbol S, Friedman S. Treatment outcome in endodontics : The Toronto study. Phase III: initial treatment. J Endod. 2006;32: 299-306.ArticlePubMed
- 9. de Chevigny C, Dao TT, Basrani BR, Marquis VL, Farzaneh M, Abitbol S, Friedman S. Treatment outcome in endodontics : The Toronto study. Phase 4: initial treatment. J Endod. 2008;34: 258-263.ArticlePubMed
- 10. Strindberg LZ. The dependence of the results of pulp therapy on certain factors. An analytic study based on radiographic and clinical follow-up examinations. Acta Odontol Scand. 1956;14: 1-175.
- 11. Peciuliene V, Balciuniene I, Eriksen HM, Haapasalo M. Isolation of Enterococcus faecalis in previously root-filled canals in a Lithuanian population. J Endod. 2000;26: 593-595.ArticlePubMed
- 12. Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J. 2001;34: 429-434.ArticlePubMedPDF
- 13. Hancock HH, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91: 579-586.ArticlePubMed
- 14. Pinheiro ET, Gomes BPFA, Ferraz CCR, Sousa ELR, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36: 1-11.ArticlePubMedPDF
- 15. Evans M, Davies K, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35: 221-228.ArticlePubMed
- 16. Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85: 86-93.ArticlePubMed
- 17. Novais C, Vital C, Ribeiro G, Coque , Peixe LV. First characterization of vancomycin-resistant enterococci from a Portuguese hospital. J Antimicrob Chemother. 2002;49: 215-217.Article
- 18. Love RM. Enterococcus faecalis--a mechanism for its role in endodontic failure. Int Endod J. 2001;34: 399-405.ArticlePubMedPDF
- 19. Figdor D, Davies JK, Sundqvist G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol Immunol. 2003;18: 234-239.ArticlePubMedPDF
- 20. Rocas IN, Siqueira JF, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004;30: 315-320.ArticlePubMed
- 21. Gomes BP, Pinheiro ET, Sousa ELR, Jacinto RC, Zaia AA, Ferraz CCR, Souza-Filho FJ. Enterococcus faecalis in dental root canals detected by culture and by polymerase chain reaction analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102: 247-253.ArticlePubMed
- 22. Jett BD, Huycke MM, Gilmore MS. Virulence of enterococci. Clin Microbiol Rev. 1994;7: 462-478.ArticlePubMedPMCPDF
- 23. Distel JW, Hatton JF, Gilespie MJ. Biofilm formation in medicated root canals. J Endod. 2002;28: 689-693.ArticlePubMed
- 24. Svensater G, Bergenholtz G. Biofilms in endodontic infections. Endod Topics. 2004;9: 27-36.Article
- 25. Duggan JM, Sedgley CM. Biofilm formation of oral endodontic Enterococcus faecalis. J Endod. 2007;33: 815-818.PubMed
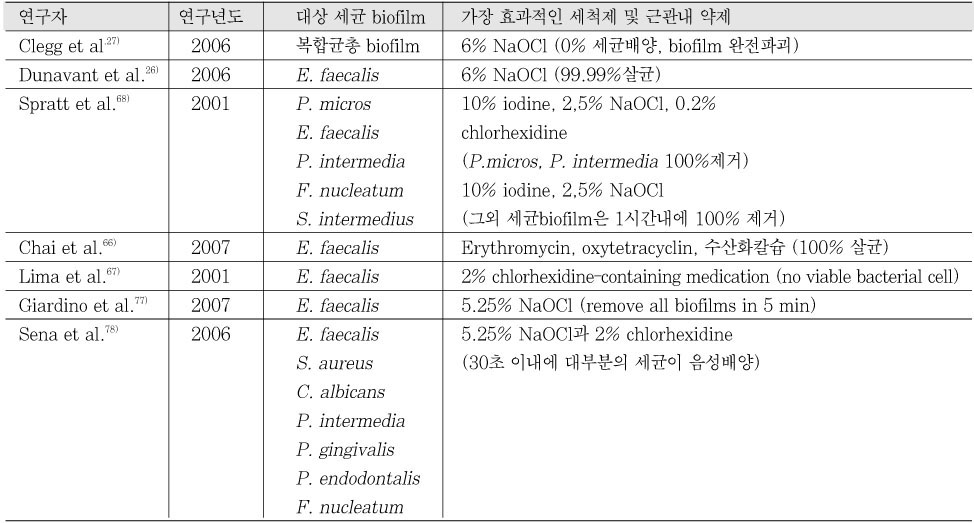
- 26. Dunavant TR, Regan JD, Glickman GN, Solomon ES, Honeyman AL. Comparative evaluation of endodontic irrigants against Enterococcus faecalis biofilms. J Endod. 2006;32: 527-531.ArticlePubMed
- 27. Clegg MS, Vertucci FJ, Walker C, Belanger M, Britto LR. The effect of exposure to irrigant solutions on apical dentin biofilms in vitro. J Endod. 2006;32: 434-437.ArticlePubMed
- 28. Kakehashi S, Stanley HR, Fitzgerald RJ. The Effects of Surgical Exposures of Dental Pulps in Germ-Free and Conventional Laboratory Rats. Oral Surg Oral Med Oral Pathol. 1965;20: 340-349.ArticlePubMed
- 29. Moller AJ, Fabricius L, Dahlen G, Ohman AE, Heyden G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89: 475-484.ArticlePubMed
- 30. Sundqvist G. Bacteriologic studies of necrotic dental pulps. 1976;Umea University; Dissertation.
- 31. Sjogren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30: 297-306.ArticlePubMed
- 32. Lin LM, Pascon EA, Skribner J, Gängler P, Langeland K. Clinical, radiographic, and histologic study of endodontic treatment failures. Oral Surg Oral Med Oral Pathol. 1991;71: 603-611.ArticlePubMed
- 33. Gomes BP, Pinheiro ET, Gade-Neto CR, Sousa ELR, Ferraz CCR, Zaia AA, Teixeira FB, Souza-Filho FJ. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19: 71-76.ArticlePubMed
- 34. Siqueira JF, Rocas IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97: 85-94.ArticlePubMed
- 35. Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31: 1-7.ArticlePubMed
- 36. Ercan E, Ozekinci T, Atakul F, Gul K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: in vivo study. J Endod. 2004;30: 84-87.ArticlePubMed
- 37. Vianna ME, Horz HP, Gomes BP, Conreds G. In vivo evaluation of microbial reduction after chemomechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J. 2006;39: 484-492.ArticlePubMed
- 38. Sassone LM, Fidel R, Fidel S, Vieira Hirata MR. The influence of organic load on the antimicrobial activity of different concentrations of NaOCl and chlorhexidine in vitro. Int Endod J. 2003;36: 848-852.ArticlePubMedPDF
- 39. Siqueira JF, Paiva SSM, Rocas IN. Reduction in the cultivable bacterial populations in infected root canals by a chlorhexidine-based antimicrobial protocol. J Endod. 2007;33: 541-547.ArticlePubMed
- 40. Ringel AM, Patterson SS, Newton CW, Miller CH, Mulhern JM. In vivo evaluation of chlorhexidine gluconate solution and sodium hypochlorite solution as root canal irrigants. J Endod. 1982;8: 200-204.ArticlePubMed
- 41. Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis-contaminated root canals of extracted human teeth. J Endod. 2003;29: 576-579.ArticlePubMed
- 42. Torabinejad M, Cho Y, Khademi AA, Bakland LK. Shahrokh Shabahang The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003;29: 233-239.PubMed
- 43. Bystrom A, Claesson R, Sundqvist G. the antibacterial effect of camphorated para-monochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1: 170-175.ArticlePubMed
- 44. Sjogren U, Figdor D, Spangberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24: 119-125.ArticlePubMed
- 45. Sathorn C, Parashos P, Messer H. Antibacterial efficacy of calcium hydroxide intracanal dressing: a systematic review and meta-analysis. Int Endod J. 2007;40: 2-4.ArticlePubMed
- 46. Haapasalo M, Orstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66: 1375-1379.ArticlePubMedPDF
- 47. Lin YH, Mickel AK, Chogle S. Effectiveness of selected materials against Enterococcus faecalis: part 3. The antibacterial effect of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2003;29: 565-566.ArticlePubMed
- 48. McHugh CP, Zhang P, Michalek S, Eleazer PD. pH required to kill Enterococcus faecalis in vitro. J Endod. 2004;30: 218-219.ArticlePubMed
- 49. Tronstad L, Andreasen JO, Hasselgren G, Kristerson L, Riis I. pH changes in dental tissues after root canal filling with calcium hydroxide. J Endod. 1981;7: 17-21.ArticlePubMed
- 50. Haapasalo HK, Sirén EK, Waltimo TMT, Órstavik D, Haapasalo MPP. Inactivation of local root canal medicaments by dentine: an in vitro study. Int Endod J. 2000;33: 126-131.ArticlePubMed
- 51. Portenier I, Haapasalo H, Rye A, Waltimo T, Ørstavik D, Haapasalo M. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J. 2001;34: 184-188.ArticlePubMedPDF
- 52. Portenier I, Haapasalo H, Ørstavik D, Yamauchi M, Haapasalo M. Inactivation of the antibacterial activity of iodine potassium iodide and chlorhexidine digluconate against Enterococcus faecalis by dentin, dentin matrix, type-I collagen, and heat-killed microbial whole cells. J Endod. 2002;28: 634-637.ArticlePubMed
- 53. Sukawat C, Srisuwan T. A comparison of the antimicrobial efficacy of three calcium hydroxide formulations on human dentin infected with Enterococcus faecalis. J Endod. 2002;28: 102-104.ArticlePubMed
- 54. Cwikla SJ, Belanger M, Giguere S, Progulske-Fox A, Vertucci FJ. Dentinal tubule disinfection using three calcium hydroxide formulations. J Endod. 2005;31: 50-52.ArticlePubMed
- 55. Evans MD, Baumgartner JC, Khemaleelakul S, Xia T. Efficacy of calcium hydroxide: chlorhexidine paste as an intracanal medication in bovine dentin. J Endod. 2003;29: 338-339.ArticlePubMed
- 56. Gomes BP, Souza SF, Ferraz CC. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003;36: 267-275.ArticlePubMedPDF
- 57. Ercan E, Dalli M, Dulgergil CT. In vitro assessment of the effectiveness of chlorhexidine gel and calcium hydroxide paste with chlorhexidine against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102: e27-e31.ArticlePubMed
- 58. Silva Garcez A, Núñez SC, Lage-Marques JL, Jorge AOC, Ribeiro MS. Efficiency of NaOCl and laser-assisted photosensitization on the reduction of Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102: e93-e98.ArticlePubMed
- 59. Eldeniz AU, Ozer F, Hadimli HH, Erganis O. Bactericidal efficacy of Er,Cr:YSGG laser irradiation against Enterococcus faecalis compared with NaOCl irrigation: an ex vivo pilot study. Int Endod J. 2007;40: 112-119.ArticlePubMed
- 60. Bergmans L, Moisiadis P, Huybrechts B, Van Meerbeek B, Quirynen M, Lambrechts P. Effect of photo-activated disinfection on endodontic pathogens ex vivo. Int Endod J. 2008;41: 227-239.ArticlePubMed
- 61. Tronstad L, Barnett F, Cervone F. Periapical bacterial plaque in teeth refractory to endodontic treatment. Endod Dent Traumatol. 1990;6: 73-77.ArticlePubMed
- 62. Larsen T. Susceptibility of Porphyromonas gingivalis in biofilms to amoxicillin, doxycyclin and metronidazole. Oral Microbiol Immunol. 2002;17: 267-271.PubMed
- 63. Ramachandran Nair PN. Light and electron microscopic studies on root canal flora and periapical lesions. J Endod. 1987;13: 29-39.PubMed
- 64. Noiri Y, katsumoto T, Azakami H, Ebisu S. Effects of Er:YAG laser irradiation on biofilm-forming bacteria associated with endodontic pathogens in vitro. J Endod. 2008;34: 826-829.ArticlePubMed
- 65. George S, Kishen A, Song KP. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J Endod. 2005;31: 867-872.ArticlePubMed
- 66. Chai WL, Hamimah H, Cheng SC, Sallam AA, Abdullah M. Susceptibility of Enterococcus faecalis biofilm to antibiotics and calcium hydroxide. J Oral Sci. 2007;49: 161-166.ArticlePubMed
- 67. Lima KC, Fava LRG, Siqueira JF. Susceptibilities of Enterococcus faecalis biofilms to some antimicrobial medications. J Endod. 2001;27: 616-619.ArticlePubMed
- 68. Spratt DA, Pratten J, Wilson M, Gulabivala K. An in vitro evaluation of the antimicrobial efficacy of irrigants on biofilms of root canal isolates. Int Endod J. 2001;34: 300-307.ArticlePubMedPDF
- 69. Chavez de paz L. Redefining the persistent infection in root canals: possible role of biofilm communities. J Endod. 2007;33: 652-662.ArticlePubMed
- 70. Shenker BJ, McArthur WP, Tsai CC. Immune suppression Induced by Actinobacillus actinomycetemcomitans. I. Effects on human peripheral blood lymphocyte responses to mitogens and antigens. J Immunol. 1982;128: 148-154.ArticlePubMedPDF
- 71. Korostoff J, Wang JF, Kieba I, Miller M, Shenker BJ, Lally ET. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect Immun. 1998;66: 4474-4483.ArticlePubMedPMCPDF
- 72. Yoshida H, Jontell M, Sundqvist G, Bergenholtz G. Effect of sonicated material from Fusobacterium nucleatum on the functional capacity of accessory cells derived from dental pulp. Oral Microbiol Immunol. 1995;10: 208-212.ArticlePubMed
- 73. Shenker BJ, Datar S. Fusobacterium nucleatum inhibits human T-cell activation by arresting cells in the mid-G1 phase of the cell cycle. Infect Immun. 1995;63: 4830-4836.ArticlePubMedPMCPDF
- 74. Lee W, Pankoski L, Zekavat A, Shenker BJ. Treponema denticola immunoinhibitory protein induces irreversible G1 arrest in activated human lymphocytes. Oral Microbiol Immunol. 2004;19: 144-149.ArticlePubMed
- 75. Lee W, Lim S, Son H, Bae K. Sonicated extract of Enterococcus faecalis induces irreversible cell cycle arrest in phytohemagglutinin-activated human lymphocytes. J Endod. 2004;30: 209-212.ArticlePubMed
- 76. Shon W, Lim S, Bae K, Baek S, Lee W. The expression of alpha4 integrins by human polymorphonuclear neutrophils in response to sonicated extracts of Enterococcus faecalis. J Endod. 2005;31: 369-372.PubMed
- 77. Giardino L, Ambu E, Savoldi E, Rimondini R, Cassanelli C, Debbia EA. Comparative evaluation of antimicrobial efficacy of sodium hypochlorite, MTAD, and tetraclean against Enterococcus faecalis biofilm. J Endod. 2007;33: 852-855.ArticlePubMed
- 78. Sena NT, Gomes BPFA, Vianna ME, Berber VB, Zaia AA, Ferraz CCR, Souza-Filho FJ. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. Int Endod J. 2006;39: 878-885.ArticlePubMed
REFERENCES
Tables & Figures
REFERENCES
Citations
Citations to this article as recorded by 

Reconsideration of treatment protocol on the reduction of Enterococcus faecalis associated with failed root canal treatment
Reconsideration of treatment protocol on the reduction of Enterococcus faecalis associated with failed root canal treatment
Table 1

 KACD
KACD
 ePub Link
ePub Link Cite
Cite

