Abstract
-
The purpose of this study was to compare the cytotoxicity by MTT test and genotoxicity by Ames test of new calcium phosphate-based root canal sealers (CAPSEAL I, CAPSEAL II) with commercially available resin-based sealers (AH 26, AH Plus), zinc oxide eugenol-based sealers (Tubliseal EWT, Pulp Canal Sealer EWT), calcium hydroxide-based sealer (Sealapex), and tricalcium phosphate based sealers (Sankin Apatite Root Canal Sealer I, II, III).
According to this study, the results were as follows:
The extracts of freshly mixed group showed higher toxicity than those of 24 h set group in MTT assay (p < 0.001).
CAPSEAL I and CAPSEAL II were less cytotoxic than AH 26, AH Plus, Tubliseal EWT, Pulp Canal Sealer EWT, Sealapex and SARCS II in freshly mixed group (p < 0.01).
AH 26 in freshly mixed group showed mutagenicity to TA98 and TA100 with and without S9 mix and AH Plus extracts also were mutagenic to TA100 with and without S9 mix.
Tubliseal EWT, Pulp Canal Sealer EWT and Sealapex in freshly mixed group were mutagenic to TA100 with S9 mix.
Among those of 24 h set groups, the extracts of SARCS II were mutagenic to TA98 with and without S9 mix and AH 26 showed mutagenic effects to TA98 with S9 mix.
No mutagenic effect of CAPSEAL I and CAPSEAL II was detected.
There is no statistically significant difference between CAPSEAL I and CAPSEAL II at MTT assay and Ames test in both freshly mixed group and 24 h set group.
-
Keywords: Root canal sealer; Cytotoxicity; Genotoxicity; Calcium phosphate
I. Introduction
The biocompatibility of root canal sealers is important for the clinical success of endodontic therapy because they may come into direct contact, especially when extruded, with surrounding soft and hard tissues for a prolonged period of time.
Various studies have revealed that eluatable substances or degradation or corrosion products from root canal filling materials may gain access to surrounding tissues (periodontal ligament, alveolar bone) through numerous connections, e.g., dentinal tubules, accessory and lateral canals, and apical foramen
1,
2). Araki et al.
3) investigated the diffusion of
14C-formaldehyde through radicular dentin 72 h after the application of formocresol into the root canal of cat canines. It was found that formaldehyde was distributed from the pulp space into the body.
There are many brands and types of root canal sealers in use today. They may be divided according to their ingredients: zinc oxide-eugenol (ZOE)-based, resin-based, calcium hydroxide-based, glass ionomer-based, and calcium phosphate-based.
For many decades the root canal sealers most frequently used were those based on ZOE, which, despite their satisfactory physico-chemical properties, do not present a favorable biological behavior. The presence of a chronic inflammatory process is observed in apical and periapical tissues after their use leading to tissue injury attributed to the presence of free eugenol, which may act as a cell depressor
4,
5).
Results from these studies showed that endodontic materials possessed both beneficial and undesirable properties. Thus biocompatibility of root canal sealers is as important as physical and chemical features.
The beginnings of the application of calcium phosphate materials as bone substitute or bone graft may be traced to Albee, who reported in 1920 that a triple calcium phosphate compound used in a bony defect promoted osteogenesis or new bone formation
6). Levin et al.
7) reported in 1974 the first dental application of a tricalcium phosphate ceramic in periodontal defects in dogs.
Brown and Chow
8) reported on a self-hardening calcium phosphate cement (CPC) that contained equimolar mixture of finely ground tetracalcium phosphate (TTCP) and dicalcium phosphate anhydrous (DCPA) or dicalcium phosphate dehydrate (DCPD) as the solid phase. When mixed with water, the cement forms hydroxyapatite (HA) as the only end product, which is the major mineral component of tooth and bone. Formation of HA in such mixture does not release acidic or basic by-products. The setting reaction of calcium phosphate cement is Eq. 1:
Because the HA is formed in an aqueous environment, it is more similar to biological apatite than is the HA formed in high temperature processes. Since CPC has a neutral pH and contains only calcium phosphates, it was found to be highly biocompatible and osteoconductive
9).
Gruninger et al.
10) reported that testing cement (containing TTCP, DCPD, HA, and sodium fluoride) was neither toxic nor mutagenic, and performed implants were well tolerated by the animals and no adverse tissue reaction was reported. Hong et al.
11) evaluated the histologic reactions to a calcium phosphate cement composed of TTCP, DCPA or DCPD in the periapical and periodontal tissues. They reported that only a limited inflammatory response to CPC was found after 6 weeks of implantation in the periodontal area, and the bone formation activity and biocompatibility in general were found to be even better in the periapical region in 16 week specimens.
As a result of its ease of use, together with excellent biocompatibility and bone replacing properties, CPC has been investigated for use in a number of medical and dental procedures, including use in reconstruction of frontal sinus and augmentation of craniofacial skeletal defects
12), pulp capping and cavity lining
13,
14), repair of periodontal bone defects
15), and endodontics
16).
In vitro studies and animal models have indicated that it is also useful in endodontics as a sealer in root canal treatment
14,
16,
17). Krell and Wefel
17) reported that CPC as a root canal sealer appeared similar to Grossman's cement sealer in apical and dentinal tubule occlusions. Sugawara et al.
14) showed that CPC had a better sealing ability than Grossman's sealer. In addition to being used as a sealer,
in vitro studies have shown that CPC can also seal a furcation perforation and could be used as an apical barrier for apexification
18). These results suggest that CPC has potential to promote the healing of bone in endodontic treatment.
Recently, we have developed the new calcium phosphate-based root canal sealers (CAPSEAL I, CAPSEAL II) composed of a mixture of TTCP, DCPD and zirconium oxide as solid phase and sodium phosphate buffer as liquid phase, complied with the standard of ISO-6876 (the International Organization for Standardization) applicable to the dental root canal sealing materials. Kim et al. showed the new sealers revealed a lower tissue response in the subcutaneous implantation test
19).
Root canal filling materials are usually in close contact with living tissue. Thus, the biological properties of those materials are important as cytotoxic materials can damage periapical tissues, and material with mutagenic potential can induce DNA mutations, possibly causing malignant transformation of the cells.
In vitro test model to determine the cellular responses is one of the methods for evaluating the biological compatibility of root canal sealers. This has the advantages that many factors and variables can be controlled and the cytotoxicity can be determined with reliability and reproducibility
20).
The short-term Ames test has been recommended as the mutagenesis-screening test for chemicals and environmental samples because of its extensive database and good correlation with carcinogenicity. Also its low cost, simplicity, and speed make the Ames test an important and widespread part of biological examinations of dental materials and of standardization protocols.
The purpose of this study was to compare the cytotoxicity and genotoxicity of newly developed calcium phosphate-based root canal sealers (CAPSEAL I, CAPSEAL II) with another type of commercially available calcium phosphate-based sealers, resin-based sealers, ZOE-based sealers and calcium hydroxide-based sealer using MTT assay and Ames test.
II. Materials and Methods
The root canal sealers used in this study were: new calcium phosphate-based sealers (CAPSEAL I, CAPSEAL II), another commercially available calcium phosphate-based sealers (Sankin Apatite Root Canal Sealer (SARCS) I, SARCS II, SARCS III, Sankin kogyo, Tokyo, Japan), resin-based sealers (AH 26, AH Plus, Dentsply DeTrey, Konstanz, Germany), ZOE-based sealers (Pulp Canal Sealer EWT, Tubliseal EWT, Kerr, Detroit, MI, USA) and calcium hydroxide-based sealer (Sealapex, Kerr, Detroit, MI, USA). Components of CAPSEAL I and CAPSEAL II are listed
Table 1.
1. Cell Culture
L929 mouse fibroblasts were grown in minimum essential medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and antibiotics (Gibco, Grand Island, NY, USA). Cells were cultivated in plastic culture flasks with vented cap at 37℃ in a humidified 5% CO2 containing incubator. Subcultivation was performed on sufficient cultures.
2. Preparation of Test Materials
The commercially available root canal sealers were prepared according to the manufacturer's instructions. Both CAPSEAL I and CAPSEAL II were mixed in 1.5 P/L ratio (g/g). The mixed materials were extracted in cell culture medium (1 g/2 ml) immediately after mixing (fresh mixed group), or after 24 h from mixing (24 h set group), for 24 h at 37℃ in a humidified 5% CO2 containing incubator. Each extracted medium was filter-sterilized through a 0.2 µm filter (Corning Incorporated, Corning, NY, USA).
3. MTT assay
This assay represents the capacity of mitochondrial dehydrogenase in viable cells to convert a yellow water-soluble tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma, St. Louis, MO, USA) into dark blue formazan crystals. 2 × 104 cells in 50 µl culture medium were seeded in flat-bottomed 96-well microplates (Costar, Corning Incorporated, Corning, NY, USA). After overnight attachment, cells were treated with various eluates of sealers (50 µl/well) for 24 h. Then 20 µl MTT solution (5 mg/ml) was added to each well and incubated for 4 h at 37℃. 50 µl dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) was added to each well to dissolve the formazan precipitate, and the plates incubated for 2 h. Subsequently, the absorbance at 570 nm was measured using a microplate spectrophotometer (PowerWave, Bio-Tek Instruments, Inc., Winooski, VT, USA). Intact cells in 50 µl of culture medium served as a control for cell viability.
B. Genotoxicity test
1. Preparation of Test Materials
The commercially available root canal sealers were prepared according to the manufacturer's instructions. Both CAPSEAL I and CAPSEAL II were mixed in 1.5 P/L ratio (g/g).
The mixed materials were extracted in DMSO (0.1 g/2 ml), immediately after mixing (fresh mixed group), or after 24 h from mixing (24 h set group), for 24 h at 37℃ in a humidified 5% CO2 containing incubator. The quantities assayed for each material were 5, 2.5, 1.25 and 0.62 mg/plate. In case of AH 26 and AH Plus, lower concentrations were tested, down to 0.15 mg/plate.
Negative control was solvent control, DMSO. Chemicals used as positive are listed in
Table 2.
2. Ames test
The Ames test was performed as the standard plate incorporation assay on minimal glucose agar (MGA) plates according to Maron and Ames
21).
Two tester strains of Salmonella typhimurium TA98 and TA100 were used to detect frame-shift and base-pair mutations respectively. The overnight culture of the bacteria was performed in nutrient broth Oxoid No. 2 (Oxoid LTD., Hampshire, England) following the standard plate incorporation assay procedure.
The extracts of test sealers and bacterial broth (0.1 ml) were added to 2 ml of molten top agar in sequence with vortexing. Then the contents of the test tubes were poured onto the surface of MGA plates. The bacteria were then incubated at 37℃ for 2 days and revertant colonies were counted. The plates were hand-counted but when the plates had above 100 colonies/plate, these were counted automatically (Chemi-Doc, BioRad, Hercules, CA, USA).
The experiments were carried out in the presence and in the absence of a metabolically active microsomal fraction (S9, Moltox, Annapolis, MD, USA) from rat liver. Tests were run in triplicate for each material's dosage.
C. Statistical analysis
The results from the MTT test and Ames test were analyzed using the Kruskal-Wallis test and Mann-Whitney U test.
III. Results
A. Cytotoxicity test
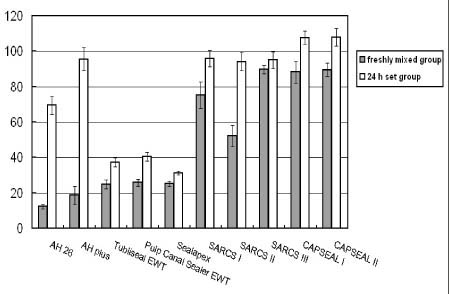
CAPSEAL I and CAPSEAL II were less cytotoxic than AH 26, AH Plus, Tubliseal EWT, Pulp Canal Sealer EWT, Sealapex and SARCS II in case of freshly mixed group (p < 0.01). The extracts of freshly mixed groups were more toxic than those of 24 h set groups (p < 0.001). SARCS II of freshly mixed group showed more cytotoxic effect than other calcium phosphate-based sealers.
AH 26, Tubliseal EWT, Pulp Canal Sealer EWT, and Sealapex of 24 h set groups showed cytotoxic effect (p < 0.05). There is no significant difference between CAPSEAL I and CAPSEAL II in both freshly mixed group and 24 h set group.
Figure 1 shows the cytotoxic effects of test root canal sealers on L929 fibroblasts.
The results of the experiments are presented in
Table 3 to
6. Colonies appearing in the absence of a background lawn were survivors of the killing effect of the test chemical and were not counted as revertants. This happened when most of the bacteria on the plate were killed because of the toxic effect of the test chemical, which allowed the survivors to grow into small colonies by using up the available histidine in the top agar.
AH 26 in freshly mixed group was mutagenic to TA98 and TA100 with and without S9 mix. AH Plus extracts also were mutagenic to TA100 with and without S9 mix (
Table 3). The extracts of Tubliseal EWT, Pulp Canal Sealer EWT and Sealapex were mutagenic to strain TA100 with S9 mix in case of freshly mixed group. The other sealers in freshly mixed group showed no mutagenic response. Pulp Canal Sealer EWT, Sealapex and SARCS II were toxic to TA98 and TA100 at higher doses (
Table 3 and
4). Among the test sealers of 24 h set group, the extracts of SARCS II were mutagenic to TA98 with and without S9 mix and AH 26 showed mutagenic effects to TA98 with S9 mix (
Table 5 and
6). There was no statistically significant difference between CAPSEAL I and CAPSEAL II in both freshly mixed group and 24 h set group.
IV. Discussion
The MTT assay is a colorimetric method for quantifying viable cell numbers. The methyltetrazolium ring is cleaved by mitochondrial dehydrogenase in viable cells to formazan, which has a blue color and can be measured with a spectrophotometer
17). The amount of formazan produced is directly proportional to the total viable cell number over wide range of cell numbers. The MTT assay reflects cell numbers at any stage in their growth cycle. Since dead cells are unable to produce the colored formazan product, this assay can be distinguished from dead cells
22). The advantages of this method are its simplicity, rapidity, and precision, in addition, it does not require radioisotopes.
In this study, the
in vitro test of newly developed calcium phosphate-based root canal sealers (CAPSEAL I, CAPSEAL II) and other commercially available root canal sealers were compared. Clinically, root canal sealers are inserted into the mouth in a freshly mixed and/or incompletely polymerized stage, but even after the setting period, it is still possible that potentially toxic constituents may be released from the materials by leaching into tissue fluids. For these reasons, in current study, cytotoxicity experiments were performed to estimate the cytotoxic potential of diffusible components of the set sealers. Because of different amounts of reactive substances in the fresh and set states, differences can be seen between the toxicity of fresh and set sealers. In a study in which cytotoxicity of eight root canal sealers were evaluated, Matsumoto et al.
23) reported that moderate and strong cytotoxicity was observed in the fresh sealers and definite toxicity was also noted in the set sealers.
Many investigators showed AH 26 had a severe cytotoxic effect
24-
27). These toxic effects of AH 26 could be caused mainly by formaldehyde, which is released primarily during the initial setting reaction
25). And the toxicity of AH 26 may be related to amines that accelerate epoxy polymerization
4).
Cohen et al.
28) reported that AH 26 and AH Plus exhibited severe reactivity by agar diffusion test using L929 cells. Tai et al.
29) also showed that AH Plus was found to be a cytotoxic agent on three different cell lines by MTT test.
On the other hand, Koulaouzidou et al
24) showed that AH Plus indeed exhibited a lower cytotoxic potential compared to AH 26.
Beltes et al.
30) tested the cytotoxicity of two glass-ionomer root canal sealers. Ketac-Endo exhibited a very low cytotoxicity in all experimental periods. It proved to be a very biocompatible material. In contrast to this study, Willershausen et al.
31) reported strong inflammatory reaction for Ketac-Endo, whereas Endion was found to evoke a low increased PGE2 release in all of the cell lines. Kolokuris et al.
32) showed mild inflammatory reaction was observed with Ketac-Endo.
Briseno and Willershausen
33) tested the four calcium hydroxide-based sealers using human gingival fibroblast. Sealapex demonstrated a relatively low cytotoxicity after 3 days of culturing. Recently several researchers investigated the biocompatibility of calcium phosphate-based sealers
34). They concluded that these materials showed mild to moderate inflammatory responses and did not exert any cytotoxic effects.
Leyhausen et al.
35) reported that no genotoxic or mutagenic effects were found with AH Plus. In other
in vitro study, Koulazodou et al.
24) reported that AH Plus exhibited a low cytotoxic potential compared with AH 26. However the results of the current study do not correlate with those obtained by Cohen et al.
28) who evaluated the toxicity of AH Plus and found severe toxicity. In this study, AH 26 and AH plus were severe cytotoxic especially in state of fresh mixed. The Tubliseal EWT and The Pulp Canal Sealer EWT, ZOE-based sealers, were also toxic. Eugenol could inhibit macrophage function and may influence inflammatory reactions in the periapical tissues
36). Eugenol liberation from eugenol-containing compounds is initially high just after mixing. Even after the sealer has set, free eugenol is still available for release over an extended period
37,
38).
The SARCS II especially revealed the most cytotoxic among the calcium phosphate-based sealers in this MTT assay. This reason may be due to the polyacrylic acid and iodoform, which it contains. Polyacrylic acid has a low pH and may leak out gradually to the surrounding tissue during the setting process
39). The CPC containing polyacrylic acid showed an imflammatory response caused by the toxicity of unreacted polyacrylic acid
40). In addition to polyacrylic acid, the poor cellular response of the SARCS II may be attributed to iodoform because the only difference between the SARCS I and the SARCS II is that the SARCS II contains iodoform. Iodoform-based tooth filling paste reportedly cause considerable tissue necrosis and have higher cytotoxicity than ZOE
41).
New calcium phosphate-based sealers (CAPSEAL I, CAPSEAL II) showed acceptable biocompatibility than the ZOE-based sealer, resin-based sealer and calcium hydroxide-based sealer by MTT assay and Ames test. The CAPSEAL does not have polyacrylic acid. The liquid phase of this is sodium phosphate solution instead of polyacrylic acid. Sodium phosphate is already known to show excellent tissue responses
43). It has pH7.4 and enhances hydroxyapatite formation compared with polyacrylic acid. The new sealers contain the Portland cement as one component of the powder phase. The Portland cement and mineral trioxide aggregate (MTA) have similar chemical constitutions, except that MTA contains bismuth oxide
44). Portland cement and white Portland cement have comparable tissue reactions to MTA and no cytotoxic and genotoxic effects
45-
46). CAPSEAL I and CAPSEAL II also had no cytotoxic effects in L-929 fibroblasts and no mutagenic responses in this study.
Although further investigation is needed for the more information on the tissue adaptabilities of CAPSEALs, the results from our study suggest that CAPSEALs have the potential to be used in clinical situations.
V. Conclusion
The purpose of this study was to compare the cytotoxicity and genotoxicity of new calcium phosphate-based root canal sealers (CAPSEAL I, CAPSEAL II) with commercially available resin-based sealers (AH 26, AH Plus), zinc oxide eugenol-based sealers (Tubliseal EWT, Pulp Canal Sealer EWT), calcium hydroxide-based sealer (Sealapex), and tricalcium phosphate based sealers (Sankin Apatite type I, Sankin Apatite type II, Sankin Apatite type III).
According to this study, the results were as follows:
The extracts of freshly mixed group showed higher toxicity than those of 24 h set group in MTT assay (p < 0.001).
CAPSEAL I and CAPSEAL II were less cytotoxic than AH 26, AH Plus, Tubliseal EWT, Pulp Canal Sealer EWT, Sealapex and SARCS II in freshly mixed group (p < 0.01).
AH 26 in freshly mixed group showed mutagenicity to TA98 and TA100 with and without S9 mix and AH Plus extracts also were mutagenic to TA100 with and without S9 mix.
Tubliseal EWT, Pulp Canal Sealer EWT and Sealapex in freshly mixed group were mutagenic to strain TA100 with S9 mix.
Among those of 24 h set groups, the extracts of SARCS II were mutagenic to TA98 with and without S9 mix and AH 26 was shown mutagenic effects to TA98 with S9 mix.
No mutagenic effect of CAPSEAL I and CAPSEAL II was detected.
There is no statistically significant difference between CAPSEAL I and CAPSEAL II at MTT assay and Ames test in both fresh mixed group and 24 h set group.
REFERENCES
- 1. Haikel Y, Wittenmeyer W, Bateman G, Bentaleb A, Allemann C. A new method for the quantitative analysis of endodontic microleakage. J Endod. 1999;25: 172-177.ArticlePubMed
- 2. Jacobson SM, von Fraunhofer JA. The investigation of microleakage in root canal therapy. An electrochemical technique. Oral Surg Oral Med Oral Pathol. 1976;42: 817-823.PubMed
- 3. Araki K, Isaka H, Ishii T, Suda H. Excretin of 14C-formaldehyde distributed systemically through root canal following pulpectomy. Endod Dent Traumatol. 1993;9: 196-199.
- 4. Mittal M, Chandra S, Chandra S. Comparative tissue toxicity evaluation of four endodontic sealers. J Endod. 1995;21: 622-624.PubMed
- 5. Pascon EA, Leonardo MR, Safavi K, Langeland K. Tissue reaction to endodontic materials: methods, criteria, assessment, and observations. Oral Surg. 1991;72: 222-237.ArticlePubMed
- 6. LeGeros RZ. Calcium phosphate materials in restorative dentistry: a review. Adv Dent Res. 1988;2: 164-180.ArticlePubMedPDF
- 7. Levin MP, Getter L, Adrian J, Cutright DE. Healing of periodontal defects with ceramic implants. J Clin Periodontol. 1974;1: 197-205.ArticlePubMed
- 8. Brown WE, Chow LC. A new calcium phosphate setting cement. J Dent Res. 1990;69: 672.PubMed
- 9. Fukase Y, Eanes ED, Tagaki S, Chow LC, Brown WE. Setting reaction and compressive strength of calcium phosphate cements. J Dent Res. 1990;69: 1852-1856.ArticlePubMedPDF
- 10. Gruninger SE, Siew C, Chow LC, O'Young A, Ts'AO NK, Brown WE. Evaluation of the biocompatibility of a new calcium phosphate setting cement[abstract]. J Dent Res. 1984;63: 200.
- 11. Hong CY, Lin SK, Kok SH, Wong MY, Hong VC. Histological reaction to a newly developed calcium phosphate cement implanted in the periapical and periodontal tissues. J Formos Med Assoc. 1990;89: 297-304.PubMed
- 12. Shindo ML, Costantino PD, Friedman CD, Chow LC. Facial skeletal augmentation using hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 1993;119: 185-190.PubMed
- 13. Matsuya Y, Antonucci JM, Matsuya S, Takagi S, Chow LC. Polymeric calcium phosphate cements derived from poly(methyl vinyl ether-maleic acid). Dent Mater. 1996;12: 2-7.ArticlePubMed
- 14. Sugawara A, Chow LC, Tagaki S, Chohayeb H. In vitro evaluation of the sealing ability of a calcium phosphate cement when used as a root canal sealer-filler. J Endod. 1990;16: 162-165.ArticlePubMed
- 15. Sugawara A, Nishiyama M, Kusama K, Moro I, Nishimura S, Kudo I, Chow LC, Takagi S. Histopathological reactions of calcium phosphate cement. Dent Mater J. 1992;11: 11-16.ArticlePubMed
- 16. Chohayeb H, Chow LC, Tsaknis PJ. Evaluation of calcium phosphate as a root canal sealer-filler material. J Endod. 1987;13: 384-387.ArticlePubMed
- 17. Krell KF, Wefel JS. Calcium phosphate cement root canal sealer-scanning electron microscopic analysis. J Endod. 1984;10: 571-576.PubMed
- 18. Chau JY, Hutter JW, Mork TO, Nicoll BK. An in vitro study of furcation perforation repair using calcium phosphate cement. J Endod. 1997;23: 588-592.ArticlePubMed
- 19. Kim JS, Baek SH, Bae KS. In vivo study on the biocompatibility of newly developed calcium phosphate-based root canal sealers. J Endod. 2004;30: 708-711.ArticlePubMed
- 20. Beltes P, Koulaouzidou E, Kotoula V, Kortsaris AH. In vitro evaluating of the cytotoxicity of calcium hydroxide-based root canal sealers. Endod Dent Traumatol. 1995;11: 245-249.PubMed
- 21. Maron DM, Ames B. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113: 173-215.ArticlePubMed
- 22. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65: 55-63.ArticlePubMed
- 23. Matsumoto K, Inoue K, Matsumoto A. The effect of newly developed root canal sealers on rat pulp cells in primary culture. J Endod. 1989;15: 60-67.PubMed
- 24. Koulaouzidou EA, Papazisis KT, Beltes P, Geromichalos GD, Kortsaris AH. Cytotoxicity of three resin-based root canal sealers: an in vitro evaluation. Endod Dent Traumatol. 1998;14: 182-185.ArticlePubMed
- 25. Spangberg L, Langeland K. Biologic effects of dental materials. I. Toxicity of root canal filling materials on HeLa cells in vitro. Oral Surg. 1973;35: 402-414.PubMed
- 26. Spangberg LS, Barosa SV, Lavigne GD. AH 26 releases formaldehyde. J Endod. 1993;19: 596-598.ArticlePubMed
- 27. Huang FM, Tai KW, Chou MY, Chang YC. Cytotoxicity of resin-, zinc oxide eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int Endod J. 2002;35: 153-158.ArticlePubMed
- 28. Cohen BI, Pagnillo MK, Musikant BL, Deutsch AS. An in vitro study of the cytotoxicity of two root canal sealers. J Endod. 2000;26: 228-229.ArticlePubMed
- 29. Tai K, Huang F, Chang Y. Cytotoxic evaluation of root canal filling materials on primary human oral fibroblast cultures and a permanent hamster cell line. J Endod. 2001;27: 571-573.ArticlePubMed
- 30. Beltes P, Koulaouzidou E, Kolokuris I, Kortsaris AH. In vitro evaluation of the cytotoxicity of two glassionomer root canal sealers. J Endod. 1997;23: 572-574.ArticlePubMed
- 31. Willershausen B, Briseno B, Schafer D, Schulze R. Cytotoxicity of root canal filling materials to three different human cell line. J Endod. 2000;26: 703-707.PubMed
- 32. Kolokuris I, Beltes P, Economides N, Vlemmas I. Experimental study of the biocompatibility of a new glass ionomer root canal sealer (Ketac Endo). J Endod. 1996;22: 395-398.ArticlePubMed
- 33. Briseno BM, Willershausen B. Root canal sealer cytotoxicity with human gingival fibroblast III. Calcium hydroxide-based sealers. J Endod. 1992;18: 110-111.PubMed
- 34. Bilginer S, Esener T, Soylemezoglu F, Tiftik AM. The investigation of biocompatibility and apical microleakage of tricalcium phosphate based root canal sealers. J Endod. 1997;23: 105-109.ArticlePubMed
- 35. Leyhausen G, Heil J, Reifferscheid G, Waldmann P, Gerutsen W. Genotoxicity and cytotoxicity of the epoxy resin based sealer AH Plus. J Endod. 1999;25: 109-113.PubMed
- 36. Segura JJ, Jmenez-Rubio A. Effect of eugenol on macropage adhesion in vivo to plastic surfaces. Endod Dent Traumatol. 1998;14: 72-74.PubMed
- 37. Economides N, Ioannis K. Experimental study of the biocompatibility of four root canal sealers and their influence on the zinc and calcium content of several tissues. J Endod. 1995;21: 122-127.ArticlePubMed
- 38. Kolokouris I, Nikolaos E. In vivo comparison of the biocompatibility of two root canal sealers implanted into the subcutaneous connective tissue of rats. J Endod. 1998;24: 82-85.ArticlePubMed
- 39. Yoshikawa M, Hayami S, Tsuji I, Toda T. Histopathological study of a newly developed root canal sealer containing tetracalcium-dicalcium phosphates and 1.0% chondroitin sulfate. J Endod. 1997;23: 162-166.ArticlePubMed
- 40. Takechi M, Miyamoto Y, Ishikawa K, Toh T, Yuasa T, Nagayama M, Suzuki K. Initial histological evaluation of anti-washout type fast-setting calcium phosphate cement following subcutaneous implantation. Biomaterials. 1998;19: 2057-2063.ArticlePubMed
- 41. Wright KJ, Barbosa SV, Araki K, Spangberg LS. In vitro antimicrobial and cytotoxic effects of Kri 1 paste and zinc oxide-eugenol used in primary tooth pulpectomies. Pediatr Dent. 1994;16: 102-106.PubMed
- 42. Yuan H, Li Y, de Bruijn JD, de Groot K, Zhang X. Tissue responses of calcium phosphate cement: a study in dogs. Biomaterials. 2000;21: 1283-1290.ArticlePubMed
- 43. Miyamoto Y, Ishikawa K, Takechi M, Yuasa M, Kon M, Nagayama M, Asaoka K. Non-decay type fast-setting calcium phosphate cement: setting behaviour in calf serum and its tissue response. Biomaterials. 1996;17: 1429-1435.ArticlePubMed
- 44. Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005;38: 834-842.ArticlePubMed
- 45. Ribeiro DA, Duarte MA, Matsumoto MA, Marques ME, Salvadori DM. Biocompatibility in vitro tests of mineral trioxide aggregate and regular and white Portland cements. J Endod. 2005;31: 605-607.ArticlePubMed
- 46. Saidon J, He J, Zhu Q, Safavi K, Spangberg LS. Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95: 483-489.ArticlePubMed
Figure 1Effect of tested root canal sealers by MTT assay. Percentage of absorbance at each concentration compared with that of control was calculated. Each bar represents a mean ± SD.
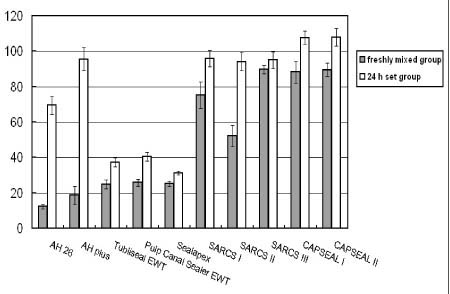
Table 1Composition of CAPSEAL I and CAPSEAL II
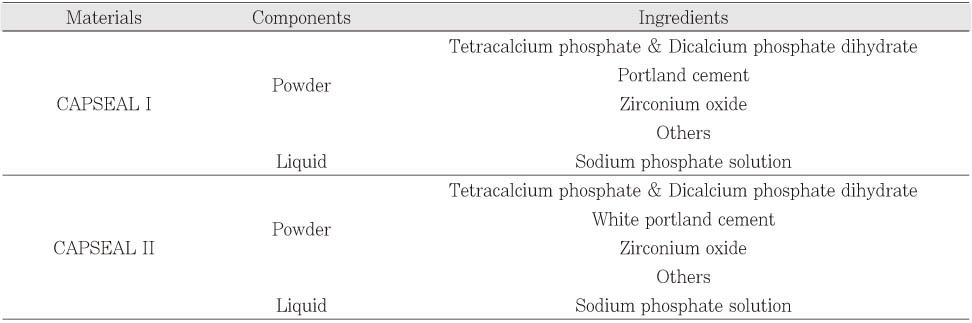
Table 2Positive control chemicals
Table 3Mutagenicity of resin-based, ZOE-based and calcium hydroxide-based sealers in freshly mixd group. The number of colonies are the mean values ± SD of triplicates.
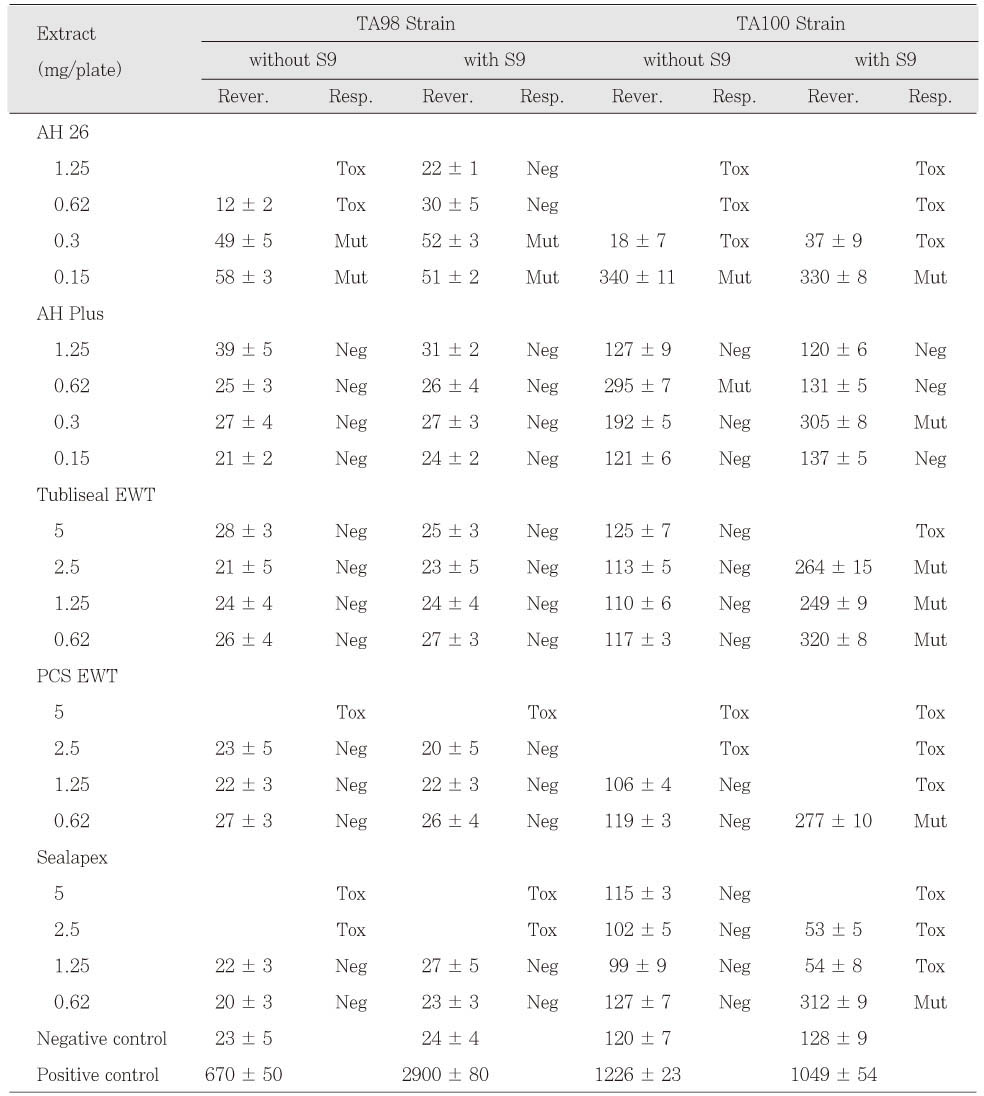
Table 4Mutagenicity of calcium phosphatebased sealers in freshly mixd group. The number of colonies are the mean values ± SD of triplicates.
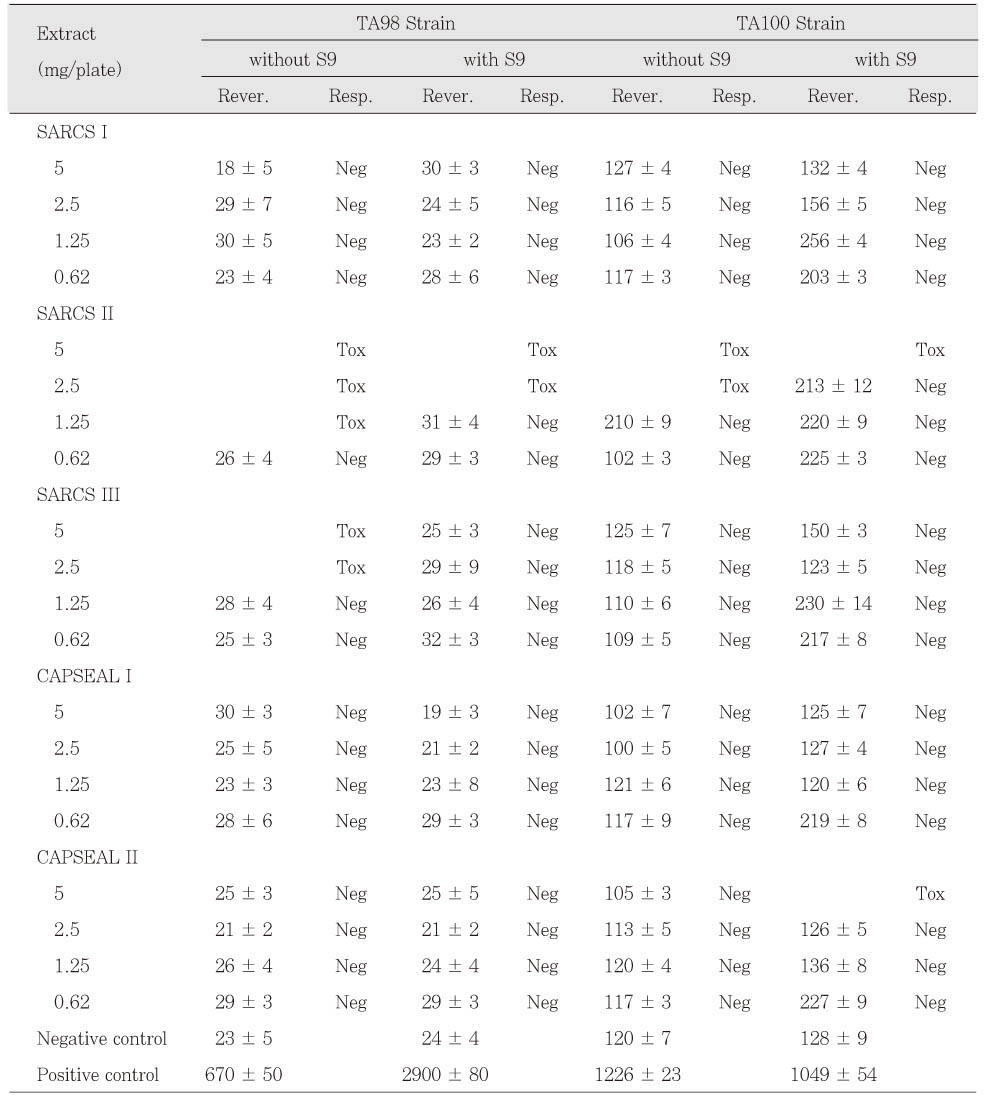
Table 5Mutagenicity of resin-based, ZOE-based and calcium hydroxide-based sealers in 24 h set group. The number of colonies are the mean values ± SD of triplicates.
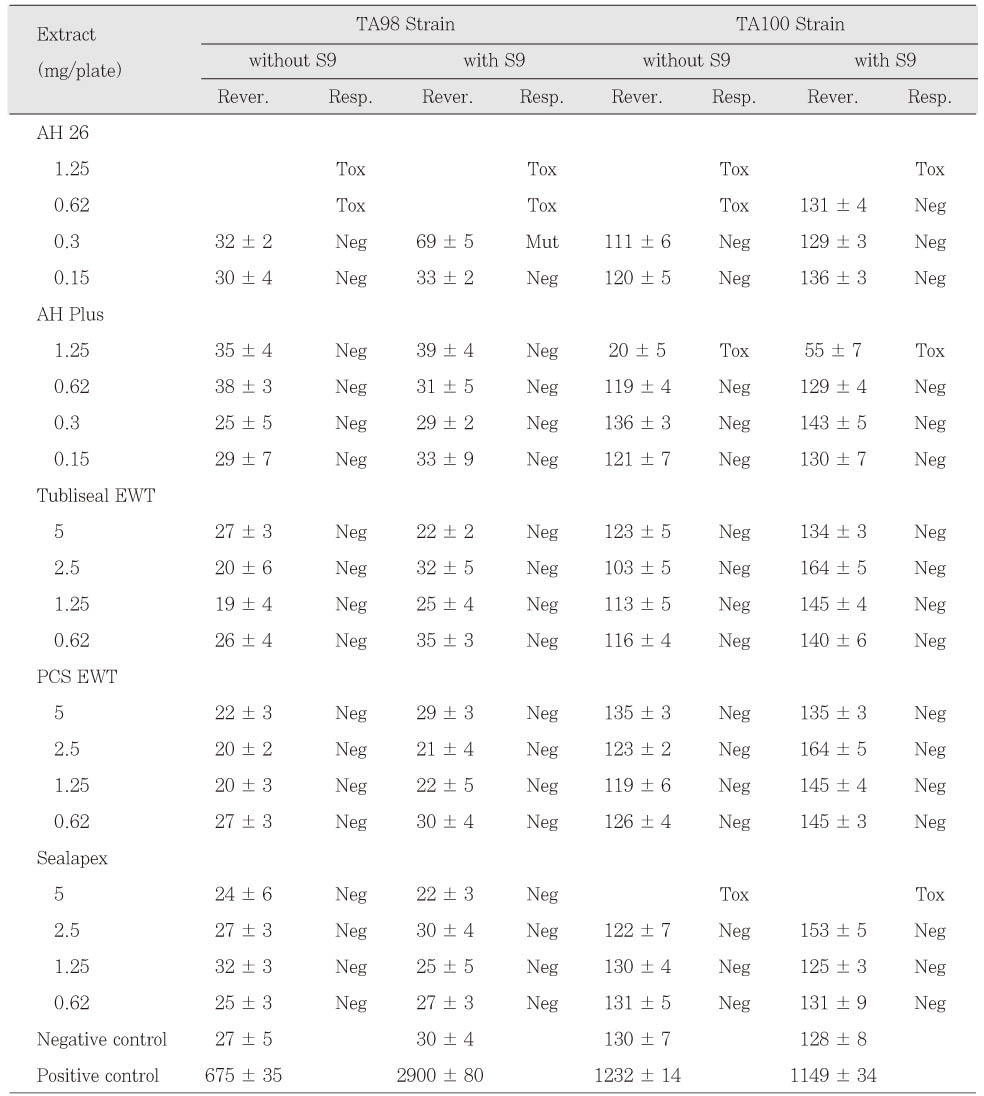
Table 6Mutagenicity of calcium phosphate-based sealers in 24 h set group. The number of colonies are the mean values ± SD of triplicates.
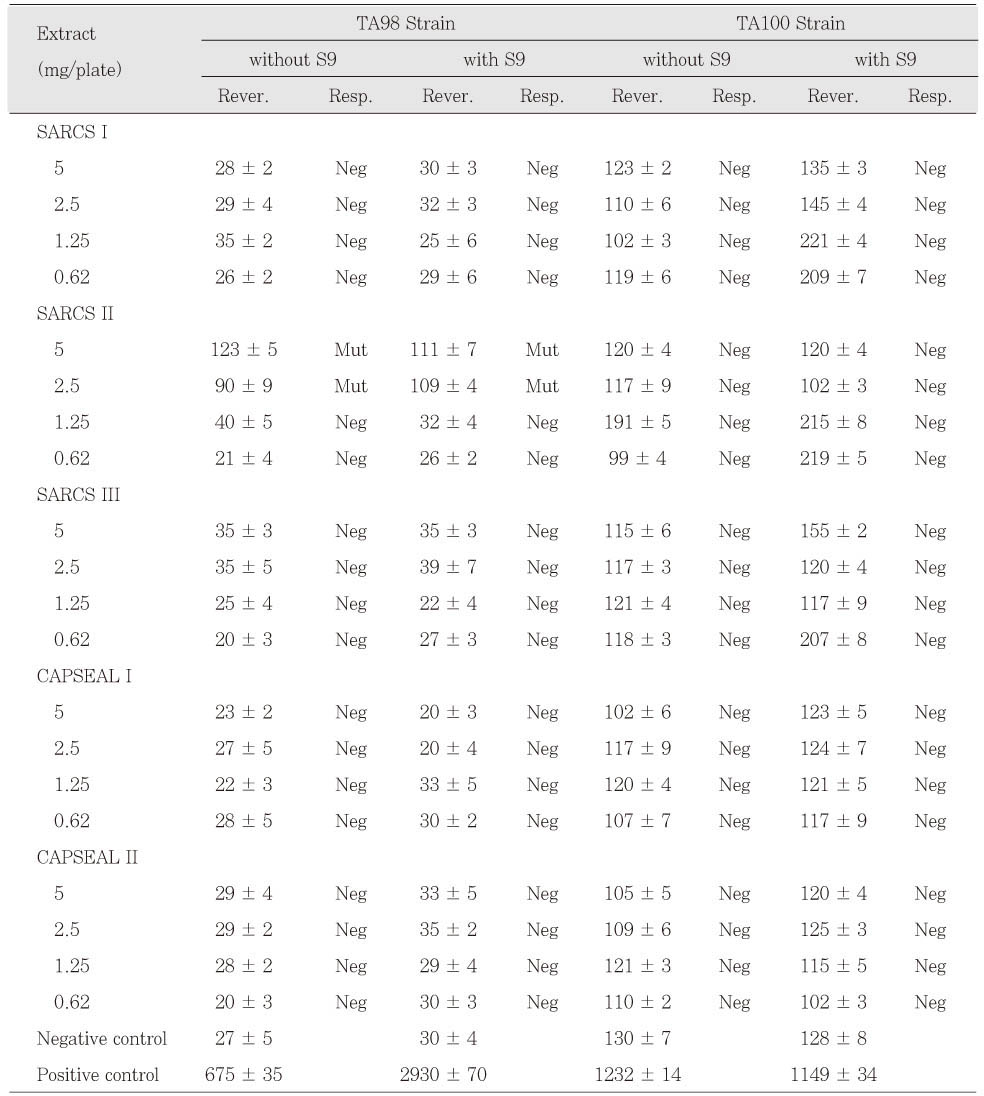





 KACD
KACD




 ePub Link
ePub Link Cite
Cite

