I. INTRODUCTION
Infection of the dental pulp commonly occurs as a consequence of caries. Pulpal infections often progress to pulp necrosis and periapical lesion development with periapical bone destruction. The severity of pulpal/periapical inflammation has been directly correlated with the total microbial content within root canals, and with the length of time the periapical tissues were exposed to the infecting microorganisms
1). Microbial studies of endodontic infections have revealed that gramnegative anaerobic bacteria are the prominent microorganisms
2,
3). Some combinations of bacterial species present in root canals are more pathogenic in inducing periapical inflammation and bone destruction
4,
5). Species of Prevotella, Porphyromonas, Fusobacterium, and Peptostreptococcus are strongly linked to increased periapical destruction
3). Sunqvist et al
5) identified 25 strains of black pigmented Bacteroides in 22 of 72 infected root canals and periapical abscesses. The most common isolate was Bacteroides intermedia, consisting of 14 strains of 25 isolated strains. Van Winkelhoff et al
6) isolated one or more species of Prevotella and Porphyromonas in all abscesses of endodontic origin. P. intermedia was the most frequently isolated strain comprising 63% of the examined abscesses.
Many of the pathogenic effects of the endodontic infections are due to bacterial by-products in addition to the effects of these products on the host-derived soluble mediators such as cytokines, rather than the direct necrotizing effects of the bacteria on the tissues
7). Soluble bacterial components, including cell wall structure, lipopolysaccaride (LPS) and toxins result in the stimulation of specific immune responses characterized by the activation of T- and B-lymphocytes
8). Polymorphonuclear leukocytes are attracted to site of infection by a number of bacteria-derived chemoattractant
9).
The species Prevotella intermedia have been subdivided into the species Prevotella intermedia and Prevotella nigrescens according to the taxonomic changes
10). Four out of fifteen isolates (27%) were re-identified as P. nigrescens, using biochemical methods and SDS-PAGE of whole cell protein analysis tentatively identified as P. intermedia in endodontic infection
11). Baumgartner et al
12) used conventional biochemical methods and molecular biologic methods, using polymerase chain reaction of specific primers for 16S rRNA, to differentiate P. nigrescens from P. intermedia collected from infected root canals. 11 of 22 (50%) of were identifed as P. nigrescens and 8 of 22 (36%) were P. intermedia.
Cytotoxicity of some Gram-negative bacterial species was studied. P. gingivalis ATCC 33277 was strongly cytotoxic to gingival fibroblasts
13). Sonicated bacterial extracts (SBEs) from anaerobic Gram-negative bacteria inhibited the growth of the periapical fibroblasts
14). In vitro testing of cytotoxicity using cell culture has the advantage of controllability, reproducibility, and rapidity compared to in vivo animal study. However, there is evidence that different cell types display varying sensitivities to different test materials
15). The cytotoxicity of growth supernatants from some bacterial species was specific against cells of various origin and from different individuals
13).
One approach to elucidate the role of the blackpigmented bacteria most commonly identifed in infected root canals as developing and progressing pulp/periapcial lesions would be to investigate the toxic effects of metabolic products, cell proteins, and LPS of those bacteria. There has been no study on the cytotoxicity of both P. nigrescens ATCC type strain and clincial isolates of P. nigrescens from infected root canals.
The purpose of this study was to investigate the toxic effects of P. nigrescens ATCC type strain and P. nigrescens isolated from infected root canals having acute signs and symptoms. Cytotoxic effects of bacterial growth medium supernatants, sonicated bacteiral extracts and LPS were analyzed. Two cell lines, MC3T3-E1 and NIH3T3, and human gingival fibroblast were used to compare the possible specific sensitivity of their cytotoxicity against cell types.
II. MATERIALS AND METHODS
1. Bacterial Sampling Procedure
The bacteria had been isolated from the root canals of patients experiencing acute clinical symptoms who were treated at the Dental Clinic at Wonkwang University, College of Dentistry. Patient's symptoms of spontaneous pain, tenderness to percussion, and swelling were recorded. Patients who had taken antibiotic medication within the preceding 3 months were excluded from this study. Briefly, the tooth was isolated using a rubber dam and disinfected. After access cavity was opened, sterile paper points were introduced into the canal and then placed in 1 ml of reduced transfer fluid.
2. DNA Extraction and PCR Amplification for Identification of P. nigrescens
Pure cultures of bacteria from blood agar plates were collected into a microcentrifuge tube and washed three times with distilled water. Cells were centrifuged for 10min at 7,500rpm and supernatant was discarded. Genomic DNA was isolated using a DNeasyTM Tissue Kit (Qiagen, Germany) The resulting pellets were stored at -20℃ before PCR amplification. PCR oligonucleotide primers were developed from published data
16) for the 16S rRNA gene of P. nigrescens ATCC 33563 (sense primer: GTGTTTCATTGACGGCATCCGATATAGAAC, antisense primer: CCACGTCTCTGTGGGCTGCGA). Cycling conditions were at 94℃ for 5min, 30 cycles at 94℃ for 1min, 65℃ for 1min, and 72℃ for 1min; and ending with 72℃ for 5min. PCR products of ~828 bps were confirmed by 1.2% agarose gel electrophoresis, visaulized by ethidium bromide fluorescence and photographed. Amplicon size was analyzed by comparison to a 1kb DNA ladder (Promega, Madison, USA).
Six bacteria isolated and identified as P. nigrescens from teeth with acute symptoms and P. nigrescens ATCC type strain were included. These bacteria were grown in BM broth containing 1% tryptone (Difco, Detroit, MI, USA), 1% proteose peptone (Difco), 0.5% yeast extract (Difco), 0.5% NaCl, 5µg/ml of hemin, and 0.5µ/ml of menadione in an anaerobic chamber (N2, 90%; H2, 5%; CO2, 5%, Sheldon Manufacturing Inc. USA ) at 37℃.
4. Bacterial Growth Medium Supernatants
Bacteria from blood agar plates were grown in BM broth for 72 hrs at 37℃ in an anaerobic condition. The optical density (OD) of bacteria was measured at 650nm. 500µl (OD 1.0) of each bacterial suspension was added to tubes containing 3 ml BM medium and allowed to grow for 72hrs at 37℃. The bacterial suspensions were centrifuged at 3000rpm for 15min. The supernatant fluids were filter-sterilized through 0.22-µm membranes (Milipore, USA). Supernatants were immediately frozen, stored at -80℃ before they were used in cytotoxicity tests.
5. Sonicated Bacterial Extracts
The bacterial cells were harvested by centrifugation (3,000rpm) for 30min then washed three times with PBS. The concentrated cell suspensions were sonicated thirty times in an ice box with bursts from a sonifier (Bio-Rad, USA) for 1 min to prevent heat generation, and the supernatants were then recovered. The SBE preparation was sterilized with a syringe filter (0.22µm). The protein contents of SBEs were determined by the Bradford Protein Assay (Bio-Rad Laboratories, USA). The SBE was diluted with PBS to a final concentration of 2.5µg of protein/ml and stored at -80℃ until used.
6. LPS Purification
LPS was extracted from 1g wet weight of Prevotella nigrescens (ATCC 33536) and clinical isolates using the Eidhin and Mouton method
17). Broth cultures were harvested by centrifugation and washed in 1/10 volume of a wash buffer (20mM Tris-HCl, pH 7.4, 0.15M NaCl, 10mM MgCl
2) to remove any residual medium. The bacteria were then reharvested, lyophilized and ground to a powder in a mortar. Twenty-five milligrammes of powder were placed in a microcentrifuge tube, and mixed with 1ml of deionised water. The tube was then placed in a boiling water bath for 15min, with vortexing at 5-min intervals. The cellular debris was then collected by centrifugation at 12,000×g for 5min, and the supernatant removed to a fresh tube. Proteinase K (1mg dissloved in 50µl water) was then added, and the tube incubated for 1hr at 60℃. The tube was then placed in boiling water for 5min to precipitate any residual proteinase K and centrifuged as before. The supernatant was dialysed against water overnight, subjected to a further round of precipitation and centrifugation, and the resulting supernatant lyophilised. The final powder was weighed in order to calculate the yield of crude LPS and redissolved to a final crude LPS concentratrion of 5mg/ml in phosphate buffered saline (PBS).
To confirm the purity of LPS preparations, samples were subjected to sodium dodecyl sulfate polyacrylamide gel electophoresis (SDS-PAGE) with Tris-buffer using 12% gels. LPS samples suspended in PBS were heated in SDS sample buffer at 100℃ for 5min. Gel electrophoresis was carried out at 90-100V through the resolving gel. The current was stopped when the dye front reached the bottom of the gel. Gels were silver stained to confirm the presence of step-ladderlike LPS bands on the gels using Bio-Rad silver stain kit (Bio-Rad Laboratories, CA, USA).
8. Cell Culture
Human gingival fibroblast was obtained from a patient undergoing elective periodontal surgery. Clinically, the healthy tissue appeared firm, non-erythmatous, non-edematous, and non-bleeding. The tissue section was washed several times with a complete culture medium α-MEM (Gibco, Grand Island, NY), 10% fetal bovine serum (Gibco) supplemented with penicillin G sodium (100 units/ml), sreptomycin sulfate (100µg/ml), and amphotericin B (0.25µg/ml). Gingival tissue was minced with a blade into small pieces and transferred into a 60mm2 culture dish (Nunc, Roskilde, Denmark). Cultures were maintained at 37℃ in a humidified atmosphere of 5% CO2 and 95% air. Confluent cells were detached with 0.25% trypsin and 0.05% EDTA for 5min, and aliquots of separated cells were subcultured. Cell cultures between the third and eighth passages were used in this study.
MC3T3-E1 osteoblast from mouse calvaria and mouse fibroblast (NIH3T3) were used as cell lines. NIH3T3 and MC3T3-E1 were grown in RPMI1640 (Gibco, Grand Island, NY) and α-MEM respectively. Other culture conditions were the same as human gingival fibroblast culture.
9. Cytotoxicity Assay
MTT assay was used to determine cell viability of three cell types exposed to each bacterial preparation. 2×105 cells were seeded in a 96-well tissue culture plate in 100µl of complete culture medium and allowed to attach for 24hr in a 5% CO2 incubator at 37℃. The cells were washed twice with phosphate-buffered saline. Cells were then exposed to various concentrations of each bacterial preparation for 24hrs. The volume of bacterial supernatants was 20µl and 100µl. The same volume of BM broth was used as the control group. The amount of SBE tested was 12.5µg/ml and 25µg/ml. The same amount of PBS was added in the control group. 1.5mg/ml and 2.5mg/ml of LPS was added in the experimental groups and the same amount of PBS was used as control. Five wells were used in each group. Cells were exposed to 50µl of MTT solution (2mg/ml, Sigma) for 4 hours in a CO2 incubator. The blue formazan precipitate was extracted using 100µl of dimethyl sulfoxide on a shaker at room temperature for 30 min. The absorption at 540nm (OD540) was determined using an ELISA reader (Spectra MAX 2500, Molecular Devices, USA). Results of the cytotoxicity experiments were expressed as a percentage of control tissues. Each measurement was performed with five replicates. Changes in cell morphology and detachment from the underlying surface were examined by inverted light microscopy at 400 x magnification.
10. Statistical Analysis
The significance of difference between the control and experimental groups was statistically analyzed by one-way ANOVA with the value of statistical sigificance at p<0.05. The significance of difference among three cell types was also anlayzed. Tukey's HSD post-hoc test was used to determine significant differences between group means.
III. RESULTS
1. Identification of P. nigrescens
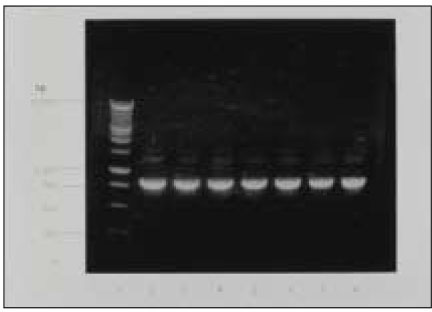
Fig. 1 depicts amplicons from PCR amplification results for colonies of P. nigrescens. Primers of 16S rRNA for P. nigrescens yielded a product of 828-bp.
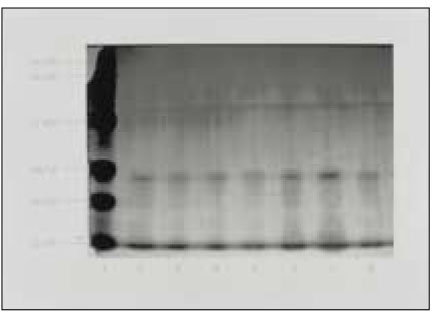
2. Electrophoresis of LPS
Silver-stained SDS-PAGE analysis of LPS prepared from ATCC type strain and clinical isolates revealed a typical LPS step-ladder like banding pattern with minimal protein (
Fig. 2).
Cytotoxicity of bacterial preparations from P. nigrescens ATCC strain and clinical isolates exposed to three cell types was compared to the control groups. The addition of 20µl of BGS to all strains used in this study significantly increased cell viability of MC3T3-E1 osteoblasts (p<0.05,
Table 1). However, the BGS from all strains was significantly (p<0.05) inhibitory at a dosage of 20 µl on NIH3T3 (
Table 2). Cell viability of human gingival fibroblasts was stimulated at the dosage of 20µl BGS of six strains except clinical isolate 2202 (
Table 3). The BGS from all strains significantly (p<0.05) inhibited cell viability of three cell types at a dosage of 100µl (
Table 1,
2,
3).
The SBEs from all strains had no statistically significant cytotoxic effect on MC3T3-E1 at a dosage of 12.5µg/ml. The SBEs from clinical isolates 304, 305, 2202, and 2302 were significantly (p<0.05) inhibitory on these cells at a dosage of 25µg/ml (
Table 4). The SBEs from all strains showed statistically significant cytotoxic effect on NIH3T3 at both dosages of 12.5µg/ml and 25µg/ml (
Table 5). There was no cytotoxic effect on human gingival fibroblasts at the dosage of 12.5µg/ml of SBE. The SBEs from all strains except clinical isolate 203 significantly (p<0.05) inhibited cell viability of human gingival fibroblasts at the dosage of 25µg/ml (
Table 6).
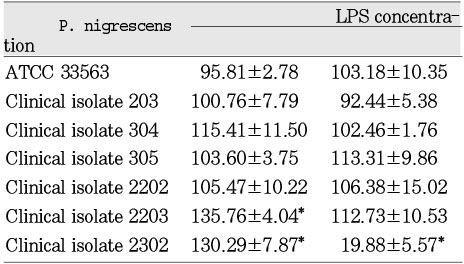
The LPS from P. nigrescens ATCC type strain significantly (p<0.05) inhibited cell viability of MC3T3-E1 at a dosage of 1.5mg/ml. LPS from clinical isolates exhibited no effect on these cells (
Table 7). LPS from clinical isolates 203, 305, 2202, and 2302 was significantly inhibitory on NIH3T3 at the dosage of 1.5mg/ml. The LPS from P. nigrescens ATCC type strain stimulated cell viability of NIH3T3 at the dosage of 1.5mg/ml. The LPS from P. nigrescens ATCC type strain , clinical isolates 203, 305, and 2302 significantly inhibited cell viability of NIH3T3 at a dosage of 2.5mg/ml (p<0.05,
Table 8). The addition of 2.5mg/ml of LPS from clinical isolate 2203 showed a stimulatory effect on NIH3T3. The LPS from clinical isolates 2203 and 2302 showed stimulatory effects on human gingival fibroblast at the dosage of 2.5mg/ml. The LPS from other five strains had no effect on human gingival fibroblast at the dosage of 2.5 mg/ml. The LPS from clinical isolate 2302 had a strong cytotoxic effect on human gingival fibroblast at the dosage of 2.5 mg/ml (p<0.05,
Table 9).
BGSs from seven bacteria had a significantly strong cytotoxic effect on NIH3T3 compared to MC3T3-E1 and human gingival fibroblast at the dosage of 20µl (p<0.05,
Table 1,
2,
3). When compared to BGSs from clinical isolates 304, 305, and 2202, NIH3T3, MC3T3-E1, and human gingival fibroblast showed a significantly different sensitivity of cytotoxicity in descending order at the dosage of 100µl (p<0.05,
Table 1,
2,
3).
SBEs from P. nigrescens ATCC type strain and all of clinical isolates were inhibitory only on NIH3T3 cells at the dosage of 12.5µg/ml, showing that those cells were the most sensitive cell type compared to MC3T3-E1 and human gingival fibroblast (p<0.05,
Table 4,
5,
6).
LPSs from seven strains had no cytotoxic effect on human gingival fibroblasts at both dosages of 1.5µg/ml and 2.5µg/ml except clinical isolate 2302. On the contrary, cell viability of MC3T3-E1 and NIH3T3 was affected by several strains of P. nigrescens at dosages of 1.5mg/ml and 2.5mg/ml showing that gingival fibroblast was the least sensitive in expression of cytotoxicity (
Table 7,
8,
9).
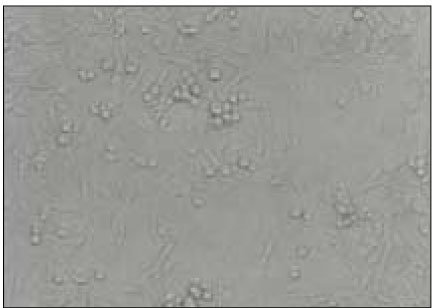
MC3T3-E1 osteoblasts exposed to 100µl of BGS from P. nigrescens ATCC type strain show morphological alteration. Cells exhibited rounded shape and granulations in the cytoplasm (
Fig. 4).
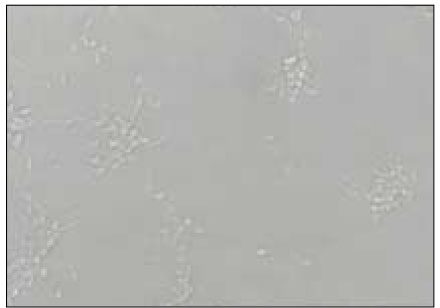
NIH 3T3 fibroblasts exposed to 100µl of BGS from P. nigrescens ATCC type strain lost intercellular connection. Cells did not maintain confluent monolayer showing rounded cellular aggregations (
Fig. 6).
Human gingival fibroblasts exposed to 2.5mg/ml of LPS from clinical isolate 2302 completely lost normal spindle-shaped morphology. Cellular aggregations were observed, and these cells tended to float into the medium (
Fig. 8).
IV. DISCUSSION
Bacterial infection of the pulp is the main etiologic agent of periapical lesion formation with the resorption of bone. Gram-negative black-pigmented anaerobic bacteria are the predominant microorganisms associated with the acute signs and symptoms of periapical lesions
16). P. nigrescens was reclassified from P. intermdia species due to the differing results of multilocus enzyme electrophoretic analysis, DNA analysis, and SDS-PAGE analysis of soluble cellular proteins
19). Bae et al
20) reported that 41 (73.2%) were identified as P. nigrescens and 15 (26.8%) as P. intermedia of the 56 strains of black pigmented Bacteroides (BPB) isolated from endodontic infections. This result suggested that P. nigrescens was the most frequently isolated BPB from infections of endodontic origin.
Van Steenbergen et al
21) observed the filtered culture extracts from B. gingivalis and B. asaccharolyticus were toxic to Vero cells. Shah et al
22) showed the culture supernatant from low speed centrifugation of P. gingivalis culture was cytotoxic on human epithelial cell lines due to the presence of the cysteine proteinase in the supernatant. The BGS from P. nigrescens ATCC type strain and clinical isolates were cytotoxic to all three types of cells tested in this study at a dosage of 100µl. Sensitivity difference among cell types were not observed in that concentration. On the contrary, the addition of 20µl of BGS stimulated cell viability of MC3T3-E1 and human gingival fibroblast, and there was no morphological alterations under the microscopy in those two cell types. These findings of stimulatory effects at a low dosage are consistent with other investigations
23,
24). Cytotoxicity was observed, however, in NIH3T3 culture at a dosage of 20µl, although the inhibitory effect was weaker than at a dosage of 100µl. This data indicates that NIH3T3 cells are more sensitive than other two cell types suggesting this cell type might be used as an early indicator of cytotoxiciy expression for BGS. The BGS contains all meabolic by-products of bacteria. It would seem that their end products are a direct cause of the cytotoxic effect on cells. These cells possibly are affected by the relatively low pH of BGS due to the breakdown ability of sucrose by saccharolytic Prevotella, as well.
The cytotoxicity of SBE was investigated using P. gingivalis and target cell was gingival fibroblast
24,
25). P. gingivalis displayed strong cytotoxic potential causing inhibition of fibroblast growth and morphological changes. Yamasaki et al
14) showed the SBE from P. intermedia ATCC 25611 had no cytotoxic effect on periapical fibroblasts at the dosages of 10µg/ml, 20µg/ml, and 30µg/ml, meaning P. intermedia was less cytotoxic compared with P. endodontalis, P. gingivalis and F. nucleatum. Cell viability of MC3T3-E1 and gingival fibroblast was not affected by 12. 5µg /ml of SBE from P. nigrescens in this study. The cells did not show any morphological change. Cytotoxicity on MC3T3-E1 and gingival fibroblasts was observed at a dosage of 25µg/ml of SBE. However, cytotoxicity of NIH3T3 was observed at both dosages of 12.5µg/ml and 25µg/ml, although there was no significant difference between two dosages. This results are similar to the cytotoxicity of BGS on NIH3T3. It seems that NIH3T3 cells were more sensitive to the toxic effects of BGS and SBE than two other cell types.
Lipopolysaccharides (LPS) can stimulate osteoclastic resorption with relatively low potency
26). The detection rate of LPS was higher in symptomatic teeth or teeth with radiolucent areas than in those without them
27). LPS, purified from several gram-negative bacteria isolated from infected root canals, increased the rate of consumption of the C3 component of the complement
28). LPS can activate B-lymphocytes to secrete antibody of diverse specificity
29). LPS also acts as a potent stimulator of macrophages, inducing them to produce bone-resorptive cytokine, interleukin-1 and tumor necrosis factor
30). Other cell wall components of gram negative bacteria such as a lipoprotein
31), muramyl dipeptide, a fragment of peptidoglycan
32), and lipid A associated protein
33) have been shown to have some biological effects similar to LPS. Human gingival fibroblasts express low levels of interleukin (IL)-6 constitutively and increased levels after stimulation with LPS34). In our experiment MC3T3-E1 cells exposed to 1.5mg/ml of LPS from ATCC type strain showed a cytotoxic effect. The addition of 2.5mg/ml of LPS from type strain, clnical isolates 203, 305, 2202, and 2302 inhibited cell viability of MC3T3-E1. On the other hand, cytotoxicity on NIH3T3 cells was observed at dosages of 1.5mg/ml and 2.5mg/ml from ATCC type strain, clinical isolates 203, 305, and 2302. The cytotoxicity on gingival fibroblasts was shown at a dosage of 2.5mg/ml of LPS from clinical isolate 2302. LPS caused less cytotoxic effects on three types of cells compared to BGS and SBE at those concentration used in this study. There were discrepances of cytotoxicity on three types of cells among the 7 tested P. nigrescens. LPS from clinical isolate 2302 had strong cytotoxicity on three types of cells compared to ATCC type strain and 5 isolates, which was not observed on those cells with the addition of BGS and SBE. Johansson et al35) observed the interstrain discrepancies of cytotoxic effects on human gingival fibroblasts between 3 strains of P. gingivalis, 33277, 381, and W50. They suggested that these discrepancies might be an effect of the genetic variation between serotypes. Further study on the serotypes of P. nigrescens could explain the differences in cytotoxicity according to the strains.
The results from microscopic observation of damaged cells indicated that BGS, SBE, and LPS can cause morphological alterations. Shrinkage of the monolayers was shown instead of normal confluent monolayers. Damaged human gingival fibroblasts lost spindle-shaped morphology and changed into round-shaped cells. Cell rounding was also observed in MC3T3-E1 and NIH3T3 cells. Detachment of the cells from each other and the surface of the cultrue dish due to the loss of intercelluar connection caused cellular aggregations. Cells were easily removed from culture dish and tened to float into culture medium without the use of trypsin-EDTA. These morphological findings are the same as other investigations
21,
24,
25). MC3T3-E1 exposed to BGS at the dosage of 100µl showed rounding deformation of individual cells and displayed granule foramation in cell cytoplasm as well.
There has been variation of expression of cytotoxicity in the choice of cell types. Browne
15) demonstrated that most materials demonstrate greater toxicity when tested with mouse peritoneal macrophages than with BHK C-21 derived fibroblasts. Pissiotis and Spangberg
23) have observed that sonicated extracts of B. gingivalis have a different effect on primary cultures of pulp fibroblasts than to established cell line of murine fibroblast L929. Johansson et al
13) showed P. gingivalis 33277 were strongly cytotoxic on human gingival fibroblasts in comparison cell lines of epithelial origin. In this study, NIH3T3 cells are specifically sensitive to BGS and SBE compared to other two cell types. There was, however, no difference in the expression of cytotoxicity on three cell types exposed to LPS.
The cytotoxicity casued by sonicated bacterial extracts, LPS and enzymes from B. gingivalis was explained by the decrease in growth and proliferation in other investigations
14,
25,36). In this study we observed that each bacterial preparation affected cell viability demonstrating that these preparations could directly affect cells. Moreover, LPS might be one of important factors in the development of pulp/pericapical lesions inducing alveolar bone resorption via production of inflammatory mediators and a variety of cytokines. Future study on the toxicity of P. nigrescens including virulence factors and production of bone resorptive cytokines would be helpful in clarifying the role of P. nigrescens in infections of endodontic origin.
V. CONCLUSION
Gram-negative black-pigmented anaerobic bacteria are the predominant microorganisms associated with acute signs and symptoms of periapical lesions; bacterial components and products are thought to be associated with the pathogenesis of periapical periodontitis. The purpose of this study was to investigate the toxic effets of P. nigrescens ATCC type strain and P. nigrescens isolated from infected root canals having acute signs and symptoms. Cytotoxic effects of bacterial growth medium supernatants, sonicated bacterial extracts and LPS were analyzed using MTT cell viability test. Two cell lines, MC3T3-E1 and NIH3T3, and human gingival fibroblast were used to compare the possible specific sensitivity of their cytotoxicity against cell types. Morphological changes due to cell damage were observed under inverted microscopy.
The bacterial growth medium supernatants significantly inhibited cell viability of the three cell types at a dosage of 100µl (p<0.05). Sonicated bacterial extracts had cytotoxicity on NIH3T3 at both dosages of 12.5µg/ml and 25µg/ml (p<0.05). Cytotoxic effects of LPS on three cell types were different according to the concentration of LPS and strain of bacteria. Shrinkage of the monolayers, cellular aggregations and detachment of cells were evident in severely damaged experimental groups under the microscopy.
These results suggest that P. nigrescens might be one of causative bacteria contributing to the development and progression of periapical lesions via modulation of host response.
REFERENCES
- 1. Korzen BH, Krakow AA, Green DB. Pulpal and periapical tissue responses in conventional and monoinfected gnotobiotic rats. Oral Surg Oral Med Oral Pathol. 1974;37: 783-802.ArticlePubMed
- 2. Farber P, Seltzer S. Endodontic microbiology. I. Etiology. J Endod. 1988;14: 363-371.ArticlePubMed
- 3. Sundqvist G. Taxonomy, ecology, and pathogenicity of the root canal flora. Oral Surg Oral Med Oral Pathol. 1994;78: 522-530.ArticlePubMed
- 4. Sundqvist G. Bacteriological studies of necrotic dental pulps. 1976;Umea, Sweden: Umea university; [thesis].
- 5. Tani-Ishii N, Wang CY, Tanner A, Stashenko P. Changes in root canal microbiota during the development of rat periapical lesions. Oral Microbiol Immunol. 1994;9: 129-135.ArticlePubMed
- 6. van Winkelhoff AJ, Carlee AW, de Graaff J. Bacteroides endodontalis and other black-pigmented Bacteroides species in odontogenic abscesses. Infect Immun. 1985;49: 494-498.ArticlePubMedPMCPDF
- 7. Stashenko P, Teles R, D'Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med. 1998;9: 498-521.ArticlePubMedPDF
- 8. Stashenko P. Regulatory effect of monocytes on T cell proliferative responses to oral microbial antigens. Infect Immun. 1982;38: 938-947.ArticlePubMedPMCPDF
- 9. Torabinejad M, Clagett J, Engel D. A cat model for evaluation of mechanisms of bone resorption: induction of bone loss by simulated immune complexes and inhibition by indomethacin. Calcif Tissue Int. 1979;29: 207-214.ArticlePubMedPDF
- 10. Shah HN, Collins DM. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int J Syst Bacteriol. 1990;40: 205-208.PubMed
- 11. Gharbia SE, Haapasalo M, Shah HN, Kotiranta A, Lounatmaa K, Pearce MA, Devine DA. Characterization of Prevotella intermedia and Prevotella nigrescens isolates from periodontic and endodontic infections. J Periodontol. 1994;65: 56-61.ArticlePubMed
- 12. Baumgartner JC, Watkins BJ, Bae KS, Xia T. Association of black-pigmented bacteria with endodontic infections. J Endod. 1999;25: 413-415.ArticlePubMed
- 13. Johansson A, Bergenholtz A, Holm SE. Strong cytotoxicity to human gingival fibroblasts by Porphyromonas gingivalis ATCC 33277. J Periodont Res. 1996;31: 477-482.Article
- 14. Yamasaki M, Nakata K, Imaisumi I, Iwama A, Nakane A, Nakamura H. Cytotoxic effect of endodontic bacteria on periapical fibroblasts. J Endod. 1998;24: 534-539.ArticlePubMed
- 15. Browne RM. The in vitro assessment of the cytotoxicity of dental materials-does it have a role? Int Endod J. 1988;21: 50-58.Article
- 16. Baumgartner JC, Watkins BJ, Bae K-S, Xia T. Assoication of black-pigmented bacteria with endodontic infections. J Endod. 1999;25: 413-415.PubMed
- 17. Eidhin DN, Mouton C. A rapid method for preparation of rough and smooth lipopolysaccjaride from Bacteroides, Porphyromonas and Prevotella. FEMS Microbiol Lett. 1993;110: 133-138.PubMed
- 18. Sundqvist G, Johansson E, Sjogren U. Prevalence of black-pigmented bacteria with endodontic infections. J Endod. 1989;15: 13-19.PubMed
- 19. Frandsen EVG, Poulsen K, Kilian M. Confirmation of the species Prevotella intermedia and Prevotella nigrescens. Int J Syst Bacteriol. 1995;45: 429-435.ArticlePubMed
- 20. Bae KS, Baumgartner JC, Shearer TR, David LL. Occurrence of Prevotella nigrescens and Prevotella intermedia in infections of endodontic origin. J Endod. 1997;23: 620-623.ArticlePubMed
- 21. van Steenbergen T, Ouden D, Touw JA, Graarr JD. Cytotoxic activity of Bacteroides gingivalis and Bacteroides asaccharolyticus. J Med Microbiol. 1982;5: 253-258.
- 22. Shah HN, Gharbia SE, O'Toole CM. Assessment of the relative cytotoxicity of Porphyromonas gingivalis cells, products, and components on human epithelial cell lines. J Periodontol. 1992;63: 44-51.ArticlePubMed
- 23. Larjava H, Uitto VJ. Effects of extracts from Bacteroides gingivalis, Bacteroides intermedius, and Bacteroides asaccharolyticus on the growth of fibroblast lines obtained from healthy and inflamed human gingiva. Oral Microbiol Immunol. 1987;2: 112-116.ArticlePubMed
- 24. Stevens RH, Hammond BF. The comparative cytotoxicity of periodontal bacteria. J Periodontol. 1988;59: 741-749.ArticlePubMed
- 25. Pissiotis E, Spangberg LSW. Toxicity of sonicated extracts of Bacteroides gingivalis on human pulpal cells and L929 cells in vitro. J Endod. 1991;17: 553-560.ArticlePubMed
- 26. Hausmann E, Weinfeld N, Miller WA. Effects of lipopolysaccharides on bone resorption in tissue culture. Calcif Tissue Res. 1972;9: 272-282.ArticlePubMedPDF
- 27. Schein B, Schilder H. Endotoxin content in endodontically involved teeth. J Endod. 1975;1: 19-21.ArticlePubMed
- 28. Horiba N, Maekawa Y, Yamauchi Y, Ito M, Matsumoto T, Nakamura H. Complement activation by lipopolysaccharides purified from gram-negative bacteria isolated from infected root canals. Oral Surg Oral Med Oral Pathol. 1992;74: 648-651.ArticlePubMed
- 29. Coutingho A, Moller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21: 113-136.PubMed
- 30. Burchett SK, Weaver WM, Westall JA, Larsen A, Kronheim S, Wilson CB. Regulation of tumor necrosis factor/cachecin and IL-1 secretion in human mononuclear phagocytes. J Immunol. 1988;140: 3473-3481.PubMed
- 31. Melchers F, Braun V, Galanos C. The lipoprotein of the outer membrane of Escherichia coli: Ab-lymphocyte mitogen. J Exp Med. 1975;142: 473-482.ArticlePubMedPMCPDF
- 32. Dewhirst FE. N-acetyl muramyl dipeptide stimulation of bone resorption in tissue culture. Infect Immun. 1982;35: 133-137.ArticlePubMedPMCPDF
- 33. Morrison DC, Betz SJ, Jacobs DM. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976;144: 840-846.ArticlePubMedPMCPDF
Fig. 1Agarose gel electrophoresis of PCR products with use of primers from the 16S rRNA gene of P. nigresences. Lane 1 shows 1kb DNA ladder. Lane 2 shows P. nigrescens ATCC 33563. Lanes 3 to 8 show clinical isolates of P. nigrescens; lane 3, 203; lane 4, 304; lane 5, 305; lane 6, 2202; lane 7, 2203;and lane 8, 2302.
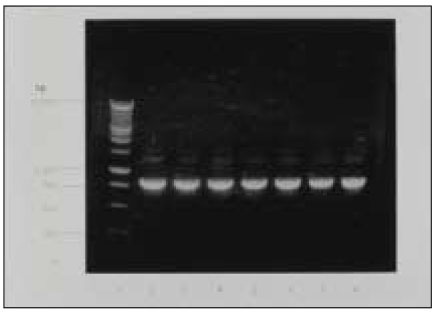
Fig. 2Silver-stained SDS-PAGE of LPS prepared from P. nigrescens. Lane 1 is size marker. Lane 2 shows P. nigrescens ATCC 33563. Lanes 3 to 8 show clinical isolates of P.nigrescens; lane 3, 203; lane 4, 304; lane 5, 305; lane 6, 2202; lane 7, 2203;and lane 8, 2302.
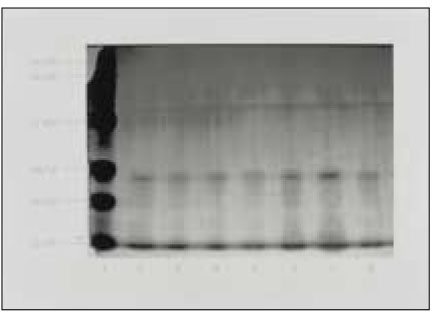
Fig. 3MC3T3-E1 osteoblasts in control culture show normal morphogy (×400).

Fig. 4MC3T3-E1 osteoblasts exposed to 100µl of bacterial growth supernatants from P. nigrescens ATCC type strain show deformation of cell morphology and display granulation of cells (×400).
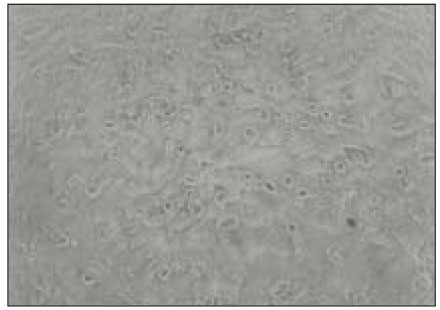
Fig. 5NIH3T3 fibroblasts in control culture show normal morpholgy (×400).

Fig. 6NIH3T3 fibroblasts exposed to 100µl of bacterial growth supernatants from P. nigrescens ATCC type strain became rounded and lost intercellular connection (×400).
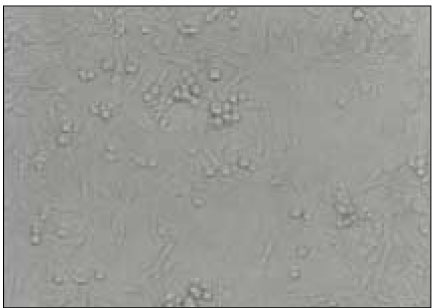
Fig. 7Human gingival fibroblasts in control culture show typical spindle-shaped morphology (×400).

Fig. 8Human gingival fibroblasts exposed to 2.5 mg/ml of LPS from clinical isolate 2302 show severe morphological alteration and cell aggregations (×400).
Table 1Cytotoxicity of P.nigrescens bacterial growth medium supernatants (BGS) to MC3T3-E1 osteoblast using MTT assay (% of control)
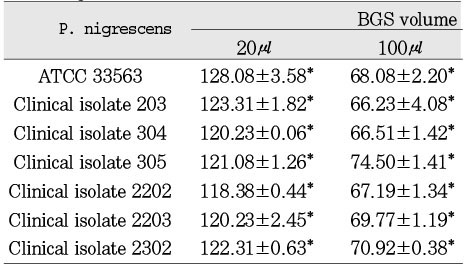
Table 2Cytotoxicity of P.nigrescens bacterial growth medium supernatants (BGS) to NIH3T3 fibroblast using MTT assay (% of control)
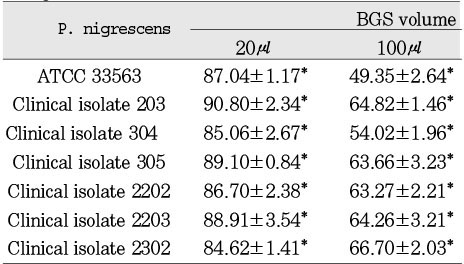
Table 3Cytotoxicity of P.nigrescens bacterial growth medium supernatants (BGS) to human gingival fibroblast using MTT assay (% of control)
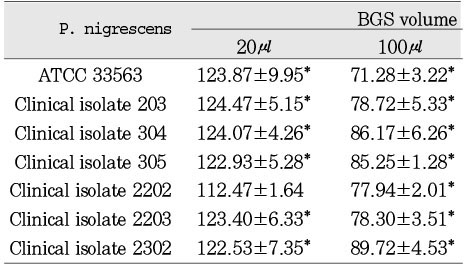
Table 4Cytotoxicity of P.nigrescens of soinicated bacterial extracts(SBE) to MC3T3-E1 osteoblast using MTT assay (% of control)
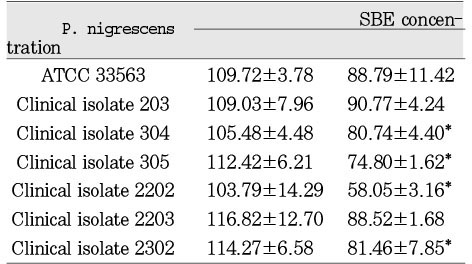
Table 5Cytotoxicity of P.nigrescens of soinicated bacterial extracts(SBE) to NIH3T3 fibroblast using MTT assay (% of control)

Table 6Cytotoxicity of P.nigrescens of soinicated bacterial extracts(SBE) to human gingival fibroblast using MTT assay (% of control)
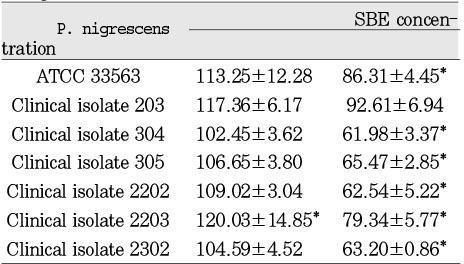
Table 7Cytotoxicity of P.nigrescens LPS to MC3T3-E1 osteoblast using MTT assay (% of control)
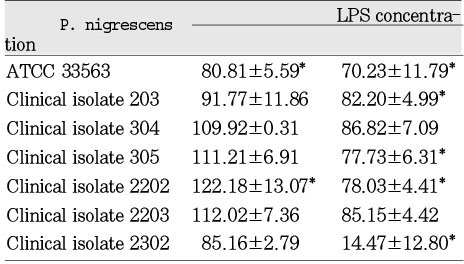
Table 8Cytotoxicity of P.nigrescens LPS to NIH3T3 fibroblast using MTT assay (% of control)
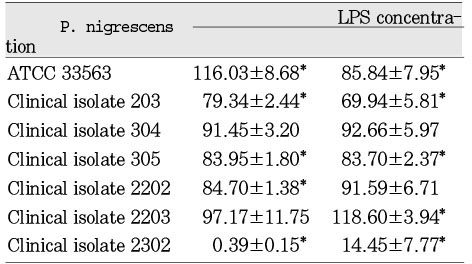
Table 9Cytotoxicity of P.nigrescens LPS to human gingival fibroblast using MTT assay (% of control)
















 KACD
KACD






 ePub Link
ePub Link Cite
Cite

