Search
- Page Path
- HOME > Search
- Biological assessment of a new ready-to-use hydraulic sealer
- Francine Benetti, João Eduardo Gomes-Filho, India Olinta de Azevedo-Queiroz, Marina Carminatti, Letícia Citelli Conti, Alexandre Henrique dos Reis-Prado, Sandra Helena Penha de Oliveira, Edilson Ervolino, Elói Dezan-Júnior, Luciano Tavares Angelo Cintra
- Restor Dent Endod 2021;46(2):e21. Published online March 24, 2021
- DOI: https://doi.org/10.5395/rde.2021.46.e21
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader ePub
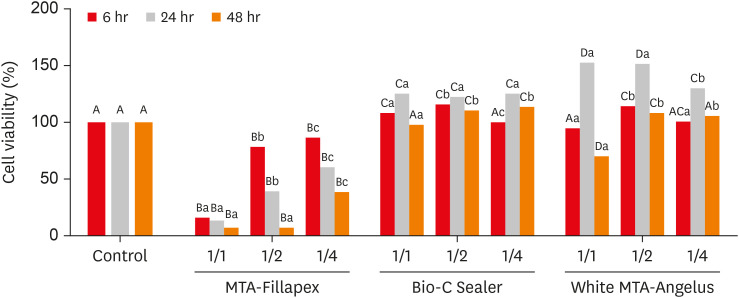
ePub Objectives This study compared the cytotoxicity, biocompatibility, and tenascin immunolabeling of a new ready-to-use hydraulic sealer (Bio-C Sealer) with MTA-Fillapex and white MTA-Angelus.
Materials and Methods L929 fibroblasts were cultivated and exposed to undiluted and diluted material extracts. Polyethylene tubes with or without (the control) the materials were implanted into the dorsa of rats. At 7 days and 30 days, the rats were euthanized, and the specimens were prepared for analysis; inflammation and immunolabeling were measured, and statistical analysis was performed (
p < 0.05).Results MTA-Fillapex exhibited greater cytotoxicity than the other materials at all time points (
p < 0.05). The undiluted Bio-C Sealer exhibited greater cytocompatibility at 6 and 48 hours than white MTA-Angelus, with higher cell viability than in the control (p < 0.05). White MTA-Angelus displayed higher cell viability than the control at 24 hours, and the one-half dilution displayed similar results at both 6 and 48 hours (p < 0.05). At 7 days and 30 days, the groups exhibited moderate inflammation with thick fibrous capsules and mild inflammation with thin fibrous capsules, respectively (p > 0.05). At 7 days, moderate to strong immunolabeling was observed (p > 0.05). After 30 days, the control and MTA-Fillapex groups exhibited strong immunolabeling, the white MTA-Angelus group exhibited moderate immunolabeling (p > 0.05), and the Bio-C Sealer group exhibited low-to-moderate immunolabeling, differing significantly from the control (p < 0.05).Conclusions Bio-C Sealer and white MTA-Angelus exhibited greater cytocompatibility than MTA-Fillapex; all materials displayed adequate biocompatibility and induced tenascin immunolabeling.
-
Citations
Citations to this article as recorded by- Clinical and radiographic assessment of mineral trioxide aggregate with platelet rich fibrin as pulp capping biomaterials: a 12-month randomized trial
Rahma Ahmed Ibrahem Hafiz Abuhashema, Mona El Saied Essa, Shereen Hafez Ibrahim, Omaima Mohamed Safwat
Scientific Reports.2025;[Epub] CrossRef - Biocompatibility and bioactivity of bioceramic endodontic sealer: NeoSealer Flo
Evelin Carine Alves SILVA, Jéssica Arielli PRADELLI, Guilherme Ferreira da SILVA, Paulo Sérgio CERRI, Mario TANOMARU-FILHO, Juliane Maria GUERREIRO-TANOMARU
Journal of Applied Oral Science.2025;[Epub] CrossRef - Influence of photoactivation on tissue response to different dyes used in photodynamic therapy and laser ablation therapy
Luciano Tavares Angelo Cintra, Cristiane Cantiga-Silva, Henrique Augusto Banci, Flávio Duarte Faria, Nathália Evelyn da Silva Machado, Carolina de Barros Morais Cardoso, Pedro Henrique Chaves de Oliveira, Lucas Rodrigues de Araújo Estrela, Gustavo Sivieri
Journal of Photochemistry and Photobiology B: Biology.2024; 251: 112843. CrossRef - Bleaching effectiveness and cytotoxicity of new experimental formulation of niobium-based bleaching gel
Camila de Sousa Caneschi, Francine Benetti, Luiz Carlos Alves de Oliveira, Jadson Cláudio Belchior, Raquel Conceição Ferreira, Allyson Nogueira Moreira, Luís Fernando dos Santos Alves Morgan
Clinical Oral Investigations.2023; 27(4): 1613. CrossRef - Biological investigation of resinous endodontic sealers containing calcium hydroxide
Carlos Roberto Emerenciano Bueno, Francine Benetti, Marina Tolomei Sandoval Cury, Ana Maria Veiga Vasques, Leopoldo Cosme-Silva, Índia Olinta de Azevedo Queiroz, Ana Cláudia Rodrigues da Silva, Rogério de Castilho Jacinto, Luciano Tavares Angelo Cintra, E
PLOS ONE.2023; 18(7): e0287890. CrossRef - Tricalcium silicate cement sealers
Anita Aminoshariae, Carolyn Primus, James C. Kulild
The Journal of the American Dental Association.2022; 153(8): 750. CrossRef - Comparative evaluation of push-out bond strength of bioceramic and epoxy sealers after using various final irrigants: An in vitro study
Chandrasekhar Veeramachaneni, Swathi Aravelli, Sreeja Dundigalla
Journal of Conservative Dentistry.2022; 25(2): 145. CrossRef
- Clinical and radiographic assessment of mineral trioxide aggregate with platelet rich fibrin as pulp capping biomaterials: a 12-month randomized trial
- 2,143 View
- 17 Download
- 7 Web of Science
- 7 Crossref


 KACD
KACD

 First
First Prev
Prev


