Laboratory model to evaluate efficacy of an experimental titanium oxide nanofibers bleaching agent
Article information
Abstract
Objectives
This study aimed to use a laboratory model to evaluate the efficacy of an experimental bleaching agent.
Materials and Methods
The model used human extracted molars that were treated and measured for bleaching efficacy. Teeth (n = 50) were distributed into 5 groups: Negative control (NC): immersion in water for 8 hours; Nanofibers (NFs): Experimental titanium dioxide nanofibers with stirring and light activation for 8 hours; Whitestrips (WS): Crest 3D White Glamorous White Whitestrips, 2 applications daily for 30 minutes, 14 days; 1% hydrogen peroxide (HP) standard: 1% hydrogen peroxide for 8 hours; and 30% HP standard: 30% hydrogen peroxide for 8 hours. Instrumental measurements were performed using a spectrophotometer. Results were recorded at baseline, 1-day post-bleaching, and 1-week post-bleaching. Kruskal-Wallis procedure was used to determine differences in color change. Pearson correlation was used to evaluate the relationship between visual and instrumental measurements. Tests of hypotheses were 2-sided with alpha = 0.05.
Results
There was no significant difference in color parameters (L1, a1, b1, and shade guide units [SGU]) at baseline (p > 0.05). There was a significant difference among the groups for overall color change (ΔE*ab) and change in shade guide units (ΔSGU) at 1-day and 1-week post-bleaching (p < 0.05). The higher the HP concentration, the higher the color change as expressed in ΔSGU and ΔE*ab. The negative control exceeded the perceptibility threshold of ΔE* = 1.2 regardless of time point. NFs showed a decrease in chroma, but were not statistically different compared to the negative control.
Conclusions
The laboratory model was successful in screening an experimental bleaching agent.
INTRODUCTION
Tooth bleaching has gained great popularity due to its high efficacy, safety, and relatively low cost. A tooth bleaching agent at its best should be effective in creating an increase in lightness and decrease in chroma of the tooth color while having minimal side effects. Additionally, since the bleaching agent comes into contact with the teeth for a considerable amount of time, it would be highly desirable to have other beneficial effects such as antimicrobial and pH modulating effects that may favorably affect the oral biofilm and improve oral health [1]. Thus, additional desired effects on plaque by means of bleaching agents may reduce caries rates, improve gingival health, and positively affect oral health care outcomes.
Hydrogen and carbamide peroxide are unequivocally effective tooth bleaching agents that readily penetrate into the tooth structure to interact with chromogens and break them down to lighten the tooth color [2]. Despite their proven effectiveness and minimal side effects, there have been concerns about the use of highly concentrated hydrogen peroxide materials and their potential to induce intense inflammation in the pulp tissue [345]. Thus, the search for new bleaching agents that may also exhibit additional oral health care benefits continues as the oral health care professionals strive to improve oral health care products that may benefit the general public. This has prompted the need for valid and reliable methods to quickly screen for tooth bleaching efficacy evaluation in vitro, prior to undergoing rigorous clinical evaluation and processes for product approval to be marketed.
Dental standards are developed to establish requirements, specifications, and guidelines that can be used consistently to ensure that materials, products, processes and services are fit for their purpose [6]. Thus, standards play an important role in patient health and safety and in the efficacy of dental products that are tested in vitro. An international standard on tooth bleaching, ‘ISO 28399’ describes test methods for laboratory assessment of tooth bleaching efficacy [7]. Despite the fact that it has been used widely, there still remain central issues to be addressed to identify the most reliable ways to measure efficacy, determine the best substrate for specimen preparation, and define how to properly interpret bleaching efficacy results. The ISO 28399 standard includes methods for tooth bleaching efficacy with the use of shade guides and instrumental devices [7]. Yet, even with the use of established recommendations, it is not clear what type of teeth, storage medium, controls, and time points to be used. Furthermore, there is no guide on what cut-off value would be appropriate for an experimental bleaching material to be considered worthwhile for further testing and development.
Based on a study, a bleaching material is deemed not effective when the overall color change (ΔE*ab) is equal or less than 1.2; with increasing effectiveness when between 1.2 and 2.7; 2.7 and 5.4; 5.4 and 8.1; and above 8.1 [8]. The application of perceptibility and acceptability thresholds to interpret bleaching efficacy may be reasonable [9]. Noteworthy though, a systematic review of in vitro studies evaluating tooth bleaching efficacy found that ΔE*ab of negative controls ranged from 2.0 to 3.8 with an estimate of 2.9. If perceptibility and acceptability thresholds were to be used for bleaching efficacy interpretation in vitro, many of the negative controls would be considered erroneously as an effective bleaching material [10]. With the current flux of new bleaching materials, it is highly desirable to evaluate an appropriate bleaching efficacy cut-off value to efficiently screen for effective experimental bleaching agents.
Indeed, there has been an increase of ‘non-hydrogen peroxide’ products such as sodium hypochlorite-based materials that are promoted over the internet and can be purchased over-the-counter. This trend of “non-hydrogen peroxide bleaching” has been partly attributed to the EU Council Directive 2011/84/EU that stated ‘Tooth whitening or bleaching products containing concentrations greater than 0.1% or less than 6% of hydrogen peroxide (HP), are to be only sold to dental practitioners [1112]. Additionally, ozone has attracted interest as it is a highly oxidizing agent rapidly releasing nascent oxygen molecules, capable of participating in diverse bleaching reactions with both organic and inorganic substances [131415]. Titanium dioxide that has been used in the industry for multiple purposes such as cosmetics, inks, special self-cleansing coatings, and air-filtration has also been used in dentistry as a photocatalyst to promote bleaching efficacy of hydrogen peroxide [16]. The transitioning of titanium dioxide particles into nanofibers (TiO2 NFs) has been claimed to allow for more electrons to be formed resulting in greater oxidation potential and the ability to degrade dyes more readily [17].
The purpose of this study was to evaluate a laboratory model that aims to quickly identify the potential of experimental agents as an effective bleaching material. The null hypotheses tested were that, first there would be no difference in bleaching efficacy among the different experimental groups. Second, there would be no correlation between subjective visual and objective instrumental color measurements.
MATERIALS AND METHODS
Sample Selection and Preparation
Extracted sound human third molars (n = 50) were collected and stored in 0.2% sodium azide solution at 4°C. The use of extracted teeth without identifiers was determined to be non-human subject research by the Institutional Review Board of Loma Linda University. All teeth were observed for the absence of developmental anomalies, caries, existing restorations, deep crack lines or severe attrition. The roots were trimmed 3 mm apical to the cemento-enamel junction and the pulp was removed. Teeth were then mounted on the top of a plastic dish with cyanoacrylate adhesive (Super glue liquid, 3M; St. Paul, MN, USA) and further stabilized with acrylic resin on the lingual side. The specimen preparation procedure is illustrated in Figure 1. The mounted specimens were then placed in artificial saliva for 24 hours at room temperature prior to initiating the experiment. Artificial saliva was prepared according to Shellis [18] and replaced weekly throughout the study.
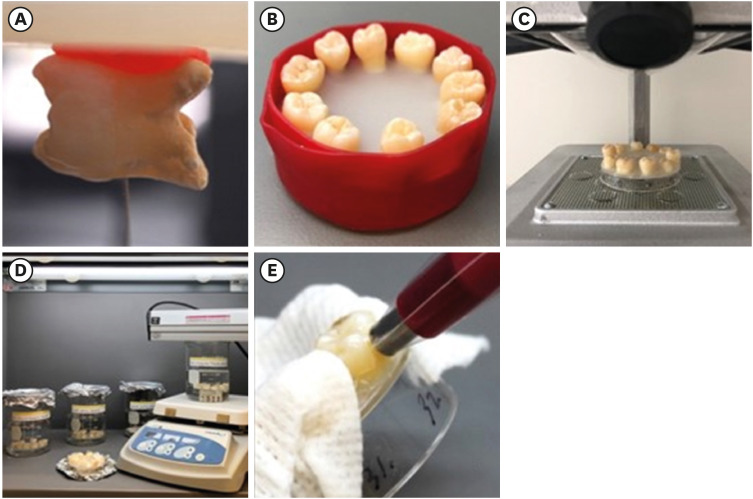
Step-by-step protocol of laboratory model for bleaching efficacy. (A) The roots are trimmed 3 mm apical to the cemento-enamel junction. (B) Teeth are mounted on a plastic dish with cyanoacrylate adhesive and further stabilized with acrylic resin on the lingual side. (C) A custom-fabricated jig is fabricated for repeated color measurements. (D) Teeth of groups NC, 1% HP, and 30% HP are immersed in respective solutions for 8 hours while the NFs group is treated in the TiO2 solution with stirring and UV-light activation. The WS group is treated with whitening strips according to manufacturer's recommendations with 2 applications daily for 30 minutes for 14 days. (E) Instrumental color measurements are performed on the middle third of the buccal surface using a contact type intraoral spectrophotometer. A custom fabricated jig is used for repeated measurements on the same area and a wet gauze is placed to prevent dehydration of the remaining teeth.
1% HP, 1% hydrogen peroxide for 8 hours; 30% HP, 30% hydrogen peroxide for 8 hours; NC, negative control; NF, experimental titanium dioxide nanofibers with stirring and light activation for 8 hours; WS, Crest 3D White Glamorous White Whitestrips, 2 applications daily for 30 minutes, 14 days.
Experimental groups
Teeth were randomly distributed into 5 groups for bleaching in the water of grade 3 (negative control; NC), Experimental 0.5 µg/mL TiO2 NFs solution (NFs) with UV light irradiation at 365 nm (Spectroline; Spectronics Corp., Westbury, NY, USA) for 8 hours, P&G Crest 3D White Glamorous White Whitestrips (WS), 1% hydrogen peroxide standard solution (1% HP), and 30% hydrogen peroxide standard solution (30% HP). The specimens were immersed in the respective bleaching solutions for 8 hours. Hydrogen peroxide standard solutions were prepared from 30% hydrogen peroxide (Sigma-Aldrich, St. Paul, MO, USA) and adjusted to a pH of 7.4 before usage. The WS group was applied according to the manufacturer's recommendations with 2 applications daily for 30 minutes for 14 days. All tests were performed at room temperature at 22°C.
Tooth color change assessment
Three examiners with superior color matching competency (ISO/TR 28642) performed the visual assessment with the VITA Bleachedguide 3D-Master (VITA Zahnfabrik, Bad Säckingen, Germany). The Vita Bleachedguide 3D-Master (BG; VITA Zahnfabrik) has been introduced in 2007, to increase the reliability and validity of visual color assessments by including more whitening shade tabs, uniform distribution between the tabs, and a visually perceivable light to dark value order [19]. The shade guide tabs are available, with each tab marked with odd numbers from 1 to 29, and with “interpolated” even numbers in between. Instrumental color measurements were performed on the middle third of the buccal surface using a contact type intraoral spectrophotometer (VITA Easyshade Compact). A custom fabricated jig was used for repeated measurements on the same area. Measurements were performed under a color-controlled lightening box (MM 4e GTI Mini Matcher; GTI Graphic Technology, Inc., Newburgh, NY, USA) at CIE D65, a color temperature of 6500K and light intensity of ≈ 1,200 lux. Results were expressed by recording L*, a*, and b* values and shade tab numbers at baseline (T1), 1-day post-bleaching (T2), and 1-week post-bleaching (T3). Additionally, the difference of shade guide units (ΔSGU) was calculated for each time interval relative to baseline (ex. ΔSGU2-1 = SGU2-SGU1). The ΔE*ab as measured with the spectrophotometer was expressed as ΔEab* from the Commission Internationale de l'Eclairage [20]. The following equation was used and calculated relative to baseline color parameters (L*1, a*1, b*1).
Statistical analysis
G*Power 3.1.9.4 (Heinrich-Heine Dusseldorf University, Germany) was used to determine the sample size based on a previous study using the following parameters: 80% power, 2.7 effect size, standard deviation (SD) of 1.9 and 5 experiment groups. A minimum sample size of 9 specimens per group was assessed to be appropriate. Measurements for tooth color assessment included L*, a*, b*, ΔSGU, ΔL*, Δb*, and ΔEab*. Kruskal-Wallis procedure was used to determine significant differences in color change among the groups. Friedman's test was used to evaluate differences in color change across the different time points. Correlations between visual and instrumental measurements were assessed with Pearson correlations. Tests of hypotheses were 2-sided with an alpha level of 0.05. Analysis was conducted with SAS v 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
The baseline lightness (L1) of teeth ranged from 78.0 to 97.3 with a mean value of 88.5. Baseline chroma in the yellow-blue ranged from 27.7 to 54.3 with a mean value of 38.8. Baseline shade guide values ranged from 10 to 29 with a mean of 17.7. There was no statistically significant difference in all color parameters among the 5 groups (L1, a1, b1, and SGU1) at baseline (p > 0.05).
Tables 1 and 2 summarize the ΔE*ab and ΔSGU relative to baseline over time by the group. The magnitude of ΔE*ab was based on an increase in lightness and decrease in chroma of a* and b*. The mean ΔE*ab at 1-week post-whitening were 3.05, 4.88, 7.82, 15.64, and 18.03 for NC, NFs, 1% HP, WS, and 30% HP, respectively indicating an increase in color change with increasing HP concentration. There was a statistically significant difference among the 5 groups for ΔE*ab and ΔSGU at 1-day and 1-week post bleaching (p < 0.001). Groups WS and 30% HP had the highest color change regardless of time point and evaluation method and did not differ from each other (p > 0.05). Groups NC and NFs had the smallest color change regardless of time point and evaluation method and did not differ from each other (p > 0.05). However, both groups exceeded the perceptibility threshold of ΔE*ab = 1.2 at both time points and the acceptability threshold of ΔE*ab = 2.7 at 1-week post bleaching.
The ΔE*ab which is a composite of ΔL, Δa, and Δb showed that 30% HP and WS had great color change while the experimental TiO2 NFs' performed somewhere in between the negative control and the 1% HP solution. There was an increase in color change for groups 1% HP, WS, and 30% HP at the 1-week follow-up compared to the 1-day post-bleaching.
The correlation between visual and instrumental measurements 1-week post-bleaching is illustrated as a scatterplot in Figure 2. Overall, a strong and inverse relationship was observed between ΔE*1 and ΔSGU1 (r, −0.742; 95% CI, −0.585 to −0.846; p < 0.001) and between ΔE*2 and ΔSGU2 (r, −0.804; 95% CI, −0.677 to −0.884; p < 0.001).
DISCUSSION
With the diverse preferences of consumers and innovative advances in product development within the field of tooth bleaching, it is important for oral health professionals to develop well-designed in vitro studies that provide evidence to support bleaching efficacy and prompt further testing in vivo. This study was based on a pilot study that introduced a laboratory model with the use of negative control of water and increasing concentrations of hydrogen peroxide solutions (1%, 6%, and 30% HP) to determine a reliable method for bleaching efficacy testing [21]. After bleaching in respective solutions, there was a difference among the 4 groups at all evaluation time points (1-day, 1-week, 2-week, and 6-week post-bleaching) [21]. More important, there was a concentration-dependent response to the bleaching solution regardless of whether measurements were made visually or instrumentally. Thus, the higher the HP concentration, the higher the color change as expressed in absolute values of ΔSGU and ΔE*ab [21]. In order to test the laboratory model, we used a negative control and a low (1%) and high (30%) HP concentration as standards in conjunction with an experimental TiO2 NF bleaching agent and an American Dental Association Seal of Acceptance over-the-counter (OTC) product [22]. A limitation of the in vitro model was that test teeth were immersed in water or hydrogen peroxide solutions, which does not simulate clinical applications.
There is no doubt that to this day, dentists and consumers recognize randomized controlled clinical trials as the gold standard to evaluate the efficacy and safety of a bleaching product. However, these trials are costly and include human subjects, and thus should be preceded by valid and reliable in vitro testing. The control group of a laboratory model consists of elements that present the same characteristics as the experimental group, except for the variable applied to the latter [2324]. Thus, the control group enables the experimental study of one variable at a time and is essential for in vitro study designs. Therefore, for the current study, the negative control of water was included to add rigor to the laboratory model.
Based on the results, we rejected our first null hypothesis. There was a difference in bleaching efficacy among the different groups. At 1-week post bleaching, there was an increase in color change with increasing HP concentration. The overall color change of the negative control group ΔE*ab = 3.05 was comparable to another study that reported a mean of ΔE*ab = 2.6. It was also well aligned with the systematic review of in vitro studies that calculated an estimate of ΔE*ab = 2.9 for negative controls [10]. The value of the negative control deserves significant considerations as it exceeds the perceptibility and acceptability threshold of 1.2 and 2.7, respectively. Thus, by solely relying on these thresholds, even the negative control could be erroneously considered to have a bleaching effect when in fact it may be due to other factors. For instance, extracted teeth are prone to dehydration and special care is required during sample preparation, storage and tooth color assessment to prevent temporary color change. Furthermore, most instrumental color measurements work best on flat surfaces and the inherent curvatures of teeth may affect the accuracy of repeated color measurements which need to be considered. The overall color change of 1% and 30% HP and the Crest Whitestrips were promising in that the values were highly comparable to other studies [2125], indicating that the results are reproducible using the suggested laboratory model. Furthermore, there was an excellent correlation between visual and instrumental color measurements that was also in accordance with another study that evaluated the correlation between the VITA Classic and VITA Bleachedguide with the VITA Easyshade [25].
Titanium dioxide nanoparticles (TiO2 NPs) have been widely used as photocatalysts in low concentration hydrogen peroxide-based products that can be activated with light to accelerate the bleaching process [16]. Interestingly, TiO2 NPs have strong oxidation potential by themselves that can be further enhanced by transitioning into TiO2 NFs [1726]. The laboratory model used in this study successfully served as a quick screening testing to evaluate the experimental TiO2 NFs' bleaching efficacy. It is worthwhile to note that the concentration of 0.5 µg/mL was based on a previous study demonstrating the high oxidation potential of the solution [17]. The overall color change was in between the negative control and the 1% HP solution. The most likely reason for the lack of efficacy may be due to the fact that with the stirring of the experimental solution, the NFs were not in direct contact with the teeth. Thus, in order to enhance performance similar to Whitening Strips, which come as a strip type that allows direct contact to the surface of teeth, there may be a need to develop an enhanced delivery method to improve the efficacy of TiO2 NFs.
CONCLUSIONS
The study evaluated the use of a laboratory model to screen for bleaching efficacy of an experimental TiO2 NFs solution. Since the purpose was quick screening, 2 time points, 1-day and 1-week post bleaching were used. It is not certain how the results may have differed if extended follow-up times were used. Within the limitation of the study, we conclude that the major strength of the model is that it enables quick screening and readily identifies the need for further improvement in the experimental agent. However, the model needs further evaluation on a wider level to test reproducibility and determine a valid threshold for bleaching efficacy assessment. Future studies should also evaluate the relevance or correlation of in vitro bleaching efficacy results to in vivo results.
Notes
Funding: The study was supported by Loma Linda University School of Dentistry Student Research Program.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Tran C, Choi E, Watu B, Kwon S.
Data curation: Oyoyo U.
Formal analysis: Oyoyo U.
Funding acquisition: Tran C, Choi E, Watu B.
Investigation: Tran C, Choi E, Watu B.
Methodology: Tran C, Choi E, Watu B.
Project administration: Kwon S.
Resources: Perry C, Oyoyo U.
Software: Oyoyo U.
Validation: Perry C.
Visualization: Tran C, Choi E, Watu B.
Writing - review & editing: Tran C, Choi E, Watu B, Oyoyo U, Perry C, Kwon S.





