Cryopreservation of mesenchymal stem cells derived from dental pulp: a systematic review
Article information
Abstract
Objectives
The aim of the present systematic review was to investigate the cryopreservation process of dental pulp mesenchymal stromal cells and whether cryopreservation is effective in promoting cell viability and recovery.
Materials and Methods
This systematic review was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the research question was determined using the population, exposure, comparison, and outcomes strategy. Electronic searches were conducted in the PubMed, Cochrane Library, Science Direct, LILACS, and SciELO databases and in the gray literature (dissertations and thesis databases and Google Scholar) for relevant articles published up to March 2019. Clinical trial studies performed with dental pulp of human permanent or primary teeth, containing concrete information regarding the cryopreservation stages, and with cryopreservation performed for a period of at least 1 week were included in this study.
Results
The search strategy resulted in the retrieval of 185 publications. After the application of the eligibility criteria, 21 articles were selected for a qualitative analysis.
Conclusions
The cryopreservation process must be carried out in 6 stages: tooth disinfection, pulp extraction, cell isolation, cell proliferation, cryopreservation, and thawing. In addition, it can be inferred that the use of dimethyl sulfoxide, programmable freezing, and storage in liquid nitrogen are associated with a high rate of cell viability after thawing and a high rate of cell proliferation in both primary and permanent teeth.
INTRODUCTION
Dental pulp, which is constituted by connective tissue, mesenchymal cells, neural fibers, and blood and lymphatic vessels, is located inside dental elements, circled by dentin and contained in a structure known as the pulp chamber [12]. The multiple functions of dental pulp include the production of dentin and its biological and physiological maintenance [2]. Dental pulp mesenchymal stem cells can be obtained from both permanent and primary teeth; however, in primary teeth, they are in a less mature stage, making the process of differentiation easier [2].
Dental pulp mesenchymal stem cells present positive expression for CD44, CD90, CD105, and CD146 surface markers and negative expression for CD34 and CD45 hematopoietic markers, a pattern that classifies them as mesenchymal stem cells or mesenchymal stromal cells [3]. Moreover, the cells that present mesenchymal surface markers have immunomodulatory properties and can differentiate into osteoblasts, chondrocytes, adipocytes and neural cells due to their origin from the same embryonic leaflet from which dental pulp originates [3]. Furthermore, the cells from dental pulp present a higher differentiation potential in odontogenic lineages and are better than bone marrow cells in terms of differentiation phenomena [245].
Dental pulp mesenchymal stem cells have shown multiple applications relevant to dentistry. These cells are able to form mineralized tissues involved in dentin-pulp complex constitution in vivo with assistance from biomaterials, matrix, or carrier materials [16]. Moreover, some studies have demonstrated that these cells can regenerate dental pulp in the presence of an irreversible inflammatory process in rats and humans [789]. It was also found that dental pulp mesenchymal stem cells participated in bone neoformation during reconstruction surgery [10].
Because of the multiple possibilities of stem cell use, the necessity to store them emerged with the goal of maintenance for future applications. With time, these cells can present a reduction in their differentiation potential and genetic alterations due to multiple passages in culture and aging, which implies the need for a rigorous process of storage to prevent or postpone these alterations [1112]. The most widely used method for conservation of these cells has been cryopreservation based on the use of extremely low temperatures with the aim of living tissue maintenance [13]. The freezing process should be carried out very carefully, following certain rules to avoid the formation of ice crystals within the cells, what could be responsible for cell lysis, culture contamination, and reduction of the cell viability rate [14]. One of the main stages involved in successful cryopreservation is previous immersion culture in a mixture of penicillin and streptomycin for disinfection, in laminar flow [12], as well as the use of a cryoprotectant, usually dimethyl sulfoxide (DMSO) [1516], with the goal of reducing sample dissolution and thereby diminishing the probability of ice crystal formation [1115]. These processes are applicable to primary and permanent teeth cells [1517].
However, there currently is no standardized protocol for the cryopreservation of cells from dental pulp. Jesus et al. [18] emphasized that the presence of dental pulp mesenchymal cells in primary teeth is a recent discovery and that, regarding the cryopreservation process, it is necessary to standardize the techniques and procedures employed in order to enhance the results. Thus, the aim of the present study was to perform a systematic review to investigate the process of cryopreservation of human dental pulp stromal mesenchymal cells and whether cryopreservation is effective in promoting cell viability and recovery.
MATERIALS AND METHODS
This systematic review was elaborated according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (www.prisma-statement.org).
PECO question
The research question was determined using the population, exposure, comparison, and outcome (PECO) strategy, as follows: population: dental pulp stromal cells; exposure and comparison: materials and methods of cryopreservation; and outcome: cell recovery and viability. Based on this method, the following research question was established: “Which are the materials and methods of cryopreservation that promote cell recovery and viability of dental pulp mesenchymal stem cells?”
Inclusion and exclusion criteria
Clinical trials with dental pulp from primary and permanent teeth, containing information about extracted teeth disinfection, pulp extraction with mesenchymal stem cell separation, cell proliferation, and cryopreservation process and thawing, in which cryopreservation was performed for at least 1 week, were included in this review. Articles, dissertations, monographs, coursework, and theses published in English and Portuguese without restriction of the year of publication were considered eligible. Case reports, case series, letters to the editor, conference summaries, literature reviews, and animal studies were excluded.
Search strategy
The systematic review was started on December 2018, and the search were performed up to March 2019 at Universidade Federal do Paraná, Curitiba-PR, Brazil. The PubMed, Cochrane Library, Science Direct, LILACS and SciELO electronic databases were searched using the keywords “stem cells” or “mesenchymal cells,” “dental pulp,” “cryopreservation,” and “cell culture” in English and Portuguese. All search terms were indexed in MeSH and there was no individualized strategy for each database. A manual search in the reference lists of the articles included in the review and in the gray literature (Google Scholar and thesis/dissertation databases) was also performed to complement the initial search. Two researchers independently performed the searches and the references were organized using the EndNote X7 software. When additional data and figures for some studies were needed, we contacted the relevant authors.
Article selection and data extraction
Two independent researchers selected articles based on title and abstract analysis (pre-selection), followed by a full-text analysis of the pre-selected articles. The primary outcome sought were the materials and methods used for cryopreservation and the secondary outcome was viability and cellular recovery after the cryopreservation process. The data extraction form was created with the following variables: author/year/country in which the study was performed, study design, type of tooth analyzed (deciduous or permanent), sample size, type of sample storage, steps involved in the process (tooth disinfection, pulp extraction, cell isolation, cell proliferation, cryopreservation, time and thawing), results, and conclusions. Data extraction was also performed independently by two reviewers and divergences of opinion were resolved by consensus between them.
Qualitative analysis
For a methodological quality analysis of the articles, the Joanna Briggs Institute Critical Appraisal Checklist for Quasi-Experimental Studies was carefully adapted and applied [19]. The instrument consists of nine questions: (Q1) Is it clear in the study what is the ‘cause’ and what is the ‘effect’ (i.e., there is no confusion about which variable comes first)?; (Q2) Were the participants included in any comparisons similar?; (Q3) Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest?; (Q4) Was there a control group?; (Q5) Were there multiple measurements of the outcome both pre and post the intervention/exposure?; (Q6) Was follow-up complete and if not, were differences between groups in terms of their follow-up adequately described and analyzed?; (Q7) Were the outcomes of participants included in any comparisons measured in the same way? (Q8) Were outcomes measured in a reliable way?; and (Q9) Was appropriate statistical analysis used? The answers “yes,” “no,” “unclear,” or “not applicable” could be given to each question. The risk of bias was rated high when the study reached up to 49% of “yes” scores, moderate when the study had 50% to 69% of “yes” scores, and low when the study had more than 70% of “yes” scores [1920].
RESULTS
Study selection
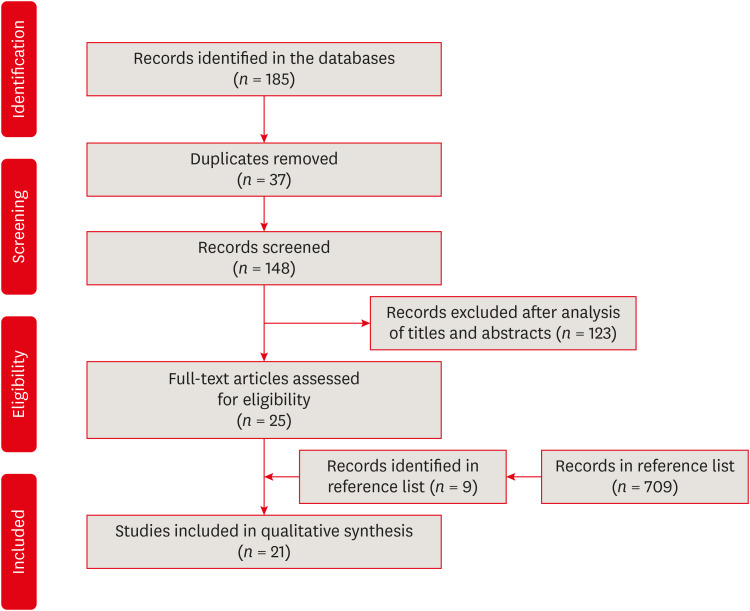
The initial search led to the retrieval of 185 articles: 17 from PubMed, none from Cochrane Library, 141 from Science Direct, 1 from LILACS, none from SciELO, and 26 from the gray literature. After the removal of 37 duplicate articles, the titles and abstracts of 148 articles were analyzed, which led to the exclusion of 123 articles, leaving 25 pre-selected articles for full-text analysis. From the manual search of the reference lists of the selected articles, 9 other papers were identified as eligible, however, they were excluded after full reading. After the full-text analysis, 21 articles were included in the review and qualitatively analyzed [141516172122232425262728293031323334353637] (Figure 1).
Extraction of article data
Table 1 shows the characteristics of the data of the 21 articles included in the review. All studies were randomized controlled trials conducted in 12 different countries, and the publication date ranged from 2006 to 2018. Sixteen articles used permanent teeth [14152122242527283031323334353637] and 5 used primary teeth [1617232629]. The sample size ranged from 3 to 122 teeth and the storage was heterogeneous among the articles: 6 articles used a supplemented culture medium [141617233537], 5 employed some type of sterile saline solution [2527323436], 3 proceeded directly to disinfection [282933] and 6 studies did not specify the type of storage used [152122243031].
Cell isolation was performed by enzymatic digestion in 8 studies [1723262932333437], by the explantation technique in 6 studies [141621252635] and a combination of the 2 techniques was used in 7 studies [15242728303137]. Cell proliferation occurred in culture medium supplemented with nutrients, such as fetal bovine serum or fetal lamb serum, as well as non-essential amino acids, and the cells were stored at 37ºC in a humid atmosphere and a low percentage of CO2 [1415161721222324252728293031323334353637].
Cryopreservation of human dental pulp cells
Table 2 shows the 3 main cryopreservation methods observed in this study. Prior to the cryopreservation process, all samples in the eligible studies were immersed in DMSO for cryoprotection [141516172122232425262728293031323334353637]. Regarding the cryopreservation process itself, the studies by Perry et al. [34], Woods et al. [36], Temmerman et al. [35], Gioventu et al. [23], Ji et al. [16], Lindemann et al. [29], Lee et al. [26], Malekfar et al. [30], Huynh et al. [14], and Mochizuki and Nakahara [31] employed programmable freezing with a cooling rate of −1°C/min to −80°C or −85°C and subsequent transfer to liquid nitrogen for storage. Eight other studies also employed programmable freezing, but with the addition of pauses at set temperatures to promote osmotic balance and reduce the risk of cell lysis. Of these studies, Lee et al. [27], Abedini et al. [21] and Lee et al. [26] employed a fixed cooling rate of −0.5°C/min starting at −5°C, maintained for 15 minutes, with subsequent cooling to −30°C or −35°C and transfer to medium storage at −150°C or −152°C. The work of Han et al. [24] had a greater variation in the cooling rate, as the samples were kept at 1°C for 30 minutes with cooling at −2°C/min to −9°C for 5 minutes, followed by subsequent cooling at −0.3°C/min to −40°C and at −10°C/min to −140°C with transfer to liquid nitrogen for storage. However, the studies by Papaccio et al. [33], Zhang et al. [37], and Munevar et al. [32] performed direct immersion in liquid nitrogen without programmable freezing.
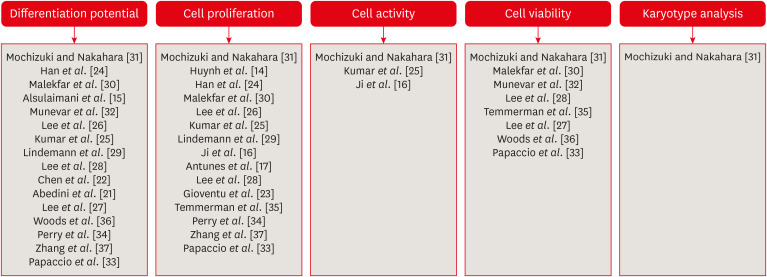
The storage time of the samples ranged from 1 week to 2 years. Defrosting was performed in a 37°C water bath in all studies [1516172122232425262728293031323334353637]. To assess the cryopreservation process, several parameters were measured to confirm stem cell characterization in the various studies. Flow cytometry or immunofluorescence were used to evaluate stem cell surface markers in all selected studies. Other parameters such as differentiation potential, cell proliferation, cell activity, and karyotype analysis were considered by the authors to verify the viability of the mesenchymal stem cells post-thawing (Figure 2).
Regarding the results obtained after the cryopreservation process, the articles by Perry et al. [34], Lee et al. [27], Chen et al. [22], Lee et al. [28], Antunes [17], Munevar et al. [32], Alsulaimani et al. [15], Malekfar et al. [30], Han et al. [24], and Huynh et al. [14] showed cell viability rates ranging from 56.2% to 100% for cryopreserved cells and from 80 to 100% for fresh-cultured cells. The lowest viability rates were presented by Munevar et al. [32], who compared 2 cryopreservation methods used in 2006 and 2007 that are not as efficient as the current ones. However, all of the included articles showed that cryopreserved cells maintained their fibroblastic shape and their differentiation capacity similar to the control group of freshly maintained culture cells [141516172122232425262728293031323334353637].
Risk of bias appraisal
As seen in Table 3, 20 studies showed a low risk of bias [1415172122232425262728293031323334353637] and 1 study showed a moderate risk of bias [16]. No studies were classified as having a high risk of bias. Five studies [1625323334] did not present a negative control group and one study did not make this comparison clear [37]. The study by Ji et al. [16] did not show how multiple measurements of the outcome were made. Question 9 of the Joanna Briggs Institute Critical Appraisal Checklist for Quasi-Experimental Studies was considered not applicable for all studies due to the heterogeneity of the data presented.
DISCUSSION
To prolong the possibility of using dental pulp stromal mesenchymal cells for regenerative procedures, a well-established and standardized cryopreservation process is required to achieve a higher cell viability rate [38]. The process of cryopreservation of dental pulp mesenchymal cells includes some essential steps, such as dental element disinfection, dental pulp extraction, cell isolation, cell proliferation, cryopreservation, time setting, and thawing [3839].
As proposed by Hilkens et al. [38], the first step in the cryopreservation process after obtaining a dental element is disinfection. Many studies presented a disinfection process involving the use of successive immersions of the dental element in phosphate-buffered saline (PBS) in combination with an antibiotic solution [1416242931333537]. These studies showed greater concern regarding the initial stage of access to the dental element, because the dental pulp framework may come into contact with it during cutting and, if not properly disinfected, it may contaminate the resident cells and induce complete loss of that material [1338]. Papaccio et al. [33] and Alsulaimani et al. [15] corroborated the use of chlorhexidine gluconate gel in combination with PBS as a potent dental surface disinfectant. Perry et al. [34] and Woods et al. [36] pointed to the use of iodopovidone and sodium thiosulfate in combination with PBS as sufficient to obtain adequate disinfection. Thus, PBS seems to be an essential element for disinfection, but alone, it is insufficient for the full accomplishment of this task and therefore requires combination with another component. The study by Gioventù et al. [23] was unprecedented in using sterile RPMI 1640 for cleansing and disinfection of the dental element, which would be a new method for this step.
An important point to be considered is the period of time between the tooth extraction and cell isolation and/or cryopreservation. Some studies recommended the use of Hank's balanced salt solution or cell culture medium during transportation [3440] or saline solution with the addition of antibiotics to prevent bacterial infections [14]. There was a time-dependent reduction in the number of pulp stem cells that could be isolated from extracted teeth as the length of time of storage increased [41], and they could remain viable for up to overnight or 12 hours [14].
The extraction of dental pulp is a critical moment because it can induce contamination if not performed properly, and the sterilization of the cutting materials helps to protect against this outcome [13]. Most of studies used a high-speed diamond drill or diamond disk for cutting the dental element at the cementoenamel junction. In general, after cutting, regardless of its shape, the pulp was excised by curettage with endodontic files or periodontal curettes. However, the details of this step at the end of the process do not seem to have an important impact. For instance, Temmerman et al. [35] compared 3 cuts at different heights of the roots of the teeth to verify whether there the region where the mesenchymal cells resided influenced their morphological characteristics and differentiation capacity beyond alterations in the cryopreservation process. Regardless of the region of the cut for pulp removal, the morphological characteristics, differentiation capacity, proliferation rate, and cell viability remained the same, whether in fresh or cryopreserved cultures; only the quantity of cells was variable. Complementarily, Abedini et al. [21], with cuts made along the long axis of the dental element, showed no difference in morphology, differentiation capacity, cell viability, and proliferation rate after cryopreservation, and the results were similar to those of the other studies selected. In addition, Gioventù et al. [23] made only 1 cavity at the height of the cementoenamel junction with a Nd:YAG laser, which could be an alternative to cutting using high rotation instruments.
Cellular isolation is the stage in which mesenchymal cells are detached from each other after pulp tissue removal [13]. It was found that 38% (n = 8) of the selected studies used the enzyme digestion technique, 28% (n = 6) the explantation technique, and 34% (n = 7) used both techniques, showing a lack of consensus on which method is the most appropriate. Studies by Temmerman et al. [35], Abedini et al. [21], Ji et al. [16], Kumar et al. [25], Lee et al. [28], and Huynh et al. [14] adopted only the explantation technique for cell isolation. This decision could be supported by the studies by Salehinejad et al. [42] and Hilkens et al. [38], who claimed that stromal mesenchymal cells obtained from explant-promoted cell isolation are purer, more heterogeneous, suffer less enzymatic damage, and have a higher proliferation rate than those obtained by enzymatic digestion. Other studies presented the use of enzymatic digestion containing collagenase type I and dispase in different concentrations, and Woods et al. [36] used type I and II collagenases associated with thermolysin bound to a neutral protein in place of dispase.
In order for dental pulp mesenchymal cells to be sufficient to carry out the experimental studies, cell proliferation in the culture medium is required [13]. Essentially, the selected studies pointed to the need to immerse these cells in a nutritive culture medium, either Dulbecco's modified Eagle's essential medium (DMEM), minimal essential alpha medium (alpha-MEM) or Mesencult, supplemented with a mixture of antibiotics and fetal bovine or calf serum (FBS or FCS, respectively). Studies prior to 2010 used FCS as a nutrient solution [333537] and, in some cases, the Mesencult medium for culture [3436]. Studies from 2010 onwards used FBS as a nutrient factor and DMEM medium for cell culture combined with a mixture of antibiotics, which is variable in types and quantities, although a combination of penicillin and streptomycin (Pen-Strep) was the most commonly used [151622232428293032333435]. In general, there was a unanimous consensus in the selected papers that, after immersion in the growth solution, the cultures should be stored in a humid incubator at 37ºC containing 5% CO2 for a variable time.
The cryopreservation process itself involves freezing the cells for later use and should only occur after immersion in cryoprotectant to prevent the formation of ice crystals within the cell, which induce plasma membrane lysis and consequent reduction of viability [113]. Except for the study by Mochizuki and Nakahara [31], which explored the formulation of serum-free compounds for cryopreservation, agreement exists on the use of 5% or 10% DMSO as a cryoprotectant for mesenchymal cell cultures. In addition, gradual freezing of cultures with a fixed cooling rate, commonly −1°C/min, has been used in studies from 2008 onwards, and was associated with a noticeable increase in the cell viability rate [14162326293031343536]. Papaccio et al. [33], Zhang et al. [37], and Munevar et al. [32] immersed the cells in liquid nitrogen immediately after cryoprotectant application and presented very low cell viability rates compared to the control group of fresh cells in culture, which proves the need for gradual freezing. There is a consensus in the selected studies that cell cultures should be immersed in liquid nitrogen at −196ºC after gradual freezing in a programmable freezer. The temperatures employed in the cryopreservation process are still debatable and explicit in the studies. There are methodologies in which the temperature has been gradually reduced and set for a few hours at some timepoints [1517212224252728] and others that only used a fixed cooling rate until an ambient temperature of the culture at −80/−85ºC, followed by storage in liquid nitrogen at −196ºC [141626293031343536].
Regarding the results of the selected studies, Papaccio et al. [33], Zhang et al. [37], and Munevar et al. [32] showed lower cell viability and recovery rates, possibly due to the direct storage of cell culture in liquid nitrogen without gradual freezing. Regarding the cell viability rate of the cryopreserved cell group after thawing, Perry et al. [34] reported a rate of 89.5%, Lee et al. [27] reported a rate of 73%, Chen et al. [22] reported a rate of 100%, Lee et al. [28] reported a rate of 73.2%, Antunes [17] reported a rate of 97.7%, Alsulaimani et al. [15] reported a rate of 85%, Malekfar et al. [30] reported a rate of 60%, Han et al. [24] reported a rate of 81.2% and Huynh et al. [14] reported a rate of 79.7%. These cell viability rates are considered high relative to those obtained in studies in which freezing was not gradual [323337]. All studies analyzed herein found that the morphophysiological characteristics, cell proliferation rate, and differentiation capacity of dental pulp mesenchymal cells were not altered after cryopreservation compared to the control group. Thus, they were positive for CD73, CD90 and CD105 surface markers, negative for CD45 and CD34, and had the ability to differentiate into chondrocytes, osteocytes, adipocytes, and fibroblast-like cells [141516172122232425262728293031323334353637].
CONCLUSIONS
According to this systematic review, the cryopreservation process involves 6 steps: dental element disinfection, pulp extraction, cell isolation, cell proliferation, cryopreservation itself, and thawing. Most studies showed a high rate of cell viability after thawing and a high rate of cell proliferation in both primary and permanent teeth using DMSO, programmable freezing, and storage in liquid nitrogen. In addition, none of the methods employed in the selected studies affected cell differentiation capacity or the fibroblastic morphology of the mesenchymal cells.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Santos CCO, Pupo YM, Fonseca-Silva T.
Data curation: Paes SM, Santos CCO.
Formal analysis: Fonseca-Silva T, Santos CCO.
Writing - original draft: Santos CCO, Cavenago BC, Paes SM.
Writing - review & editing: Santos CCO, Pupo YM, Fonseca-Silva T, Paes SM, Cavenago BC.