Influence of reciprocating and rotary instrumentation on microbial reduction: a systematic review and meta-analysis of in vitro studies
Article information
Abstract
Objectives
The purpose of this study was to conduct a systematic review and meta-analysis of in vitro studies regarding the effectiveness of reciprocating and rotary instrumentation on microbial reduction in root canals.
Materials and Methods
PubMed, Scopus, Web of Science, the Cochrane Library, and the gray literature were searched through December 2019. Studies comparing the influence of reciprocating and rotary instrumentation on the removal of microorganisms from root canals that quantified the antimicrobial effect were included. Data extraction was completed using a systematic form for data collection. The risk of bias of the studies was evaluated. Standardized mean differences (SMDs) and confidence intervals (CIs) were calculated using a random effects meta-analysis.
Results
Seventeen in vitro studies were included in this systematic review, of which 7 provided adequate data for inclusion in the meta-analysis. Both reciprocating and rotary systems were similarly effective in reducing the microbial load in infected root canals (SMD [95% CI], 0.0481 [−0.271, 0.367]). Three studies showed a low risk of bias, whereas most of the studies (82%) presented a medium risk.
Conclusions
Although both techniques decrease the microbial content (with reductions of 23.32%–88.47% and 23.33%–89.86% for reciprocating and rotary instrumentation, respectively), they are not able to provide complete disinfection of root canals.
INTRODUCTION
The removal of microorganisms and their byproducts from the root canal system is one of the main goals of root canal treatment, since the remaining infection is an important predisposing factor for persistent apical periodontitis [1]. To achieve this goal, chemomechanical preparation is a critical step in root canal treatment, and is performed by using irrigants and instruments to eliminate microorganisms [2].
Mechanical instrumentation of the root canal system is commonly performed using nickel-titanium (NiTi) rotary files because they shorten the treatment time, create more centered preparations, and produce less debris extrusion than hand files [3456]. Chemomechanical preparation using antimicrobial irrigants and rotary NiTi files can provide an endotoxin load reduction of more than 90% in infected root canals [7]. However, especially if the anatomy is complex, many areas of the root canal system may remain untouched and microorganisms may remain lodged in such areas [8]. New instruments and techniques have been introduced to achieve more effective instrumentation and disinfection. Most NiTi systems operate using a rotary motion and involve a large number of files; thus, the root canal preparation requires several steps and an extended time when the full sequence is used [9]. Recently, reciprocating motion in root canal instrumentation was introduced to increase the cyclic fatigue resistance of instruments compared with rotary systems, reducing the incidence of instrument fracture [10]. Reciprocating systems, including WaveOne (Dentsply Maillefer, Ballaigues, Switzerland) and Reciproc (VDW, Munich, Germany), are designed to enable instrumentation of the entire root canal with only 1 instrument [48].
In several studies, the effectiveness of reciprocating systems has been compared with rotary systems in terms of microorganism removal from infected root canals, with promising results [349]. In a recent systematic review of in vivo studies, the effects of reciprocating and rotary instrumentation on the reduction of microbial load were compared, and similar microbial reduction was found for both types of motion [11]. However, only 3 studies could be included in that review, and those studies presented a high risk of bias. Although systematic reviews of in vivo studies should provide a higher level of evidence, if the number of studies is low and the risk of bias is high, they do not provide concrete evidence and do not allow a meta-analysis to be performed. Therefore, the analysis of a number of current in vitro studies published in the literature on this subject may reveal important data and shed light on the methodological design of future studies. Thus, the purpose of this study was to systematically review in vitro studies regarding the effectiveness of reciprocating and rotary instrumentation on microbial load reduction in infected root canals.
MATERIALS AND METHODS
The protocol of the present study was registered in the PROSPERO international prospective database of systematic reviews. This systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [12].
Data sources and search strategy
The research question was as follows: “In extracted teeth undergoing root canal treatment, is reciprocating instrumentation more effective than rotary instrumentation for the removal of microbial content from experimentally infected root canals?” For the structured review question, the population, intervention, comparison, and outcome strategy was used. The population included extracted teeth experimentally contaminated with microorganisms following a sterilization procedure. The intervention was endodontic treatment using reciprocating instrumentation. The comparison was endodontic treatment using rotary instrumentation, and the outcome included the effect of the instrumentation technique on the removal of microorganisms from infected root canals.
The search was performed using electronic databases to identify articles published through December 2019. No limit was set on language or publication year. The electronic databases searched were PubMed (MEDLINE), Scopus, Web of Science (all databases) and the Cochrane Library. GreyLit (http://www.greylit.org) and OpenGrey (http://www.opengrey.eu) were used to search the gray literature. The main search terms were “WaveOne,” “Reciproc,” “microorganism,” “bacteria,” “polymerase chain reaction,” “colony forming unit,” “infection,” and “toxin.” These keywords were chosen from articles published in the International Endodontic Journal, Journal of Endodontics, and Australian Endodontic Journal, and enriched during the database searches. To identify additional articles, a hand search of the reference lists of eligible articles was also performed. The search strategy used is presented in Table 1.
Screening and selection of the studies
Two reviewers first independently scanned the titles identified in electronic and hand searches and decided whether they were relevant to the topic. If the title showed the potential for inclusion, the abstract was reviewed. If there was any doubt, the full text of the article was read. The full text of all eligible studies was obtained and further examined independently by each reviewer to determine whether they were eligible for this study based on the following inclusion criteria:
1. In vitro studies performed on fully formed human permanent teeth
2. Teeth that had not received any endodontic treatment previously
3. Teeth contaminated with microorganisms
4. Studies comparing the efficacy of reciprocating and rotary instrumentation for the removal of microorganisms from root canals
5. Studies that quantified the antimicrobial effect and reported the outcome as reduction in microbial load
The inclusion of each study was determined based on consensus between the 2 reviewers. Studies failing to meet any of the above criteria, including studies that analyzed microbial load reduction during retreatment, studies that examined the apical extrusion of bacteria, and studies that evaluated microorganism removal qualitatively, were excluded.
Data extraction
Data were extracted independently from the included studies by the 2 reviewers using a data collection form designed to summarize each study. Any disagreements were resolved through consensus between the reviewers. All aspects of interventions that could potentially influence the study outcomes were identified and included in the form. The data collection form was composed of specific details about the populations, interventions, study methods, and outcomes. The details extracted from studies are shown in Tables 2 and 3 [348913141516171819202122232425].
Quality assessment (risk of bias)
The quality of each study was assessed according to the following parameters:
1. Was the calculation of the required minimum sample size performed before experiments?
2. Were the samples randomly distributed to groups?
3. Was specimen sterilization confirmed after the sterilization procedures?
4. Was specimen contamination confirmed after the procedure of root canal contamination with microorganisms?
5. Were the root canal preparation procedures performed by a single operator?
6. Was the total irrigant volume standard in all groups?
7. Were the analyses performed by evaluators blinded to the groups?
8. Were one or more outcomes of interest reported incompletely?
The 2 reviewers assessed the studies independently according to the above criteria and classified the included studies as having a low, moderate, or high risk of bias. Any disagreements were resolved based on consensus between the reviewers. Studies that failed to report 2 items or fewer were classified as low risk, studies that failed to report 3 to 5 items were classified as moderate risk, and studies that failed to report 6 items or more were classified as high risk.
Meta-analysis
Quantitative data synthesis was carried out as a meta-analysis to combine comparable results using a software program (MedCalc Statistical Software version 19.0.5, MedCalc Software, Ostend, Belgium). Microbial reduction was selected as the outcome. The number of specimens in each group and the mean and standard deviation for microbial content at the initial sampling before root canal preparation (S1) and sampling immediately after root canal preparation (S2) were extracted from the studies. The standardized mean difference was calculated for each study.
Statistical heterogeneity between studies was analyzed using the I2 value, with low, medium, and high heterogeneity indicated by values of 25%, 50%, and 75%, respectively [26]. If the I2 score was closer to 0%, a fixed-effects model was used, whereas a random-effects model was used if the I2 score was closer to 100%. The results of the comparisons are shown with a forest plot.
RESULTS
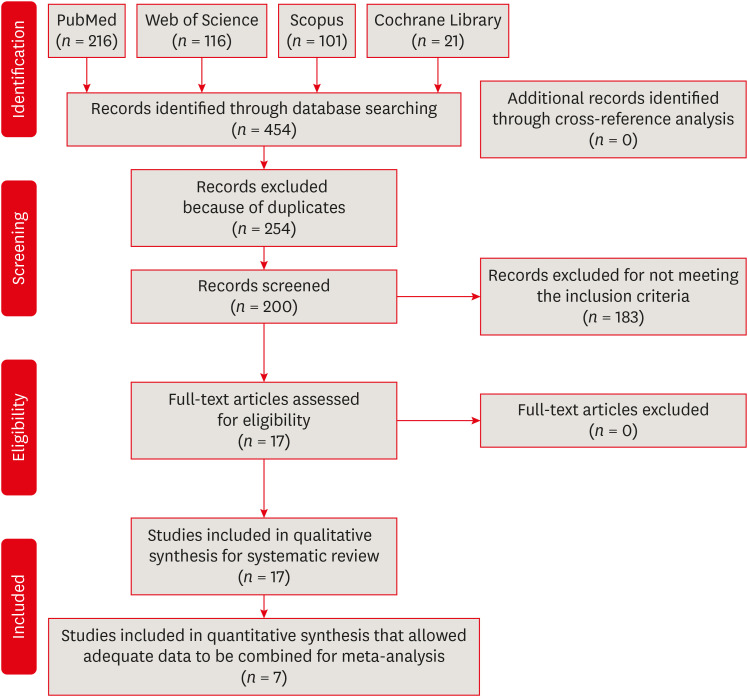
The search strategy is depicted as a flow diagram in Figure 1. The main characteristics of the included studies are shown in Tables 2 and 3. All specimens in the included studies were sterilized either with an autoclave or ethylene oxide before contamination with the chosen microorganisms [348913141516171819202122232425]. Sterilization was generally confirmed with the cultures of specimens serving as negative controls. This procedure was not mentioned in 5 studies [38142021]. Enterococcus faecalis was the most commonly used bacterium for the contamination of root canals [348131415161819202122232425]. In 1 study, Escherichia coli was used [9], while a mixture of microorganisms was used in another study [17]. Contamination was confirmed by either scanning electron microscopy or culture techniques such as Gram staining [348913141516171819202122232425]. The specimens were incubated with microorganisms for periods ranging between 24 hours and 30 days [38131421]. The number of colony-forming units (CFUs) was the most commonly assessed outcome measure [3489141516171819202122232425]. Rotary motion was superior to reciprocating motion for removing microorganisms in 5 studies [1317212425] while there were no significant differences in 11 studies regardless of the irrigant used during instrumentation [348914151819202223]. In 1 study, there was no significant difference between the motions in terms of bacteria reduction when NaOCl was used as an irrigant, whereas rotary motion was superior to reciprocating motion when saline solution was used [16]. In all studies, samples were collected before and immediately after root canal preparation. In addition, a third collection was performed after a period of time following root canal preparation in 3 studies [41819]. The tested tooth type, final apical diameter, and type and amount of irrigant used were different among the studies.
Risk of bias and meta-analysis
The methodological risk of bias of the included studies is presented in Table 4. Three studies presented selective reporting bias due to a lack of information on initial CFU values [152025]. Three studies showed a low risk of bias, whereas most of the studies (82%) presented a medium risk (Table 4).
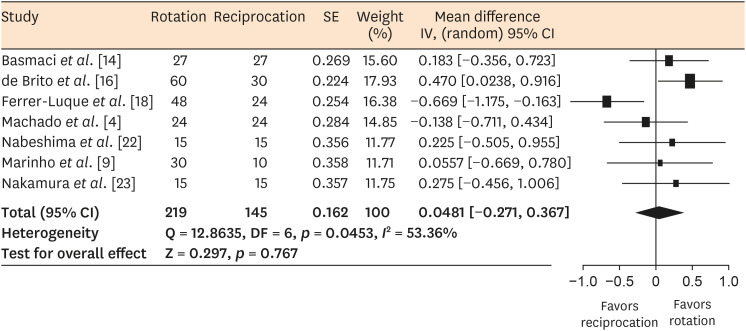
Seven studies were included in the meta-analysis as they provided adequate data to be combined [491416182223]. Significant heterogeneity was found (I2 = 53.36%, p < 0.05). Therefore, a random-effects model was used to perform the meta-analysis. No significant difference was found in the amount of microbial reduction between reciprocating and rotary motion (with reductions of 23.32%–88.47% and 23.33%–89.86% for reciprocating and rotary instrumentation, respectively) (p > 0.05) (Figure 2).
DISCUSSION
The complete elimination of microorganisms from the root canal system before obturation has been reported as a factor significantly related to successful treatment outcomes [2728]. Thus, it is important to identify more effective cleaning and shaping protocols in order to improve treatment outcomes. In this review, the effect of reciprocating and rotary instrumentation on the removal of microorganisms from infected root canals was evaluated. Based on the present findings, both rotary and reciprocating systems were equally effective in reducing the microbial load during root canal treatment. However, neither system resulted in complete eradication of microorganisms in root canals.
Single-file reciprocating techniques have become popular, since it has been claimed that they simplify and shorten the instrumentation process [38]. The only concern with this technique is its ability to clean the root canal due to the shorter contact time of the instruments with dentin walls. Moreover, the preparation of the root canals in a shorter time may lead to the use of disinfecting solutions at lower amounts or with shorter contact times [3]. According to previous studies, the shaping capability of reciprocating systems is comparable with that of rotary systems using a full range of instruments [829]. However, as different numbers of instruments are used with each system, it is difficult to standardize the duration of irrigation and volume of irrigant in the root canal. When the duration of irrigation and volume of irrigant are similar, the cleaning efficacy of reciprocating systems is also comparable with that of rotary systems [3]. Irrigation protocols, especially in terms of irrigant type and volume, varied among the studies included in the present review. Some studies used only saline solution or distilled water to directly compare the mechanical effects of the instrumentation systems and eliminated the influence of an antimicrobial solution [4131920222425]. Although the total irrigant volume was kept similar among the groups in the majority of the studies, there were some differences in the final irrigation protocols due to the different number of instruments. There were also differences in the diameter and taper of the final instruments used among the groups. The majority of these studies reported similar microbial reductions in root canals with both instrumentation techniques. Thus, the final taper or diameter of instrumentation may not be significantly associated with antimicrobial efficacy.
All studies included in the present review performed microbiological sampling using paper points. This technique has some limitations; for example, only the microorganisms in the root canal can be detected by sampling, while those inside the dentin tubules cannot be sampled [30]. Despite the significant reduction of microorganisms with the tested instrumentation systems, regrowth might have occurred in the root canals due to the remaining microorganisms in dentin tubules. Three studies evaluated regrowth and found that bacterial regrowth took place after instrumentation with all systems [41819]. The most commonly tested microorganism type was E. faecalis, and it was incubated in root canals for different time periods (between 24 hours and 30 days). In a previous study, it was reported that E. faecalis entered the growth phase after 3 hours of incubation, the stationary phase at 12 hours, and finally the starvation phase, the most resistant phase, at 48 hours [31]. Therefore, the incubation period chosen in in vitro studies can also affect the removal of microorganisms from root canals.
The included studies also analyzed some other variables. The most commonly applied technique to evaluate the microbial content was CFU calculation using culturing techniques, followed by molecular methods based on DNA detection. Molecular methods such as polymerase chain reaction exhibit higher sensitivity and can detect uncultivated microorganisms, unlike culture techniques [32]. However, microorganisms that are no longer viable in the root canal can also be detected with molecular methods. This may pose a problem when investigating samples taken immediately after treatment procedures [33]. It is well known that primary endodontic disease involves several Gram-negative bacteria species, the cells of which contain lipopolysaccharide (also known as endotoxin) [34]. Because endotoxin plays a role in the initiation and maintenance of disease, it is also important to assess endotoxin reduction in the root canal system; however, only 1 study included in this review did so [9]. Although no significant difference was found between the groups, there was a significant reduction in the amount of endotoxin after root canal preparation.
The present review revealed that the specimens were randomly distributed among groups in most studies, although the details of how random sequencing was performed unclear. Proper randomization should ensure that the chances of allocation to different groups are the same for all samples [35]. Allocation concealment is important and ensures that the operator does not have information about which group the specimen will be placed in [35]. It is assumed that the blinding of the operator could not be achieved in these studies due to the inherent differences in the instrumentation techniques; therefore, it was not considered an important factor. However, the blinding of outcome evaluators is important because ensuring that the evaluator does not know which intervention group a sample belongs to avoids a potential source of bias in the outcome measurement [35]. In most of the studies, blinding of the evaluator and sample size calculation were not performed and the procedures were not carried out by a single operator, all of which increased the risk of bias. The quality assessment of the included studies revealed that the studies had a low or moderate risk of bias. The results of the present review were obtained from in vitro studies, so it is difficult to draw direct conclusions regarding clinical applications. Although the highest level of evidence is provided by randomized controlled clinical trials, well-designed in vitro studies could also produce useful solutions for clinical problems and guide future research by identifying areas with knowledge gaps meriting further study and by revealing the limitations of previous studies [36].
Meta-analysis is a research tool designed to analyze and combine the results of randomized clinical trials in particular. This method can also be used to analyze in vitro studies. In the present review, only 7 in vitro studies could be combined for a meta-analysis due to the high level of heterogeneity in reporting the treatment outcomes. Variations in sample size, tooth type, irrigation protocol, tested microorganism type, and incubation period may have been the reason for statistical heterogeneity. Publication bias could not be evaluated due to the small number of studies included in the meta-analysis. Despite these limitations, the findings of this meta-analysis indicate that reciprocating and rotary instrumentation had similar efficacy for microbial load reduction.
For future in vitro studies evaluating microbial reduction in root canals, power analysis should be performed to determine the minimum sample size required before starting the experiments, and the randomization procedure should be described clearly. The irrigation protocol throughout root canal preparation should be kept similar in all groups when comparing the effects of preparation techniques. To make sure that the test set-up is working properly, specimen sterilization and contamination should be confirmed after the procedures of sterilization and contamination of root canals with microorganisms, respectively. The procedures including root canal preparation, irrigation, and sample collection from root canals should be performed by a single operator to avoid interoperator variability. Furthermore, the evaluator should be blinded to groups during the analysis to avoid detection bias. Such aspects of standardization would increase the quality of results reported in future studies.
CONCLUSIONS
Based on the findings of this review, reciprocating and rotary instrumentation are equally effective for microbial load reduction in infected root canals. Although both techniques decrease the microbial content, they cannot provide complete disinfection of root canals.
ACKNOWLEDGEMENTS
This review was registered in the PROSPERO database with the number of CRD42018089555.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Küçükkaya Eren S, Uzunoğlu-Özyürek E.
Data curation: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Formal analysis: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Funding acquisition: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Investigation: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Methodology: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Project administration: Küçükkaya Eren S.
Resources: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.
Software: Karahan S.
Supervision: Küçükkaya Eren S.
Validation: Karahan S.
Visualization: Küçükkaya Eren S, Uzunoğlu-Özyürek E.
Writing - original draft: Küçükkaya Eren S.
Writing - review & editing: Küçükkaya Eren S, Uzunoğlu-Özyürek E, Karahan S.