Cytotoxicity and biocompatibility of high mol% yttria containing zirconia
Article information
Abstract
Objectives
Yttria-stabilized tetragonal phase zirconia has been used as a dental restorative material for over a decade. While it is still the strongest and toughest ceramic, its translucency remains as a significant drawback. To overcome this, stabilizing the translucency zirconia to a significant cubic crystalline phase by increasing the yttria content to more than 8 mol% (8YTZP). However, the biocompatibility of a high amount of yttria is still an important topic that needs to be investigated.
Materials and Methods
Commercially available 8YTZP plates were used. To enhance cell adhesion, proliferation, and differentiation, the surface of the 8YTZP is sequentially polished with a SiC-coated abrasive paper and surface coating with type I collagen. Fibroblast-like cells L929 used for cell adherence and cell proliferation analysis, and mouse bone marrow-derived mesenchymal stem cells (BMSC) used for cell differentiation analysis.
Results
The results revealed that all samples, regardless of the surface treatment, are hydrophilic and showed a strong affinity for water. Even the cell culture results indicate that simple surface polishing and coating can affect cellular behavior by enhancing cell adhesion and proliferation. Both L929 cells and BMSC were nicely adhered to and proliferated in all conditions.
Conclusions
The results demonstrate the biocompatibility of the cubic phase zirconia with 8 mol% yttria and suggest that yttria with a higher zirconia content are not toxic to the cells, support a strong adhesion of cells on their surfaces, and promote cell proliferation and differentiation. All these confirm its potential use in tissue engineering.
INTRODUCTION
An extensive group of materials, including metals, ceramics, polymers, and composites as medical and dental prostheses, are used in medicine and dentistry. Materials used in medical and dental devices must possess both mechanical functions, such as wear and fatigue resistance, and biomedical capabilities. In dentistry, over the last decade, it has been observed that there is an increasing interest in ceramic materials made of partially stabilized tetragonal zirconia (3YTZP, 3 percent yttria, 97 percent zirconia, by weight) because of the patients' demands for improved esthetics [1234]. Even though this original high-strength tetragonal zirconia possesses excellent physical properties, it has a relatively high refractive index with a white hue and opacity [5]. The main goal in dentistry regarding the use of all-ceramic restorations are minimal or no application of a multistep technique to maintain esthetics. This is one of the key aims for developing a material with optical properties closely resembling the natural tooth. Zhang et al. used a combined approach to produce a more translucent version of zirconia by reducing the alumina content from 0.25% to less than 0.05% and improve the processing techniques [6]. Despite the enhanced esthetic appearance, these materials still lack the necessary optical feature for use on the anterior tooth. To overcome this drawback, researchers stabilized the pure zirconia with a significant cubic crystalline phase by raising the yttria amount to 8–12 mol% [5]. In the cubic phase zirconia, its crystallographic directions are different from the 3YTZ. So, the light scattering is less, resultant more translucent [57]. Cubic zirconium oxide (ZrO2) shows high mechanical strength, erosion, thermal insulation, ability to prevent crack propagation, alkali, and wear resistance [8].
The capability of a material coexistence with the living tissue without any deleterious effects termed biocompatibility [9]. The biocompatibility of materials used in the dental crown prosthesis is a critical issue because these materials are placed inside the oral cavity, and generates an active interface between the body and materials. In recent years, the biocompatibility of the 3YTZP was vastly examined using both in vitro and in vivo analysis, and in all these studies, 3YTZP showed remarkable biocompatibility [101112]. However, the biocompatibility of the high amount of yttria is still an important topic that needs to be investigated. The yttria (Y2O3) is thought to be 1 of the essential yttrium compounds and has a wide area of utilization, including effective growth inhibitors of various microorganisms and antimicrobial control systems [13]. The current research data regarding the biocompatibility and cytotoxic effects of Y2O3 are limited and controversial. Researchers reported that the Y2O3 nanoparticles (NPs) exhibit potential cytotoxicity to human foreskin fibroblasts cells and human embryonic kidney (HEK293) cells [1415]. It was also demonstrated that in osteoblast, the cytotoxicity produced by Y2O3 NPs was in a dose and size-dependent manner [16]. In the case of dental crown or restoration, the most critical problems are local toxicity, by substance release from dental materials, which causes inflammation or necrosis in adjacent tissues. Though it is not yet clear whether yttria-stabilized zirconia releases any substances or not, it is crucial to evaluate the cell and tissue compatibility of this higher mol% yttria containing zirconia before any biological application.
Zirconia ceramics showed excellent tissue reaction; however, this material has no direct cellular adhesion properties. Superficial adhesion and cell proliferation represent the first step of cell–biomaterial interactions. So, researchers modifying the material surface properties such as surface roughness and composition, though it has been considered the most critical parameters for enhancing the cell-material interactions.
In this study, we evaluated the biocompatibility of the commercially available 8 mol% yttria containing zirconia plate with or without surface polishing and collagen coating. Assessments involved cell adhesion, proliferation, and differentiation.
MATERIALS AND METHODS
Sample preparation
Commercially available 8 mol% yttria-stabilized zirconia (8YTZP) plates (91.5% ZrO2・8% Y2O2 dense plate, As One, Osaka, Japan), cut to a dimension of 25 × 25 × 2 (mm) used as the substrate for this study. According to the polishing technique, the samples were divided into 3 groups. To maintain the uniformity and minimize the experimental error, they were sequentially polished either only in a horizontal direction (1 G) or horizontal and vertical direction (2 G) each for 10 times using SiC-coated abrasive papers. Samples without polishing were referred to as No G group. After surface polishing of zirconia to restore the material to its initial stress-free state, the 8YTZP plates were thoroughly washed with deionized water, wiped, and place in an electric oven (HPM-0 G, As One) for 1 hour at 800ºC. Followed by dry heat sterilization at 180ºC for 2 hours. After heat treatment and sterilization, samples were coated with type I collagen (Corning, Corning, NY, USA). Briefly, 8YTZP plates were coated with 1 mL of type 1 collagen (1:5) for 10 minutes at 37ºC temperature. We removed the remaining coating solution and rinsed the plate at least 3 times with phosphate buffer saline (PBS, Takara Bio, Shiga, Japan). Finally, we dried the coated surface for 30 minutes inside the incubator and kept it in 4ºC temperature until use. We prepared 6 different types of zirconia samples; 1 G with or without coating (n = 12 each), 2 G with or without coating (n = 12 each), and samples without any polishing considered as negative control and named No G with or without coating (n = 12 each).
Characterizations
The crystal phase of the zirconia before and after heat treatment was determined by the X-ray diffraction (XRD) technique (Ultima IV, Rigaku, Tokyo, Japan). After collagen coating, we evaluated the surface topography of the 1 G, 2 G, and No G (with or without coating) samples using scanning electron microscopy (SEM) (JCM-5100, JEOL, Tokyo, Japan). We measured the average surface roughness at a magnification of 10× with Color 3D Laser Scanning Microscope (VK-9700, Keyence, Osaka, Japan). The surface wettability of each sample was also measured. Briefly, 3 µL distilled water was dropped onto the prepared zirconia surface from a height of 3 mm. Immediately after dropping, we captured the image with an HDMI digital microscope (UM6-cam, 3R Solution Co., Ltd., Fukuoka, Japan), and we measured the contact angle (CA) was measured from the obtained image by the drop tangent method.
In vitro analysis
Mouse fibroblast-like cells L929 was purchased from The European Collection of Authenticated Cell Cultures and used for cell adherence and cell proliferation analysis. The L929 cells were seeded 2.4 × 105 cells/surface-modified zirconia plates, either 1 G, 2 G, or No G with or without collagen coating. The cells were then cultured in alpha minimal essential medium (α-MEM, Wako Pure Chemical, Osaka, Japan) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA) and 1% penicillin-streptomycin (PS, Nacalai Tesque, Kyoto, Japan) for 7 days. Then, the medium of the cultured cells was removed, and the cells were fixed with 4% paraformaldehyde (Wako Pure Chemical). Finally, the cell nucleus was stained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO, USA) to determine the cell adherence, density of cells and cell proliferation after culturing for 2 hours, 3 days and 7 days using an image analysis program (Image J, NIH, Bethesda, MD, USA).
Bone marrow-derived mesenchymal stem cells (BMSC) were used for cell differentiation analysis and isolated from the bone marrow of BALB/C1 mice (CLEA, Tokyo, Japan) as previously described [17]. Animal experiment procedures strictly adhered to the Guidelines for Animal Experiments of Yamagata University and carried out with the approval of the “Animal Use and Care Committee of Yamagata University (Approval No. 31149)”. Briefly, mesenchymal stem cells (MSCs) were generated from tibia and femur bone marrow of 6-week-old mice. Similar to the L929 cells, MSCs were cultured in basic medium α-MEM supplemented with 10% heat-inactivated FBS and 1% PS. Non-adherent cells were removed after 24 hours, and adherent cells were harvested and re-cultured in 100 mm2 dishes for 3–4 passages before using. The medium was replaced every 3 days. BMSCs were characterized by immunostaining analysis using a monoclonal CD44 antibody (1:300) (Abcam, Cambridge, UK). BMSC were cultured to examine osteoblastic differentiation. They were supplemented with either standard culture medium (α-MEM + 1% PS + 10% FBS) or with osteogenic medium, containing standard culture medium with β glycerophosphate (1 × 10−2 M) (Sigma-Aldrich), L-ascorbic acid (50 mg/mL) (Sigma-Aldrich), and dexamethasone (1 × 10−6 M) (Sigma-Aldrich). The cells were maintained at 37ºC in humidified air with 5% CO2. The culture media was replenished every 3 days. For analyzing the mineralized nodule formation, BMSCs were cultured either at 6-well plates or the prepared zirconia plate for up to 28 days, and alizarin red S staining was performed. Briefly, cells were washed with PBS and fixed with 95% ethanol at 37°C for 15 minutes. We washed the cells with distilled water to neutralize the acidic condition and subsequently stained with 1% alizarin red S (Wako Pure Chemical) solution for 5 minutes. The remaining staining dye was washed away with distilled water and visualized by an optical microscope. We quantified the percentage area of mineralization with Image J according to the protocol explained by Shah et al. [18].
Statistical analysis
The experimental data of all groups were analyzed independently using a 1-way analysis of variance to ascertain the differences in the variables among them. A value of p < 0.05 was considered statistically significant.
RESULTS
Surface characterization
XRD pattern analysis
The XRD pattern analysis of the 8 mol% zirconia was performed before and after heat treatment at 800ºC using Cu Kα radiation at 40 kV and 200 mA. All the obtained diffraction peaks are similar to the standard data of pure cubic ZrO2 assigned by the “Joint Committee on Powder Diffraction Standards.” The full width at half maximum of the peaks corresponding to (111), (200), (220), and (311) reflections of cubic zirconia were measured (Figure 1A). The average crystallite size before and after heat treatment calculated from Scherrer equation was 12.94 nm and 12.92 nm, respectively.
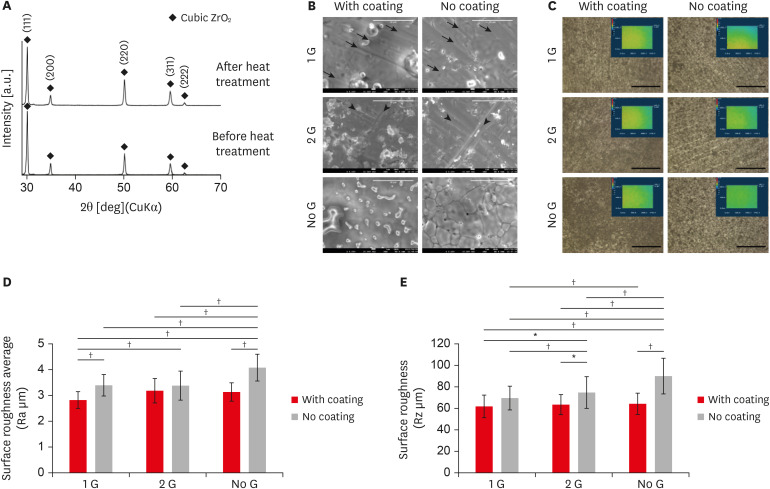
(A) X-ray diffraction patterns of zirconia before and after heat treatment. All X-ray diffraction peaks are comparable with the standard data of the pure cubic phase containing ZrO2. (B) SEM showed the microstructure of zirconia plates. All coating groups showed collagen coating along with the polished line. The sample without any polishing and coating showed a typical feature pattern of larger grains consisting of clusters of smaller grains (scale bar: 10 µm; arrow and arrowhead: grinding line). (C) Characterized the topography of the zirconia with or without coating using 3-dimensional laser microscopy (scale bar: 100 µm). (D and E) Graph depicting the quantification of the surface roughness Ra (μm) and Rz (μm) of all zirconia samples.
SEM, scanning electron microscopy.
*p ≤ 0.05; †p ≤ 0.001.
SEM analysis
The microstructure of the 6-zirconia test groups is shown in Figure 1B. All coating group showed clear collagen coating and polished line, 1 G (black arrow), and 2 G (black arrowhead). The sample without polishing and without coating showed a typical feature pattern of larger grains consisting of clusters of smaller grains of the zirconia (Figure 1B).
Surface roughness
The topography of the zirconia with or without coating, characterized using 3D laser microscopy and 3D laser microscopy (Figure 1C). The amplitude parameters Ra and Rz decreased in the coating surface. Ra and Rz values of all coating specimens ranged from 2.792 to 3.125 μm and 61.69 to 63.68 μm, respectively (Figure 1D and E). Those of all without coating samples were significantly higher and ranged from 3.402 to 4.0808 μm and 69.804 to 90.058 μm, respectively (Figure 1D and E). Among all samples, 1 G sample was associated with significantly lower Ra and Rz values.
Water CAs
The water CA data are shown in Figure 2A and B. Specimens without coating showed significantly lower CAs than the collagen-coated group (p < 0.001). Within the coated specimen groups, the Ra values decreased (Figure 1D), and the CA (Figure 2B) values increased (up to 74.0°) in polished samples.
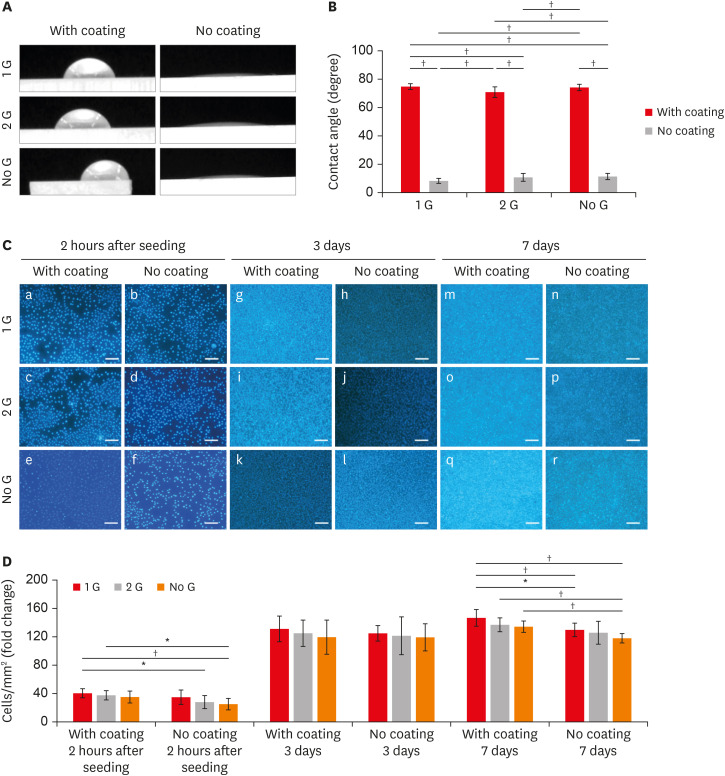
(A) Water CA images with or without coating/polishing zirconia surface. (B) CA measurement of samples with or without coating/polishing. (C) Fluorescent staining of L929 cell cultures of the cubic phase containing zirconia with or with coating/polishing in the different time intervals (a-f: 2 hours after seeding; g-l: 3 days; m-r: 7 days) (scale bar: 100 µm). (D) Graph depicting the numbered cell proliferate in different zirconia plates.
CA, contact angle.
*p ≤ 0.05; †p ≤ 0.001.
In-vitro analysis
We evaluated the cellular response of L929 cells cultured on samples to elucidate the effects of coating and surface grinding.
Cell attachment and proliferation
L929 cells were cultured on zirconia plates for 2 hours to evaluate the cell attachment behavior and followed by 3 days and 7 days to evaluate the cell proliferation ability. Cells were attached more to the coated 1 G surface than to the corresponding no coat surfaces at all culture times. A significantly higher attachment of cells is observed for the coated surfaces compared to the surface of the without coating after 2 hours of culture (Figure 2C). The cell number steadily increased on all coating surfaces. The proliferation of L929 cells cultured on coated 1 G surfaces was higher than that on the surfaces of the corresponding without coating surface at all culture time points (Figure 2D). Even though there was no significant difference can be observed on day 3, the value for the coated surface was significantly higher. It is noticeable that no significant differences in cell attachment and proliferation were detected within the 1 G and 2 G in both coated and no coated groups at any culture time-point.
Responses of BMSCs
We examine the mineralization ability to assess the role of surface modification in cell functions. In the present study, BMSCs were cultured for 28 days with a differentiation medium. The mineralized area was detected by alizarin red staining after 28 days of culture. Both alizarin red staining (Figure 3A) and the amount of calcium mineral deposits (Figure 3B) showed that the coated samples with polishing were considerably higher compared to that on without coating and polishing.
DISCUSSION
Over the last decade, there has been an increasing interest in the ceramic materials in dentistry for improved esthetics [1920]. A perfect dental ceramic should meet 2 main requirements: excellent mechanical property and optimum esthetics. The most commonly used ceramics are the high-strength tetragonal crystalline phase, partially stabilized zirconia with 3 mol% yttria (3YTZP) [421]. However, due to the insufficient translucency and opacity of these 3YTZP, many researchers attempt to develop the more transparent, fully stabilized cubic phase zirconia with 8 mol% or more yttria [5722232425]. 3YTZP is a chemically stable dental ceramics [26]; however, the consequences of various environments in a human body, the dissolution of yttria, have considered before applying as a biomedical material. It has already been reported that yttria is a cytotoxic element depending on the magnitude of exposure and is the size and concentration-dependent [1416]. Therefore, the biocompatibility of those fully stabilized zirconia with higher yttria content still needs to be evaluated.
Given that biological tissues interact with only the outer surface of the materials, several methods have been used to improve the phlegmatic properties of the ZrO2 surfaces to enhancing the cell-material interaction [2728293031]. Micro size surface roughness plays a conductive role in the initial cellular adhesion [18]. To provide a friendly microenvironment for cellular adhesion and proliferation in vitro, Type I collagen is one of the most widely used coating materials. Immobilized type I collagen coating on the zirconia surface showed better cell responses, including adhesion, proliferation, and mineralization potential of the BMSCs [3233]. In this study, we polished the 8YTZP plate with SiC-coated paper and coated it with type I collagen and emphasized the cell response to zirconia ceramics.
Measuring the angle between a liquid droplet on a solid surface assesses surface wettability. In a hydrophilic surface, the water droplet spreads along the surface, resulting in a lower CA [343536]. In a biomaterial, hydrophilic surfaces are the desirable properties for the interactions between the materials and biological fluids, cells, and tissues [3537]. In our study, both coating and without coating samples showed a strong affinity for water, and the cellular attachment was higher regardless of the wettability. Our data agreed with other in vitro studies stating that the wettability actively promotes cell attachment, proliferation, and differentiation [3537].
To evaluate the biocompatibility of a biomaterial's potential strongly depends on interferences between the cell and the host. Cellular growth, adhesion, and its functional activities depend on the physicochemical properties of the materials used [38]. We elucidate the effects of surface roughness and surface coating on cell adhesion, proliferation, and differentiation, by evaluating the biological functions of fibroblastic and bone marrow-derived mesenchymal stem cells cultured on samples.
The initial cellular adhesion is a prerequisite to successive cell proliferation, and cell morphology influences the cell differentiation potentialities [3940]. In this study, 8TYZP showed integrated cellular adherence in all samples. A significantly higher attachment of L929 cells was observed for the treated surfaces than the control after 2 hours. All samples showed a continual proliferation pattern. No significant differences in cell proliferation were detected between the 2 groups on day 3. However, on day 7, unidirectional polished and coated surfaces showed significantly higher cell proliferation tendency compared to others.
The technique for modifying the material surfaces usually affects cell-material interactions, which in turn influence the cellular functional activity. To see the effects of the modified surfaces of the materials on cell differentiation, we cultured the BMSCs with or without collagen-coated ZrO2. After 28 days, both cases showed strong cellular differentiation and alizarin red positive staining. However, mineralized sample quantification revealed a significantly higher differentiation rate in the coated groups compared with the without coating groups. This data confirms that comparatively less hydrophilic surfaces serve as a platform to deliver stable BMSC populations and may remarkably improve the efficiency of stem cell differentiation [41]. All these findings evidenced that regardless of the surface treatment, high yttria contained zirconia exhibit excellent cell attachment, proliferation, and cell differentiation, indicating a promising restorative material with biological properties.
CONCLUSIONS
According to the analyses of cell adhesion, proliferation, and differentiation demonstrate a suitable biological response of the fully stabilized cubic phase zirconia with 8 mol% yttria. As a dental restorative material, 8 YTZP shows a promising future in clinical applications. However, this is a pilot study. Research should continue to fully characterize the materials before any medical application using more comprehensive test methods.
ACKNOWLEDGMENTS
The authors would like to thank Professor Yamamoto Osamu for the assistance provided during the experimental procedure.
Notes
Funding: This research was partly supported by scientific research grant subsidies from Yamagata University.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Kazi GAS.
Data curation: Kazi GAS.
Formal analysis: Kazi GAS, Yamagiwa R.
Funding acquisition: Kazi GAS.
Investigation: Kazi GAS, Yamagiwa R.
Methodology: Kazi GAS.
Project administration: Kazi GAS.
Resources: Kazi GAS.
Software: Kazi GAS, Yamagiwa R.
Supervision: Kazi GAS.
Validation: Kazi GAS.
Visualization: Kazi GAS.
Writing - original draft: Kazi GAS.
Writing - review & editing: Kazi GAS.

