The effect of root canal irrigants on dentin: a focused review
Article information
Abstract
Despite the vast literature on the effects of root canal irrigants on the dentin characteristics, the precise effects of clinically relevant irrigation sequences remain unclear. In this review, we systematically dissect the role of different sequential irrigation approaches that are used in clinical endodontics. Using a systematic search strategy, we attempt to answer the question: ‘Which irrigating sequence has the most deleterious effects on dentin structure and properties?’ The effect of irrigants on the dentin composition and mechanical properties have been reviewed. A wide variety of concentrations, duration and techniques have been employed to characterize the effects of chemicals on dentin properties, thus making it impossible to draw guidelines or recommendations of irrigant sequences to be followed clinically. It was apparent that all the studied irrigation sequences potentially result in some deleterious effects on dentin such as decrease in the flexural strength, microhardness, modulus of elasticity and inorganic content and organic-inorganic ratio of the dentin. However, the literature still lacks comprehensive investigations to compare the deleterious effect of different irrigation sequences, using a wide variety of qualitative and quantitative methods. Such investigations are essential to make clinical recommendations and strategize efforts to minimize chemically-induced damage to dentin characteristics.
INTRODUCTION
Dentin is a unique composite material constituting the bulk of the tooth structure and absorbing the mechanical loads acting on the tooth [1]. Microscopically, this mineralized connective tissue is abundant with organic ground substances or the extracellular matrix [2]. Type I collagen fibers predominate the organic constituents, playing a pivotal role in distributing the external stress applied to the tooth. The inorganic components of dentin include hydroxyapatite crystals and other mineral salts, such as carbonates and amorphous calcium phosphates [3]. Microstructurally, dentin contains tubular spaces (dentinal tubules), which contain cytoplasmic processes and dentinal fluid [4], making it sensitive to structural and biological alterations. Bacterial presence has been demonstrated within the dentinal tubules, to as deep as 300 to 500 µm [567]. Therefore, irrigants should be able to diffuse into the dentinal tubules to elicit antimicrobial effects [789]. The organic collagen fibers per se, are vulnerable to tissue-derived and microbial-derived enzymatic activities [101112]. Furthermore, the use of non-specific proteolytic chemicals such as sodium hypochlorite (NaOCl) to disinfect the infected dentin may exemplify this damage to collagen [13].
The primary goal of mechanical instrumentation (shaping) of the root canals is to “scrape” and remove the septic load, including infected dentin. However, current evidence shows that endodontic instruments are unable to contact all the parts of the root canal wall, leaving behind untouched areas [1415]. Thus, the use of chemical adjuncts is considered a sine qua non in root canal treatment. These chemical adjuncts fall into one of the following categories: Proteolytic agents, demineralizing agents/chelating agents, antiseptics, topical antibiotics. Common examples include NaOCl (proteolytic agent), ethylenediaminetetraacetic acid (EDTA; demineralizing agent), chlorhexidine (antiseptic) and doxycycline (antibiotic) [1617]. Of these, NaOCl and EDTA are the most common clinically used root canal irrigants [1618]. Evidence shows that these irrigants, adversely affect the physical and mechanical properties of the dentin [192021].
NaOCl is the most commonly used irrigant due to its antimicrobial, antibiofilm and tissue-dissolving properties. However, it is unable to remove mineralized debris such as calcospherites, and the hard tissue debris that is formed during root canal instrumentation [22]. Hence, a demineralizing agent is used after the use of NaOCl, to dissolve the inorganic salts by forming metal chelates. The most commonly used chelator is EDTA [23]. However, the chemical interaction between NaOCl and EDTA leads to a decrease in the availability of chlorine, decreasing the tissue dissolving capacity and/or antimicrobial properties of NaOCl [24]. Moreover, the NaOCl/EDTA irrigation regimen is unable to completely remove the accumulated hard tissue debris (AHTD) produced during root canal instrumentation [2526]. Therefore, a new irrigation regimen termed continuous chelation was introduced, in which, a weak chelator, 1-hydroxyethylidene-1,1-bisphosphonate or etidronic acid (HEBP or EA) is mixed with NaOCl, to be used as an all-in-one irrigating solution. This strategy prevents the formation of hard tissue debris [27]. There is evidence to show that this regimen demonstrates comparable AHTD removal [28] and superior antibiofilm effects [2930] compared to the NaOCl/EDTA sequence.
In the clinical setting, these chemical adjuncts are used in a sequence, to enhance the root canal disinfection and condition the dentin to optimize the interactions with root fillings [31]. A recent review attempted to address the effect of different irrigating solutions on the mechanical properties of the dentin [32]. However, the exact effects of sequential irrigation with proteolytic and demineralizing agents are less understood. Understanding the effects of sequential chemical application on dentin will help in elucidating its effects on dentin collagen and, thereby explaining why structural failures are common in endodontically treated teeth. Therefore, the aim of this focused review was to discuss the effects of irrigating sequences on dentin.
REVIEW
This focused review adopted a structured search strategy, but discussed the results as a narrative, owing to the nature of the topic. An advanced search was carried on the PubMed database using the keywords and the search strategy as shown in Figure 1. The files were generated and evaluated in the EndNote X8.2 software (Clarivate Analytics, Philadelphia, PA, USA). A total of 593 articles were retrieved from the search. Following exclusion, 42 studies were included for further analysis. The full-text review showed that only 20 studies [3334353637383940414243444546474849505152] investigated the effect of irrigating sequences. Of these, 2 studies [3852] used only qualitative scoring methods and were hence excluded. Thus, 18 studies were included in this review. 5 studies [3334353637] investigated the effect of irrigation sequences on dentin composition, while 13 [39404142434445464748495051] analyzed the effect of irrigating sequences on the mechanical properties of dentin (Figure 1).
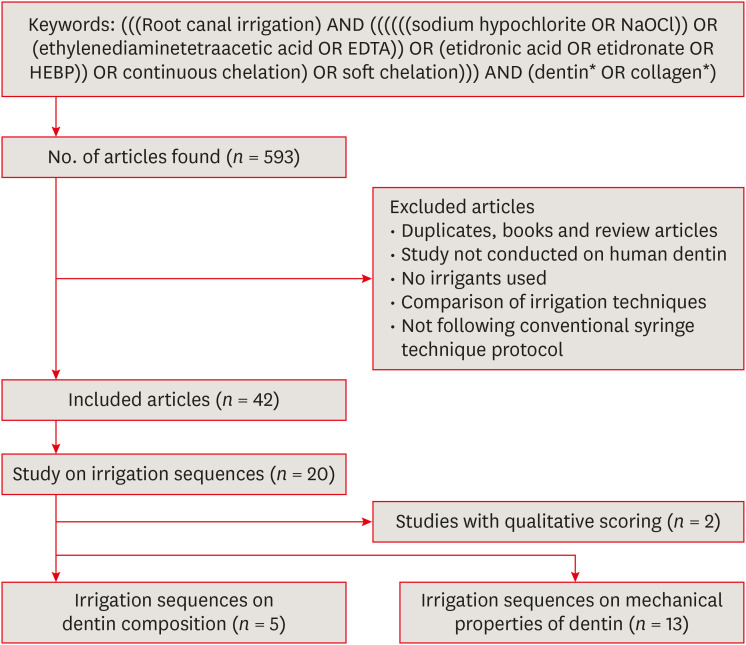
Search strategy and article selection for the review.
NaOCl, sodium hypochlorite; EDTA, ethylenediaminetetraacetic acid; HEBP, 1-hydroxyethylidene-1, 1-bis-phosphonate.
Effect of irrigation sequences on dentin composition
Three studies investigated the change in mineral content following chemical treatment of dentin, while 2 analyzed the collagen-apatite ratio (Table 1). Sequential irrigation of EDTA/NaOCl significantly increases the calcium (Ca)/phosphorus (P) and magnesium (Mg) level compared to saline irrigation on the dentin surface [33]. However, the increase in Ca/P level (i.e., the inorganic component ratio) was highest when only NaOCl irrigation is used. NaOCl, being a proteolytic agent, dissolves the organic ground substance, thus increasing the hydroxyapatite proportions on the treated dentin surface. Similarly, dentin treated with EDTA and EDTA/NaOCl showed maximum calcium loss from the dentin surface with 1 minute and 5 minutes of the treatment when compared to distilled water treatment [34]. A similar pattern of compositional effects was also observed when NaOCl/EDTA was compared with NaOCl/EDTA/NaOCl, using different concentrations of NaOCl and EDTA for different duration [35]. NaOCl/EDTA/NaOCl sequence significantly decreased the Ca and P composition compared to the NaOCl/EDTA group. Interestingly, sequences involving higher concentrations of NaOCl and EDTA (5% and 17%, respectively) showed the highest reduction of Ca and P values with maximum oxygen distribution on the dentinal surface, compared to 3% NaOCl and 8% EDTA. It should be noted that duration of treatment also had an impact on the elemental distribution. There was an increase in C and a significant decrease in Ca, P and O percentage, when duration of 5% NaOCl/17% EDTA/5% NaOCl is increased from 2-2-1 minutes to 5-5-5 minutes of exposure, respectively.
Zhang et al. [3637] calculated the apatite/collagen ratio of dentin powder treated with different irrigating solutions using Fourier-transform infrared spectroscopy. They compared different concentrations of NaOCl for different time periods, with and without a final rinse of 17% EDTA. The dentin was subjected to different concentrations of NaOCl for variable times, while concentration and duration of EDTA exposure remained the same, thus making NaOCl treatment to be the variable factor in both the studies. Irrigation with EDTA alone decreased the apatite/collagen ratio, compared to the untreated control group, as there is a demineralization of the hydroxyapatite components with EDTA treatment. The increase in NaOCl concentration from 1.3% to 5.25% showed a significant increase in the apatite/collagen ratio compared to that of the control group with no treatment. This infers that the increase in duration and concentration of NaOCl dissolves more of organic content thus exposing higher inorganic content to the external surface of the dentin. There was no significant difference in the flexural strength value with increase in the duration of exposure from 10 minutes to 240 minutes. However, 5.25% NaOCl for a duration of 120 minutes or above and a 2-minute exposure of 17% EDTA showed a significant increase in the ratio compared to that of the control group, groups treated with 1.3% NaOCl.
These studies indicate that the sequences involving NaOCl (a non-specific organic dissolving agent) such as NaOCl/EDTA, EDTA/NaOCl and NaOCl/EDTA/NaOCl, significantly decrease the Ca and P, or the inorganic content of the dentin when compared to irrigation with EDTA alone.
Effect of irrigation sequences on mechanical properties of dentin
A variety of mechanical properties have been tested including microhardness [39404142434445464748], flexural strength [495051], modulus of elasticity [51] and ultimate tensile strength [50] (Table 2). Six studies tested the microhardness of dentin after the use of 17% EDTA/NaOCl (2.5%, 2.6% or 5.25%) sequence [394041424344]. The time period of irrigants used, the load, the duration of load, and the depth of measurement were different in different studies. Although these studies varied in the duration of irrigation, load duration and depths of measurement, they all concluded that the EDTA/NaOCl sequence resulted in a significant reduction in dentin microhardness compared to the control group (saline, deionized water or NaOCl).
Three studies [464748] measured the microhardness of dentin after treatment with NaOCl/EDTA. Zaparolli et al. [46] and Dineshkumar et al. [47] used a concentration of 1% to 1.3% NaOCl with 17% EDTA and showed a significant reduction in the microhardness compared to the control (deionized water irrigation). However, as the studies have followed different durations of treatment, the hardness values cannot be compared to each other. Dineshkumar et al. [47] also compared 1.3% NaOCl/17% EDTA to that of 1.3% NaOCl/18% HEBP, where there is no significant difference in hardness values. A key concern with this study is that HEBP is meant to be used in a mixture with NaOCl and not independently.
When different concentrations of NaOCl (2% and 5%) are used for 30 minutes followed by 17% EDTA for 2 minutes, there appear to be no significant changes in the hardness of the dentin sub-surface, compared to the distilled water control [48]. Moreover, the dentin closer to the root canal lumen of the NaOCl/EDTA treated group did not show any significant difference in the microhardness values when compared to NaOCl group or control group.
Three studies [495051] investigated the flexural strength of dentin treated with different chemicals. Mai et al. [49] and Cecchin et al. [50] have used varying concentrations of NaOCl for different duration, followed by EDTA rinsing. Mai et al. [49] showed that a 10 minutes exposure of NaOCl followed by 2 minutes EDTA rinsing did not decrease the strength, while a 60 minutes exposure of NaOCl significantly reduced the flexural strength of the dentin. Cecchin et al. [50] also noted a significant reduction of flexural strength, ultimate tensile strength and fracture resistance of dentin treated with 30 minutes of NaOCl and 1 minute of EDTA. These results were also supported by another work which compared the flexural strength of dentin treated with NaOCl/EDTA, NaOCl/EDTA/NaOCl and EDTA [51]. This study concluded that a total of 24 minutes exposure of NaOCl significantly decreased the flexural strength of the dentin and EDTA had no substantial role in this effect.
The use of NaOCl/EDTA increases the apatite/collagen ratio [37], thus disturbing the homogenous arrangement of organic and inorganic components of dentin. Such disturbance eventually affects the flexural strength and elastic modulus of the dentin [53]. The NaOCl/EDTA irrigation sequence was the most studied, followed by EDTA/NaOCl. However, only 2 studies investigated the NaOCl/EDTA/NaOCl and 1 study investigated the NaOCl/HEBP sequence. Notably, the studies have shown that NaOCl alone had a significant effect on the dentin, immaterial of the use of EDTA [36374951].
Concentration and time-dependent effects of NaOCl
NaOCl is a chlorine-releasing oxidant [1836]. It undergoes saponification reaction to neutralize the amino acids of the organic compounds, that is eventually degraded by the hypochlorous acid [53]. Collagen fibers are triple helical structures of polypeptide chains [5455], encapsulated within hydroxyapatite crystals. NaOCl per se has no effect on the mineral salts. Thus, as long as the collagen fibers are protected by hydroxyapatite, they are not directly affected by the proteolytic agent [5556]. The penetration of the low molecular size hypochlorite anion may still contribute to collagen degradation. Therefore, the effect of NaOCl on mineralized collagen fibers is time and concentration-dependent [1337].
NaOCl being proteolytic in nature, breaks the collagen fibers to smaller peptide chains, thus degrading the fibers [57]. In addition to a decrease in the flexural strength and modulus of elasticity [58]. It is also responsible for surface erosion and inefficient removal of the smear layer [59]. NaOCl (5% and 10%) cause an irregular surface erosive pattern of the peritubular and intertubular dentin in deciduous teeth, thus being dubbed as “corroded surfaces” [60]. Higher concentrations of NaOCl (5% for instance) results in severe alterations of the dentin matrix peripherally [43]. This is clinically important, considering that higher concentrations of NaOCl (6%) penetrated significantly deeper (up to 300 µm) into the dentinal tubules, compared to lower concentrations [61]. How this impacts the dentin ultrastructure and properties remain unknown. Furthermore, NaOCl may also slowly dissolve the encapsulated collagen fibers [61]. It is evident that a concentration > 3% for 15 minutes may degrade the inorganic matrix embedded-collagen fibers. The use of NaOCl alone has demonstrated surface areas with high concentrations of the organic matrix of dentin [62], and such deproteination is a slow process and non-uniform process, creating vertical channels, termed as deproteinization channels [62].
AUTHORS' PERSPECTIVES
The clinical challenge in root canal treatment remains in our ability to balance the “biological” goals with the “mechanical” objectives of the therapy. Ideally, it is recommended to follow an irrigation regimen that achieves optimum antimicrobial efficacy via biofilm elimination, yet, does not presents detrimental effect on the mechanical integrity of the tooth. This requirement is further complicated by the compromised root canal fillings-dentin interface post-treatment. While numerous studies have characterized the “adhesion” and “leakage” mechanisms at the root filling-dentin interface using surrogate outcomes such as bond strength/dislocation resistance, deeper understanding of root filling-dentin interface is definitely lacking.
In this review, we sought to find an answer to the question: ‘Which irrigating sequence has the most deleterious effects on dentin structure and properties?’ From the biological standpoint, the NaOCl/EDTA/NaOCl sequence combined with ideal irrigation methods appears to be more effective than other sequences in root canal disinfection. NaOCl/EDTA sequence is suggested to result in exposed collagen fibrils on the root canal surface. Another important sequence to consider is the continuous chelation strategy (NaOCl/HEBP). Despite its introduction in 2009, detailed investigations on the effect of this sequence on dentin, are lacking in literature. Surprisingly, thorough investigations on the dentin ultrastructure after different irrigation regimens are still limited.
These are matters of conjecture at this point: ‘What are the clinical implications of these findings?’ ‘Do these chemical effects on dentin matrix lead to bulk material property changes?’ ‘Which irrigating sequence maintains the ultrastructural integrity of dentin as well as the mechanical integrity of root-filled teeth?’ ‘What happens to that exposed collagen or degraded collagen?’
CONCLUSIONS
Root canal irrigants, when used individually and in sequence, appear to decrease the flexural strength and microhardness of dentin, with disproportionation of the inorganic and organic components. NaOCl dissolves the pulp tissue and microbial biofilms, at the same time non-uniformly creating and spreading throughout the deproteinization channels. When EDTA is used subsequently, it removes the hard tissue debris, opens up the dentinal tubules and exposes the collagen fibers. Interestingly, the changes in the dentin properties such as microhardness or apatite-collagen ratio are dependent on NaOCl, rather than EDTA. However, the effects of a subsequent NaOCl rinse (NaOCl/EDTA/NaOCl) or the newer irrigating regimens such as continuous chelation (NaOCl/HEBP), on the dentin at an ultrastructural level remains to be investigated.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions:
Conceptualization: Neelakantan P, Kishen A.
Data curation: Rath PP.
Formal analysis: Rath PP, Neelakantan P.
Supervision: Neelakantan P, Kishen A, Yiu CKY, Matinlinna JP.
Writing - original draft: Rath PP, Kishen A, Neelakantan P, Yiu CKY, Matinlinna JP.
Writing - review & editing: Rath PP, Neelakantan P, Yiu CKY, Matinlinna JP.




